Microstructural Study of Arc Beads in Aluminum Alloy Wires with an Overcurrent Fault
Abstract
:1. Introduction
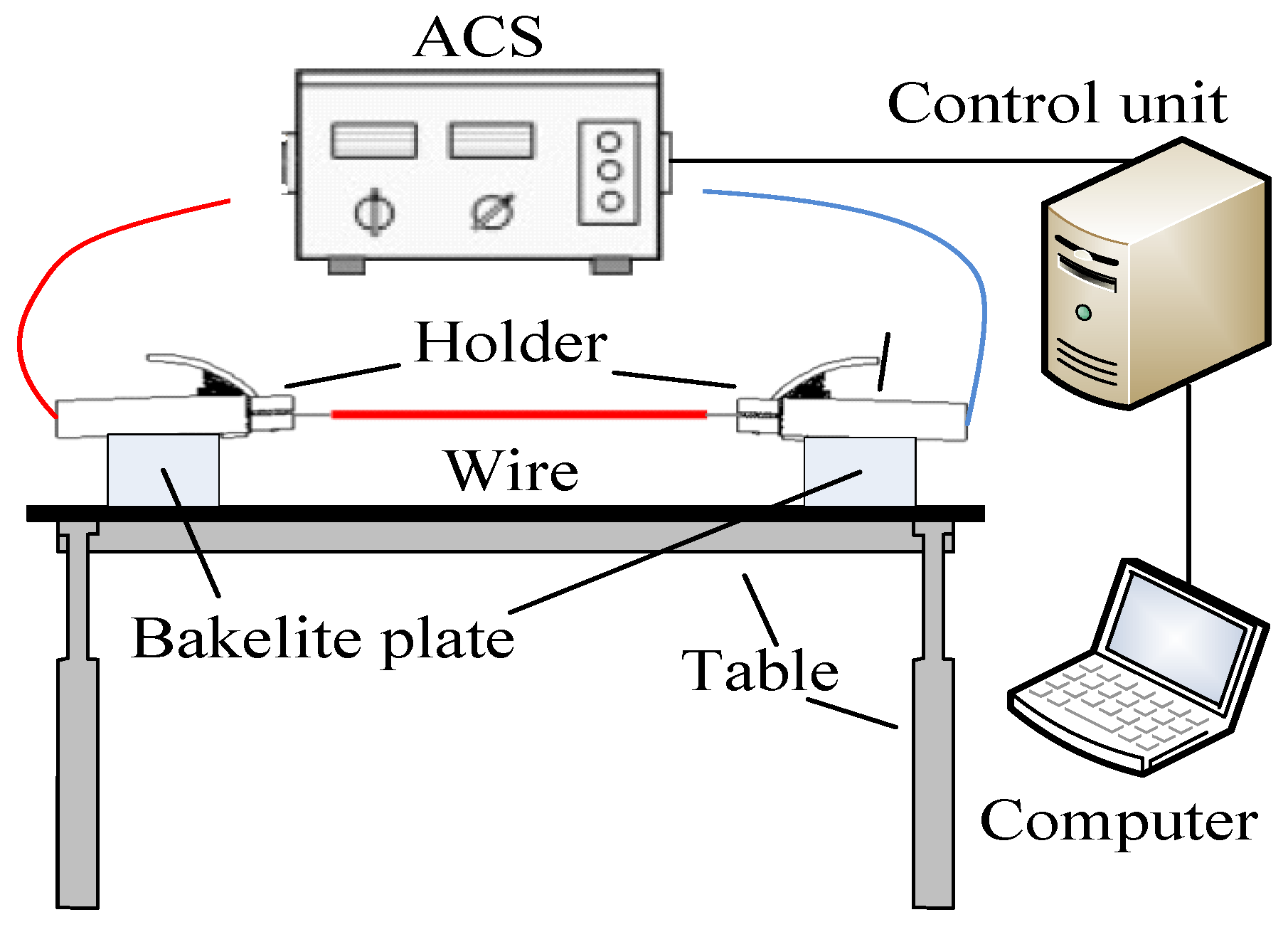
2. Materials and Methods
3. Results and Discussion
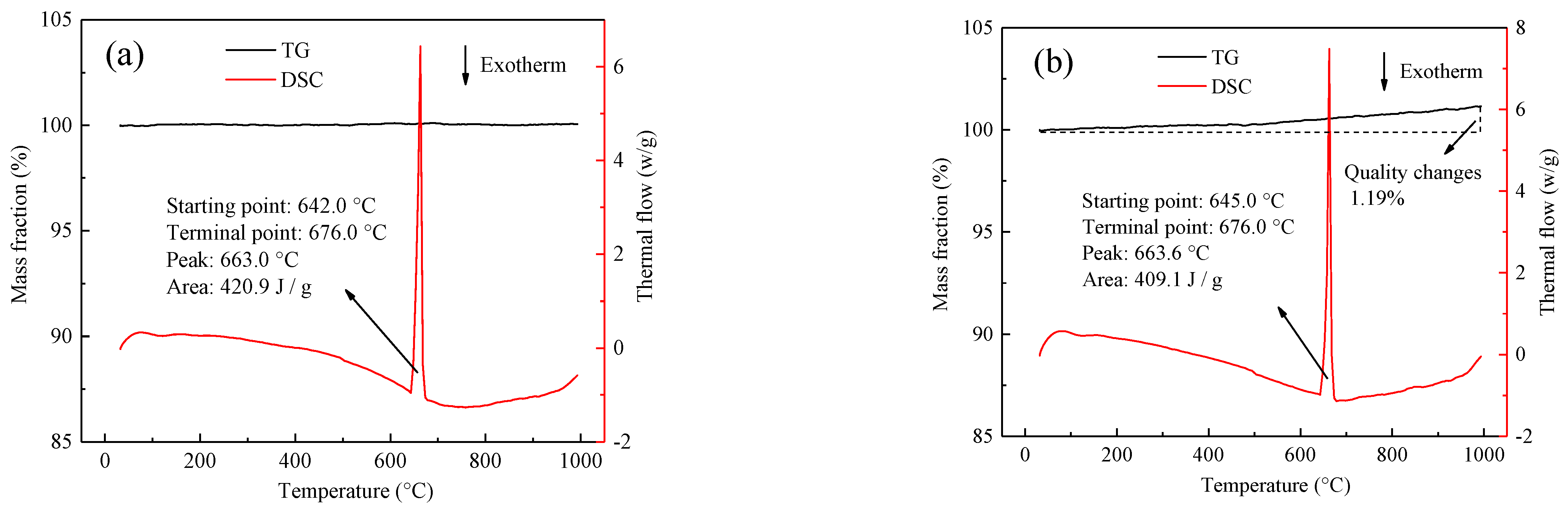
3.1. Properties of Wires at the Rated Current
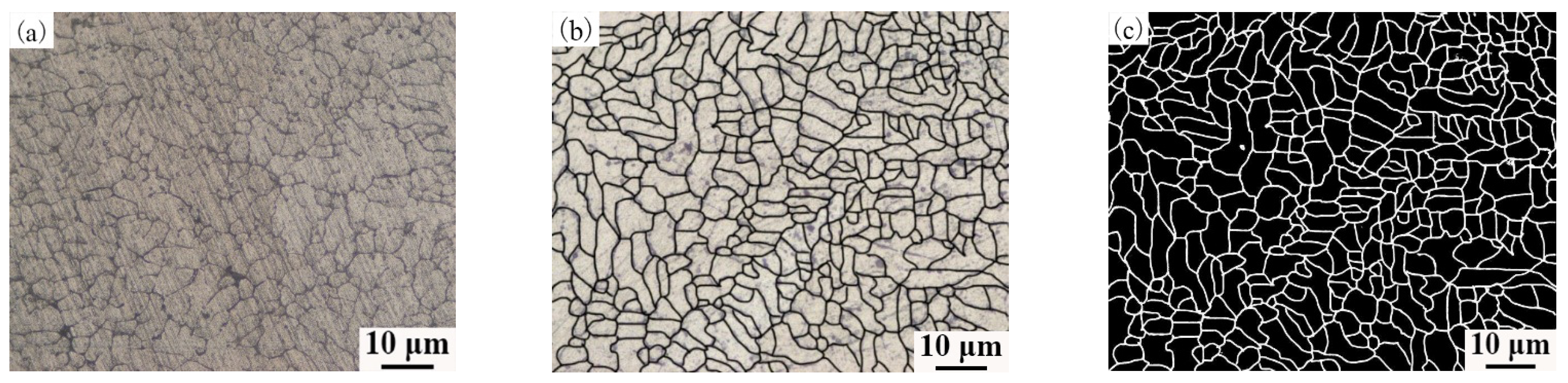
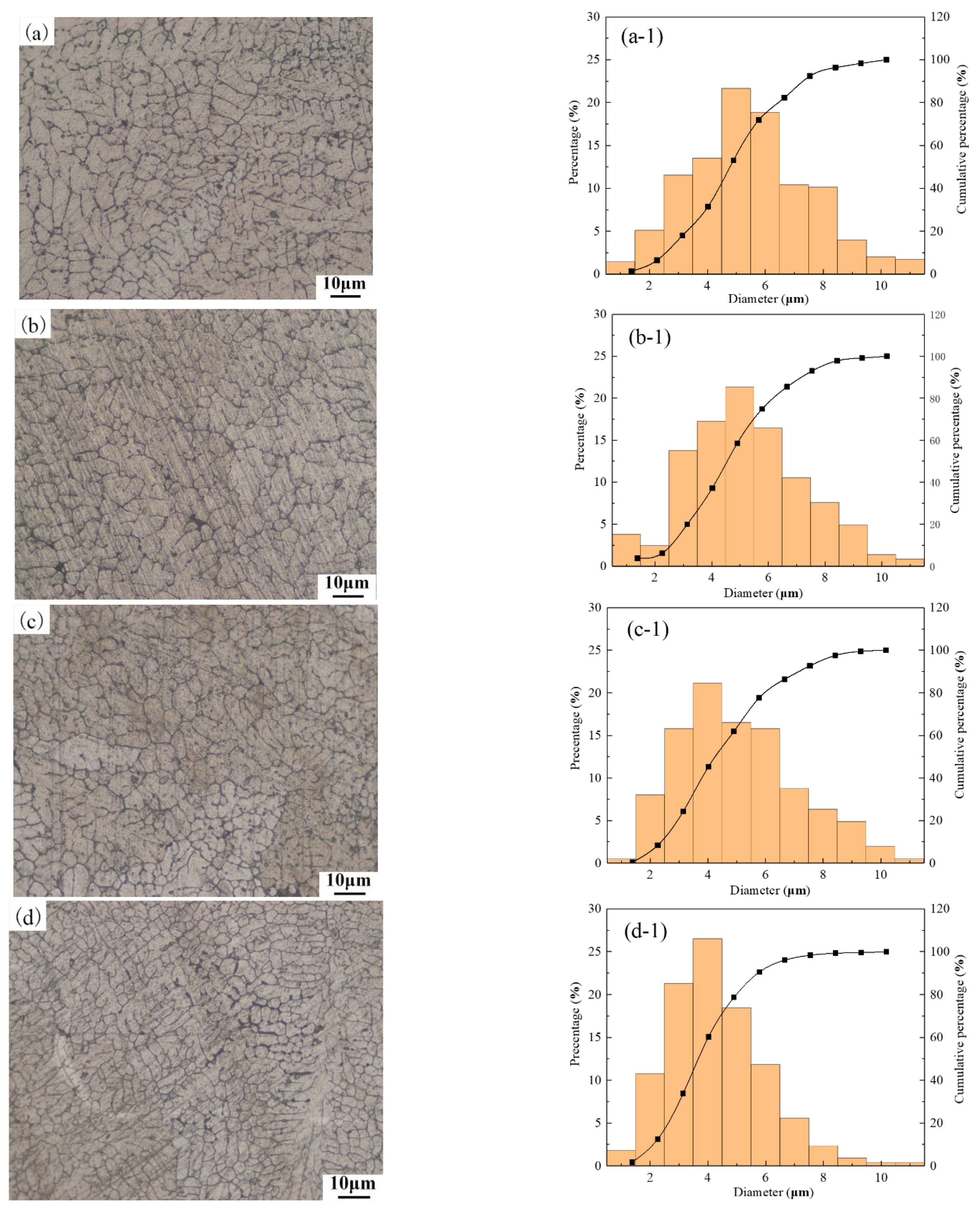
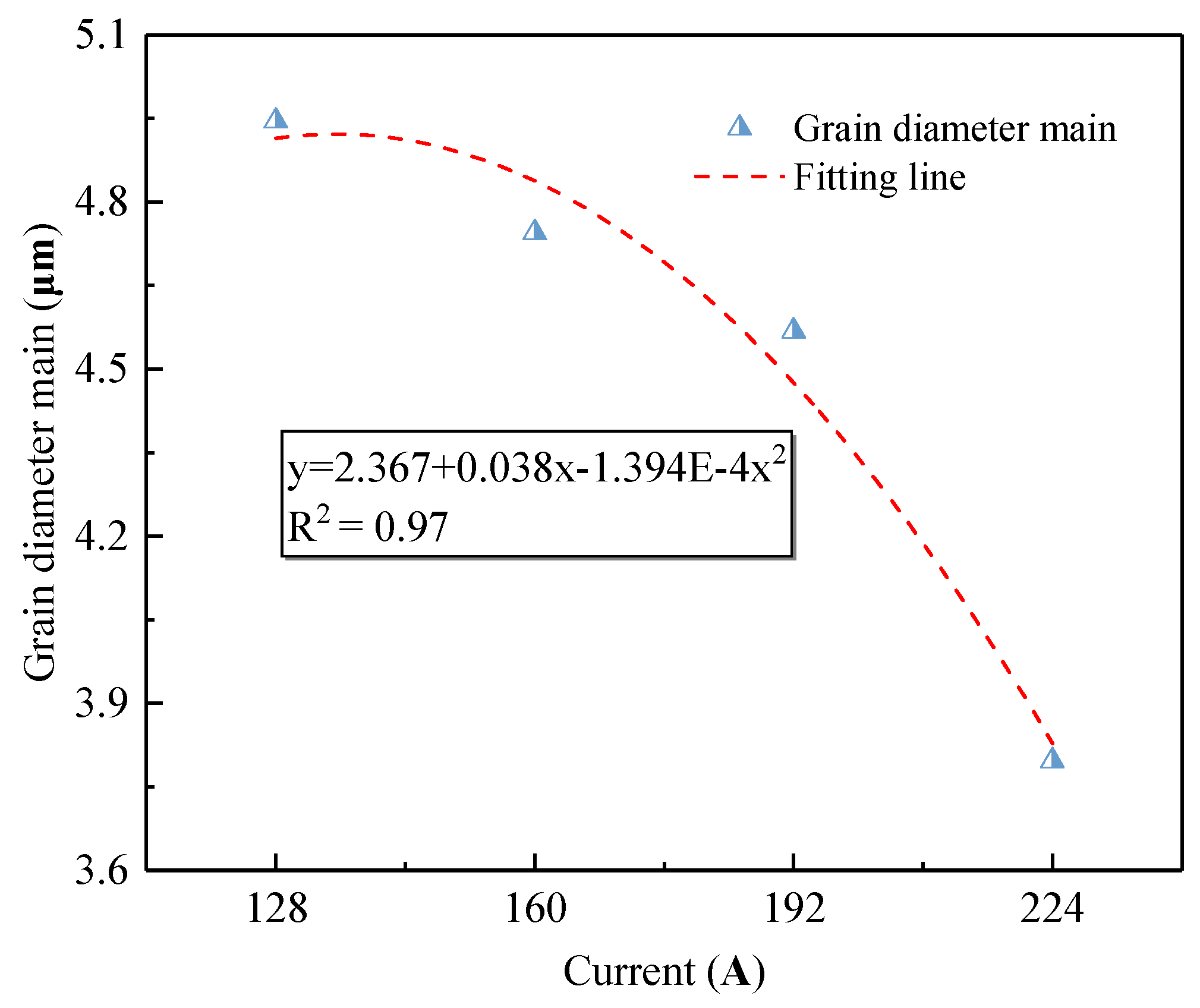
3.2. Metallographic Structure of Aluminum Alloy OABs
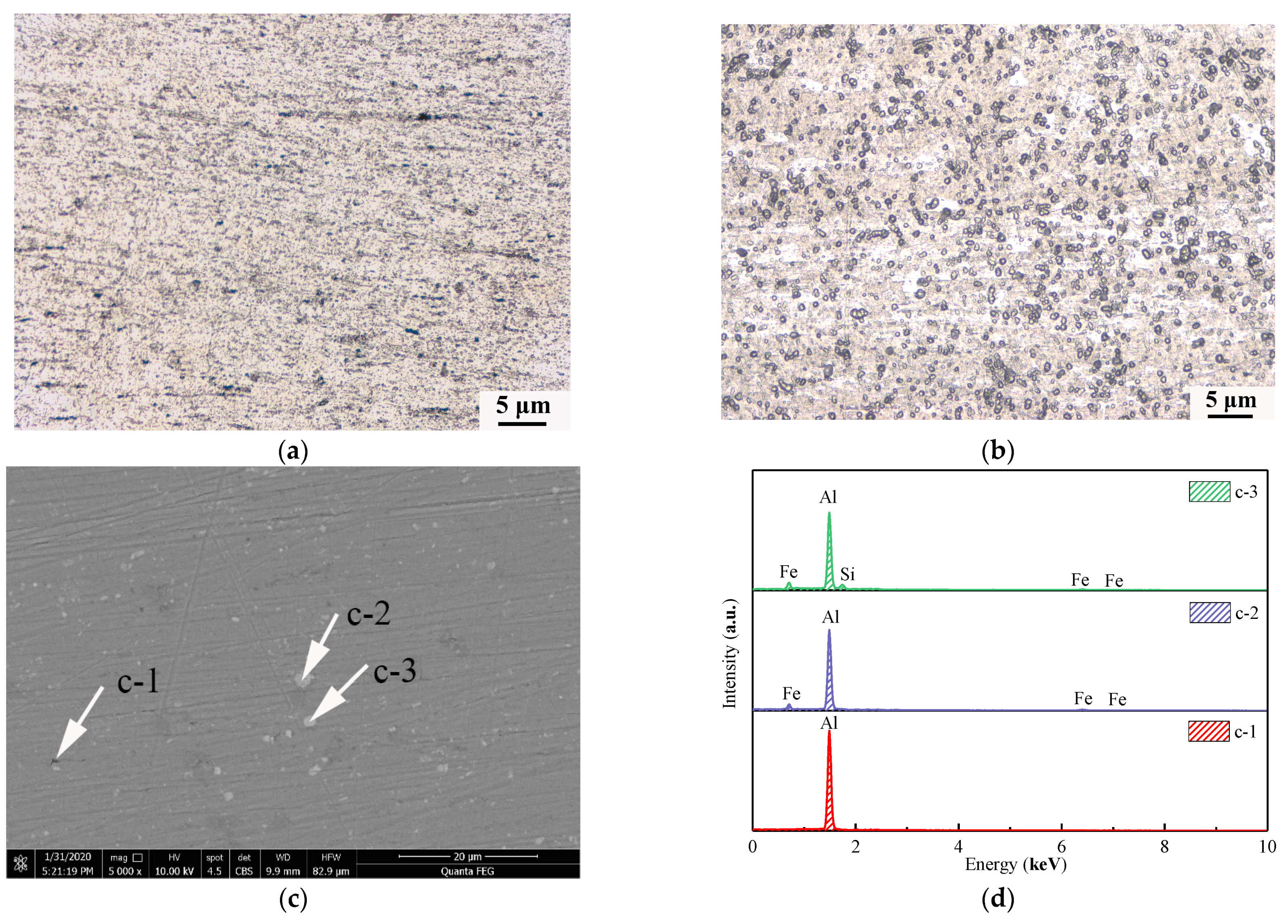
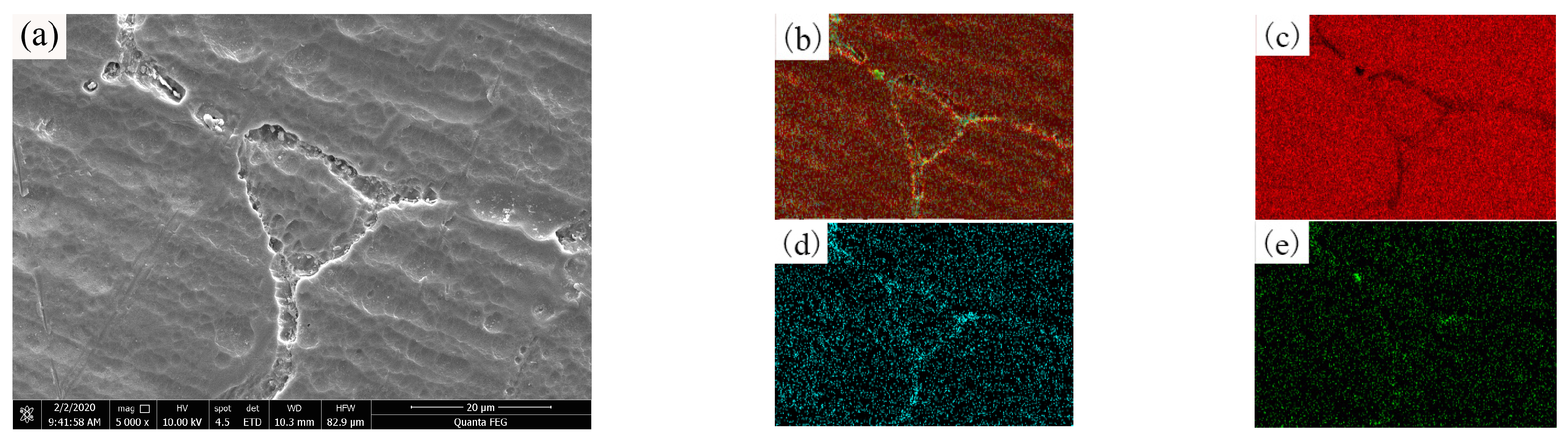
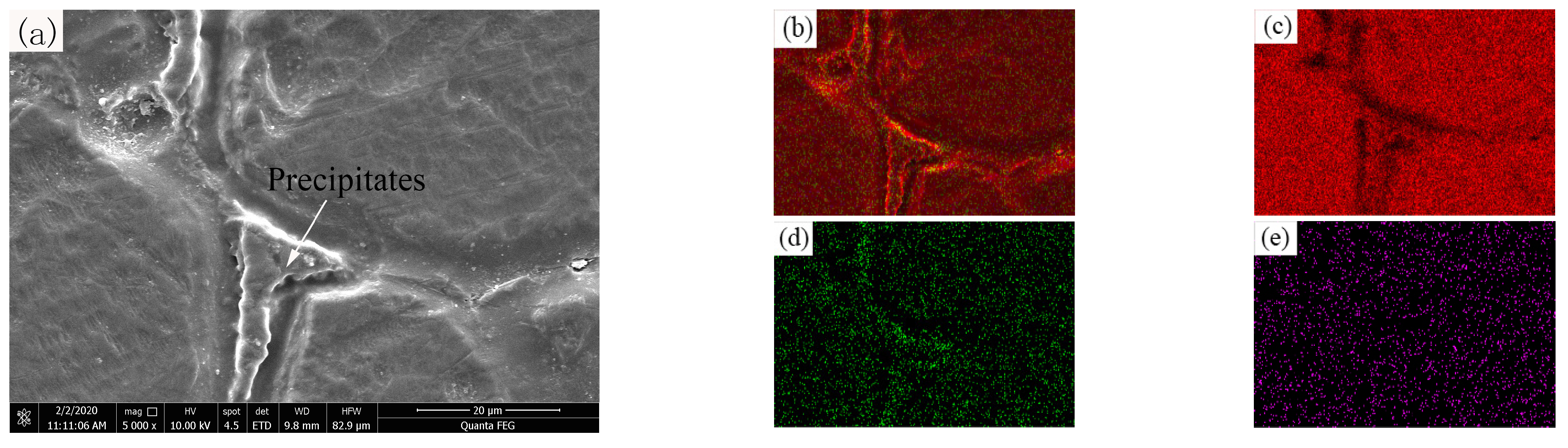
3.3. Influence of Alloying Elements on the Microscopic Morphology of OABs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.Y.; Li, W.J.; Han, Y.; Gao, Q.; Ma, G. Alloying design of a thermal-resistant aluminum alloy conductor material with high conductivity. Trans. Mater. Heat Treat. 2014, 35, 17–21. [Google Scholar]
- Sambor, W.; Miroslaw, O.; Lukasz, W. Development of innovative aluminum alloys for production of overhead electrical conductors. Light Met. Age 2015, 73, 26–29. [Google Scholar]
- Han, X.; Li, Y.; Pang, H.; Li, C.; Cui, L.; Wang, J.; Liu, Y. Effect of isothermal annealing on microstructure and properties of 8030 aluminum alloy wire. Heat Treat. Metals. 2020, 45, 154–160. [Google Scholar]
- Tremblay, K.J. Poor electrical connection ignites church fire. Epigenomics 2015, 7, 5–13. [Google Scholar]
- Hong, X.Y. The Comparative research of melted trace due to fire-burning and short-circuit. J. Liaoning Univ. 2014, 41, 230–234. [Google Scholar]
- Babrauskas, V. Ignition Handbook; Fire Science Publishers; Society of Fire Protection Engineers: Issaquah WA, USA, 2003. [Google Scholar]
- Babrauskas, V. Mechanisms and Modes for Ignition of Low-voltage, PVC-insulated Elec-trotechnical Products. Fire Mater. 2006, 30, 150–174. [Google Scholar] [CrossRef]
- Xiao, K.C.; Xue, Y.X.; Yang, L.; Wei, F.W.; Lei, B.; Liu, B.W.; Bo, W. Investigation of evolution process and molten marks characteristics of overcurrent fault. J. Xian Univ. Sci. Technol. 2020, 40, 393–399. [Google Scholar]
- Iwashita, T.; Hagimoto, Y.; Sugawa, O. Characterization of arc beads on energized conductors exposed to radiant heat. Fire Mater. 2017, 41, 1072–1078. [Google Scholar] [CrossRef]
- Babrauskas, V. Research on electrical fires: The state of the art. Fire Saf. Sci. 2008, 9, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Babrauskas, V. Arc Mapping: A Critical Review. Fire Technol. 2018 54, 1–32. [CrossRef]
- NFPA921. Guide for Fire and Explosion Investigations; National Fire Protection Association: Quincy, MA, USA, 2017. [Google Scholar]
- Erlandsson, R.; Strand, G. An investigation of physical characteristics indicating primary or secondary electrical damage. Fire Saf. J. 1985, 8, 97–103. [Google Scholar] [CrossRef]
- Takaki, A. On the Effect of Thermal Histories upon the Metallographic Structure of Electric Wires; National Research Institute of Police Science: Kashiwa, Japan, 1971; Volume 24, pp. 48–56. [Google Scholar]
- Babrauskas, V. Arc Beads from Fires: Can ‘Cause’ Beads be Distinguished from ‘Victim’ Beads by Physical or Chemical Testing? J. Fire Prot. Eng. 2004, 14, 125–147. [Google Scholar] [CrossRef]
- Wei, G.; Ying, W.; Shujun, L.; Liantie, W. Study on the in-depth composition of beads formed by fuse breaking of electric wire at different oxygen concentrations by auger electron spectroscopy. Spectrosc. Spectr. Anal. 2010, 30, 2003–2005. [Google Scholar]
- Ying, W.; Qing, S.M.; Xin, M.W.; Wei, G.; Man, D. XPS Analysis of beads formed by fuse breaking of electric copper wire. Spectrosc. Spectr. Anal. 2010, 30, 1408–1412. [Google Scholar]
- Hai, R.W.; Jian, Y.L.; Hao, W.Y.; Dong, L. The Microstructure and phase composition of metal melting marks caused by different fire. Spectrosc. Spectr. Anal. 2012, 32, 1984–1988. [Google Scholar]
- Park, J.; Kang, J.H.; Lee, E.P.; Young, H.K.; Sun, B.B. New approach to distinguish copper molten marks based on quantitative microstructure analysis using electron backscatter diffraction. Fire Technol. 2021, 57, 1667–1682. [Google Scholar] [CrossRef]
- Kuan, H.L.; Yung, H.S.; Guo, J.C.; Jaw, M.C. Microstructural study on molten marks of fire-causing copper wires. Materials 2015, 8, 3776–3790. [Google Scholar]
- Chen, C.Y.; Ling, Y.C.; Wang, J.T.; Chen, H. SIMS depth profiling analysis of electrical arc residues in fire investigation. Appl. Surf. Sci. 2003, 203, 779–784. [Google Scholar] [CrossRef]
- Dongbai, X.; Wen, W.; Shilei, L.; Shi, D. Visual and oxide analysis for identification of electrical fire scene. Forensic Sci. Int. 2018, 285, 21–24. [Google Scholar]
- Chungseog, C.; Hyangkon, K.; Kilmok, S.A. Study on the short-circuit characteristics of vinyl cords damaged by external flame. Fire Sci. Eng. 2004, 18, 72–77. [Google Scholar]
- Babrauskas, V. How do electrical wiring faults lead to structure ignitions? Fire Mater. 2001, 52, 39–49. [Google Scholar]
- Xin, J.; Huang, C.F. Fire risk assessment of residential buildings based on fire statistics from China. Fire Technol. 2014, 50, 1147–1161. [Google Scholar] [CrossRef]
- Zhi, J.Y.; Shuang, S.C.; Jun, D.; Xue, X.X.; Wei, F.W. Microstructural Characteristics of Arc Beads with Overcurrent Fault in the Fire Scene. Materials 2020, 13, 4521. [Google Scholar]
- Xue, X.X. Investigation of the Evolution Process and Microstructure of Molten Marks after Overcurrent Fault of Aluminum Alloy Wire; Xi’an University of Science and Technology: Xi’an, China, 2020. [Google Scholar]
- ASTM E3-11. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM E407-07. Standard Practice for Macroetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Feng, M.; Guang, Y.Y.; Zhen, J.X.; Zhi, Q.C.; Tong, M.W. Effect of Eu addition on the microstructures and mechanical properties of A356 aluminum alloys. J. Alloy. Compd. 2015, 650, 896–906. [Google Scholar]
- Fang, H.; Sun, J.; Wang, H.; Deng, Y. Influence of alloy added trace cerium on microstructure and properties of 7136 aluminum. J. Chin. Soc. Rare Earths 2016, 34, 313–319. [Google Scholar]
- Hurley, M.J.; Gottuk, D.T.; Kuligowski, E.D.; Puchovsky, M.; Torero, J.L.; Watts, J.M.; Wiczorek, C.J. SFPE Handbook of Fire Protection Engineering, Society of Fire Protection Engineers; Springer: Berlin, Germany, 2016. [Google Scholar]



| Sample | Chemical Compositions (Mass Fraction/%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mg | Zn | B | Else | Al | ||
| Single | Total | ||||||||
| AA8176 | 0.03–0.15 | 0.40–1.0 | - | - | 0.10 | - | 0.05 | 0.15 | Balance |
| Al | - | - | - | - | - | - | - | - | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yu, Z.; Li, Y.; Wang, W.; Xu, L. Microstructural Study of Arc Beads in Aluminum Alloy Wires with an Overcurrent Fault. Materials 2021, 14, 4133. https://doi.org/10.3390/ma14154133
Xu X, Yu Z, Li Y, Wang W, Xu L. Microstructural Study of Arc Beads in Aluminum Alloy Wires with an Overcurrent Fault. Materials. 2021; 14(15):4133. https://doi.org/10.3390/ma14154133
Chicago/Turabian StyleXu, Xueyan, Zhijin Yu, Yang Li, Weifeng Wang, and Lan Xu. 2021. "Microstructural Study of Arc Beads in Aluminum Alloy Wires with an Overcurrent Fault" Materials 14, no. 15: 4133. https://doi.org/10.3390/ma14154133
APA StyleXu, X., Yu, Z., Li, Y., Wang, W., & Xu, L. (2021). Microstructural Study of Arc Beads in Aluminum Alloy Wires with an Overcurrent Fault. Materials, 14(15), 4133. https://doi.org/10.3390/ma14154133





