Rosehip Extract-Functionalized Magnesium Hydroxide Nanoparticles and Its Effect on Osteoblastic and Osteoclastic Cells
Abstract
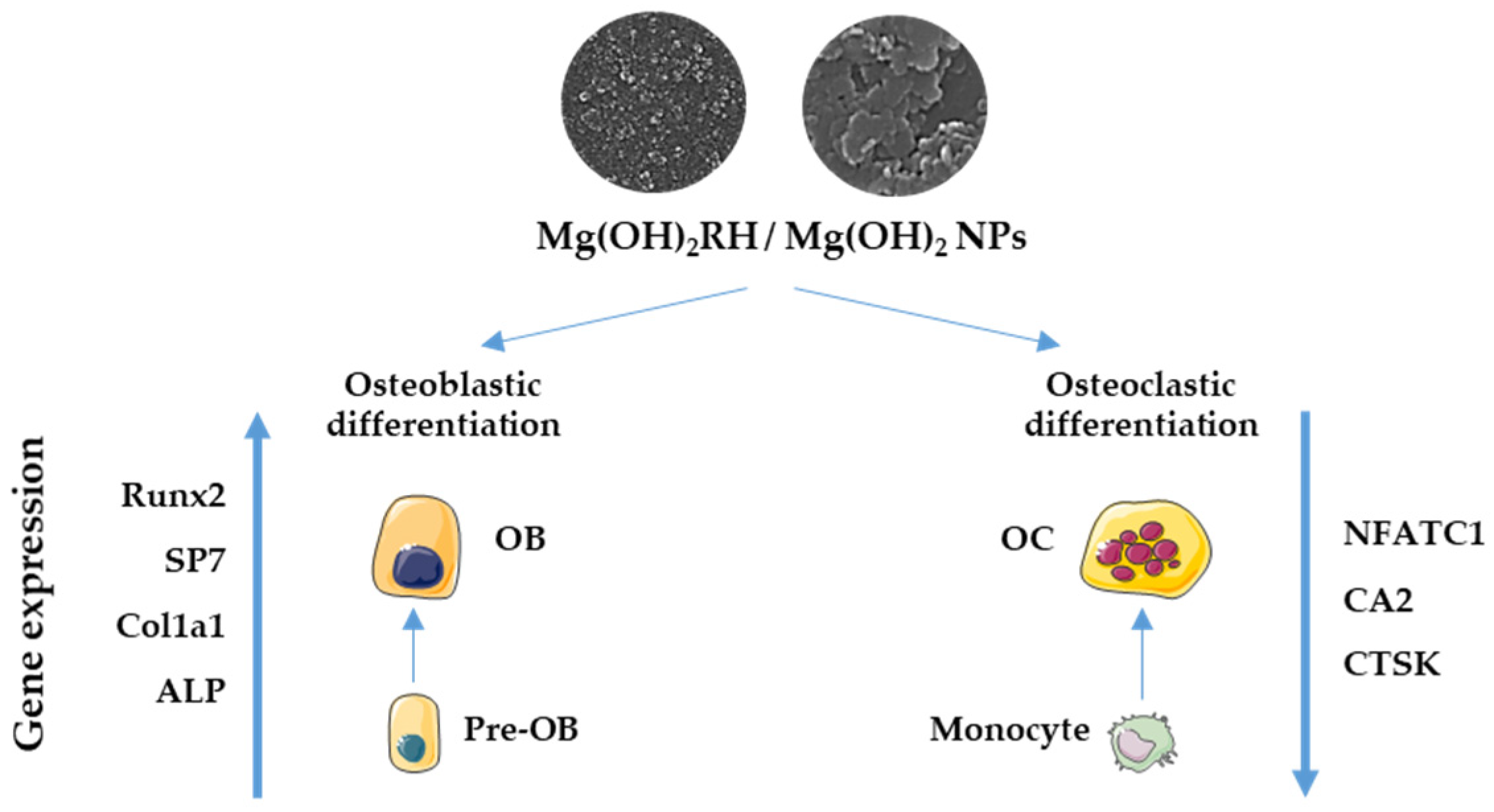
:1. Introduction
2. Materials and Methods
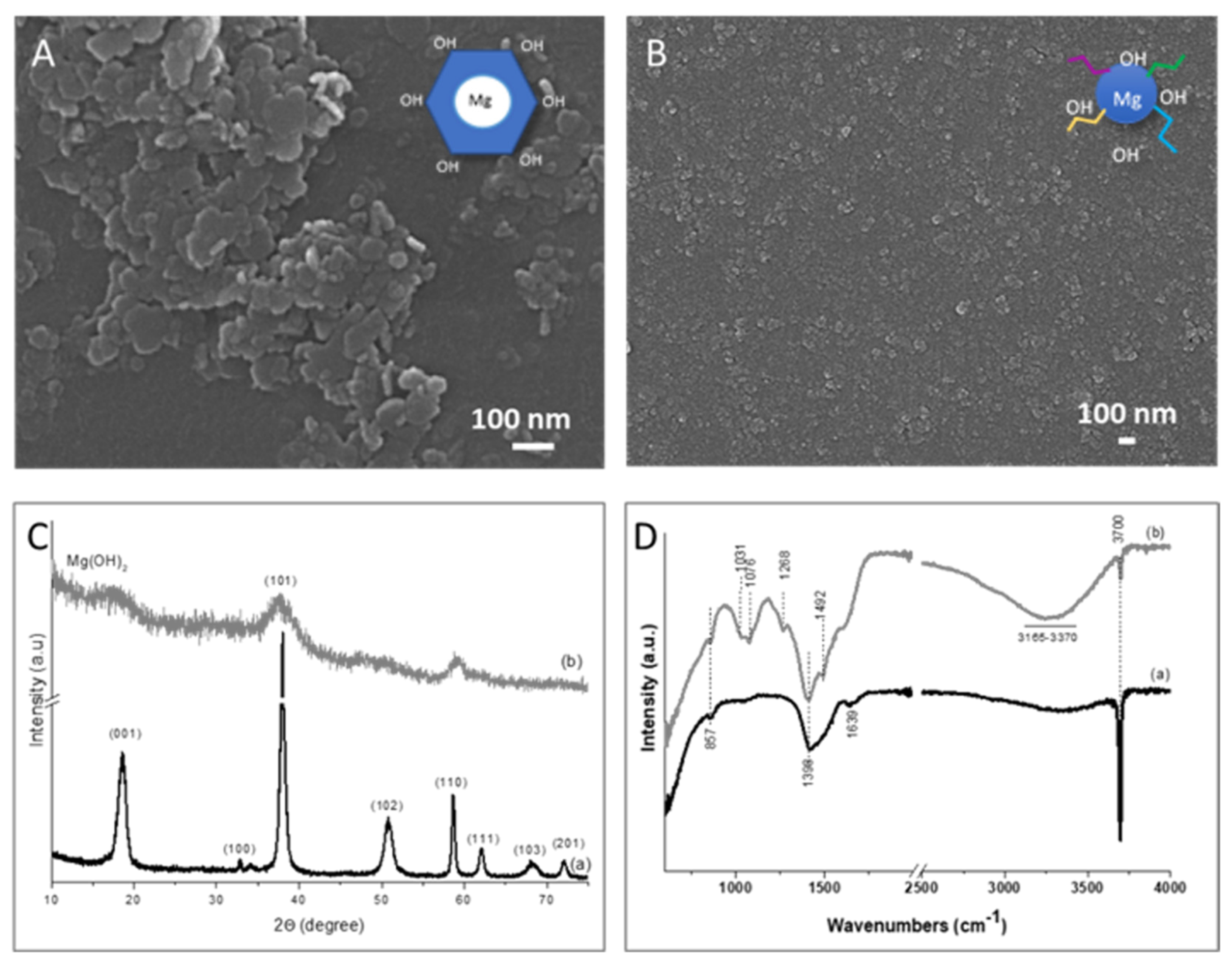
2.1. Synthesis and Physicochemical Characterization of Mg(OH)2 Nanoparticles
2.2. Cell Cultures
2.2.1. MG-63 Cell Cultures and Exposure to Mg(OH)2 Nanoparticles
2.2.2. THP-1 Cell Cultures and Exposure to Mg(OH)2 Nanoparticles
2.3. Characterization of Cell Response
2.3.1. Metabolic Activity
2.3.2. Total Protein Content
2.3.3. Alkaline Phosphatase Activity and Staining
2.3.4. Tartrate-Resistant Acid Phosphatase Activity and Staining
2.3.5. Immunostaining of F-Actin Cytoskeleton and Nucleus
2.3.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. Statistical Analysis
3. Results
3.1. Mg(OH)2 NPs
3.2. Effect of Mg(OH)2 NPs in the Osteoblastic Behavior
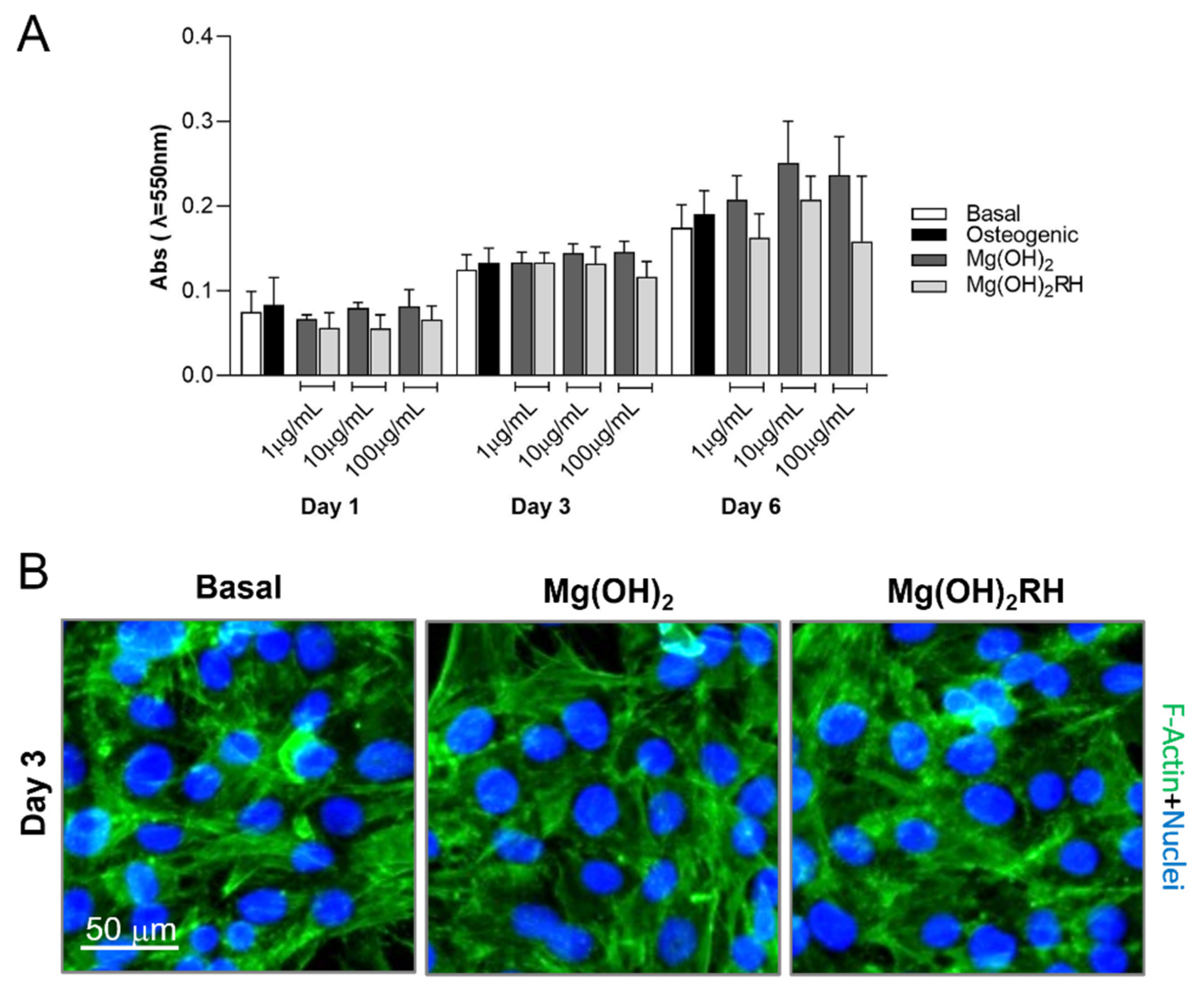
3.2.1. Metabolic Activity/Proliferation and Morphology
3.2.2. ALP Activity and Expression of Osteoblastic Genes
3.3. Effect of Mg(OH)2 NPs in Osteoclastic Behavior
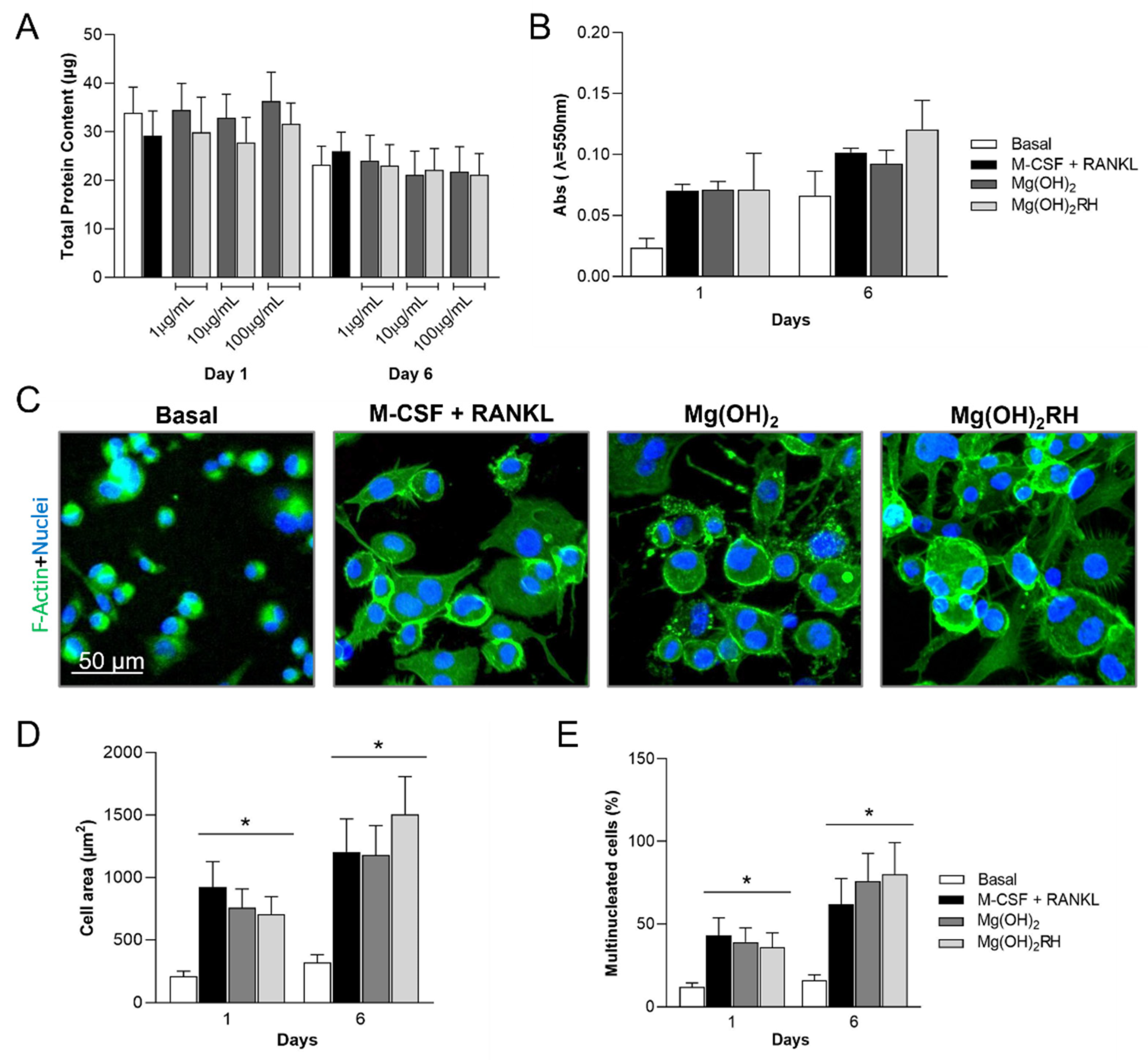
3.3.1. Total Protein Content, Metabolic Activity, and Cell Morphology
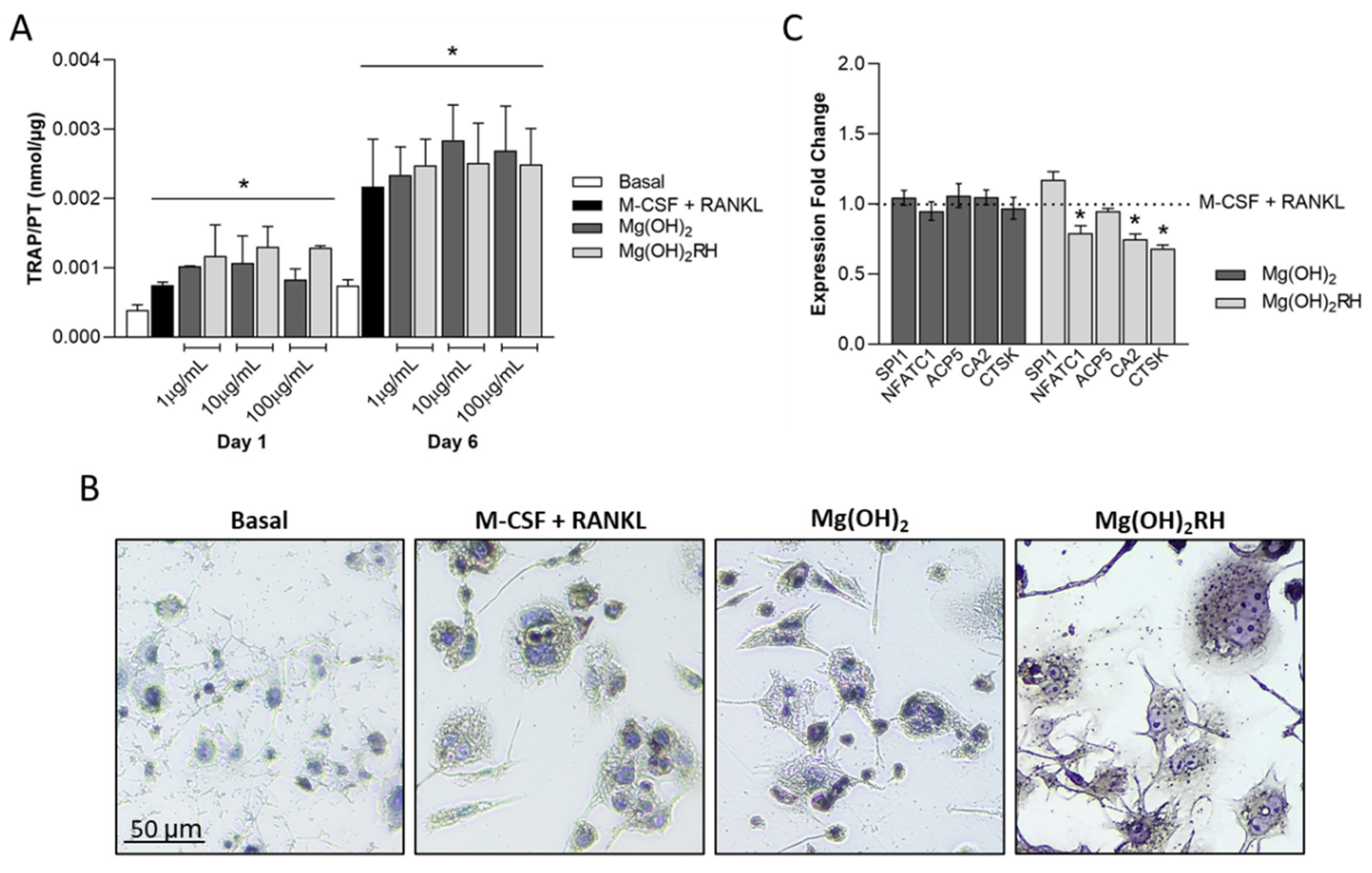
3.3.2. TRAP Activity and Expression of Osteoclastic Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L. ’Bone’, in Junqueira’s Basic Histology: Text and Atlas, 15th ed.; McGraw-Hill Education LLC.: New York, NY, USA, 2018; pp. 138–159. [Google Scholar]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef]
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 2017, 26, e12740. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.S.; Dempster, D.W. Chapter 2—Bone Remodeling: Cellular Activities in Bone. In Osteoporosis in Men, 2nd ed.; Orwoll, E.S., Bilezikian, J.P., Vanderschueren, D., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 15–24. [Google Scholar]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef]
- Hussain, A.; Bessho, K.; Takahashi, K.; Tabata, Y. Magnesium calcium phosphate as a novel component enhances mechanical/physical properties of gelatin scaffold and osteogenic differentiation of bone marrow mesenchymal stem cells. Tissue Eng. Part. A 2012, 18, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, X.; Huang, Z.; You, P.; Chen, C.; Kang, Y.; Yin, G. Synthesis and characterization of novel multiphase bioactive glass-ceramics in the CaO-MgO-SiO(2) system. J. Biomed. Mater. Res. B Appl Biomater. 2010, 93, 194–202. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [Green Version]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Dhivya, S.; Ajita, J.; Selvamurugan, N. Metallic Nanomaterials for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2015, 11, 1675–1700. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Song, H. Osteoblast response to magnesium ion-incorporated nanoporous titanium oxide surfaces. Clin. Oral. Implants Res. 2010, 21, 1278–1287. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016, 49, e5257. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Zhang, W.; Xu, L.; Pan, H.; Wen, J.; Wu, Q.; She, W.; Jiao, T.; Liu, X.; et al. Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014, 9, 2387–2398. [Google Scholar] [CrossRef] [Green Version]
- Go, E.J.; Kang, E.Y.; Lee, S.K.; Park, S.; Kim, J.H.; Park, W.; Kim, I.H.; Choi, B.; Han, D.K. An osteoconductive PLGA scaffold with bioactive β-TCP and anti-inflammatory Mg(OH)(2) to improve in vivo bone regeneration. Biomater. Sci. 2020, 8, 937–948. [Google Scholar] [CrossRef]
- Bedair, T.M.; Lee, C.K.; Kim, D.-S.; Baek, S.-W.; Bedair, H.M.; Joshi, H.P.; Choi, U.Y.; Park, K.-H.; Park, W.; Han, I.; et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J. Tissue Eng. 2020, 11, 2041731420967591. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Chen, Z.; Pan, D.; Cheng, Y.; Liu, Z.; Lin, Z.; Guan, X. Investigation of Antibacterial Activity and Related Mechanism of a Series of Nano-Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Bedair, T.M.; Heo, Y.; Ryu, J.; Bedair, H.M.; Park, W.; Han, D.K. Biocompatible and functional inorganic magnesium ceramic particles for biomedical applications. Biomater. Sci. 2021, 9, 1903–1923. [Google Scholar] [CrossRef] [PubMed]
- Sanaa, G.A.A.; Faten, A.-H.; Alwafi, R.; Alahmadi, A.A.; Al-Quwaie, D.A.H.; Bahieldin, A.; Edris, S. Bactericidal Efficacy of New Types of Magnesium Hydroxide and Calcium Carbonate Nanoparticles. Mol. Genet. Microbiol. Virol. 2019, 34, 252–262. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Patel, S. Rose hips as complementary and alternative medicine: Overview of the present status and prospects. Mediterr. J. Nutr. Metab. 2013, 6, 89–97. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhieva, S.; Georgieva, S. Rosehip extraction: Process optimization and antioxidant capacity of extracts. Open Chem. 2014, 12, 502–508. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci 2017, 18, 1137. [Google Scholar] [CrossRef]
- Marstrand, K.; Campbell-Tofte, J. The role of rose hip (Rosa canina L.) powder in alleviating arthritis pain and inflammation—Part II animal and human studies. Bot. Targets Ther. 2015, 2016, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Sabokbar, A.; Millett, P.J.; Myer, B.; Rushton, N. A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner. 1994, 27, 57–67. [Google Scholar] [CrossRef]
- Leonés, A.; Lieblich, M.; Benavente, R.; Gonzalez, J.L.; Peponi, L. Potential Applications of Magnesium-Based Polymeric Nanocomposites Obtained by Electrospinning Technique. Nanomaterials 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Controlling the Antimicrobial Action of Surface Modified Magnesium Hydroxide Nanoparticles. Biomimetics 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Zhao, H.; Liu, X.; Luo, H.-H.; Liu, R. Preparation of petal-like magnesium hydroxide particles by adding sulfate ions. J. Cryst. Growth 2020, 550, 125841. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Fazio, M.; Mastronardo, E.; Lanza, M.; Milone, C. Tuning Mg(OH)(2) Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application. Materials 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; Fan, B.; Wang, J. A novel preparation of nanosized hexagonal Mg(OH)2 as a flame retardant. Particuology 2016, 24, 177–182. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Wilkesmann, S.; Fellenberg, J.; Nawaz, Q.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: Which human cell types are (most) suitable for characterizing 45S5-bioactive glass? J. Biomed. Mater. Res. A 2020, 108, 663–674. [Google Scholar] [CrossRef]
- Owen, R.; Reilly, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front. Bioeng. Biotechnol. 2018, 6, 134. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallavi, M.; Waterman, J.; Koo, Y.; Sankar, J.; Yun, Y. Assessment of Cytotoxicity of Magnesium Oxide and Magnesium Hydroxide Nanoparticles using the Electric Cell-Substrate Impedance Sensing. Appl. Sci. 2020, 10, 2114. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wei, J.; Yan, Y.; Li, H.; Jia, J.; Wei, S.; Guo, H.; Xiao, T.; Liu, C. Preparation and preliminary cytocompatibility of magnesium doped apatite cement with degradability for bone regeneration. J. Mater. Sci. Mater. Med. 2011, 22, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthc. Mater. 2019, 8, e1901030. [Google Scholar] [CrossRef] [PubMed]
- Onder, S.; Calikoglu-Koyuncu, A.C.; Kazmanli, K.; Urgen, M.; Kok, F.N.; Torun-Kose, G. Magnesium doping on TiN coatings affects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Bio-Med Mater. Eng. 2018, 29, 427–438. [Google Scholar] [CrossRef]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodetska, L.; Natrus, L.; Lisakovska, O.; Kaniura, O.; Iakovenko, L.; Skrypnyk, I.; Flis, P. The regulatory role of the RANKL/RANK/OPG signaling pathway in the mechanisms of tooth eruption in patients with impacted teeth. BMC Oral. Health 2020, 20, 261. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Winther, K.; Campbell-Tofte, J.; Hansen, A. Bioactive ingredients of rose hips (Rosa canina L) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory Effects of Plant Polyphenols on Bone Remodeling: A Prospective View From the Bench to Bedside. Front. Endocrinol 2019, 10, 494. [Google Scholar] [CrossRef]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo-Evidence from Human Studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Hardcastle, A.C. The Effects of Flavonoids on Bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [Green Version]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Alesi, N.; Charles, J.F.; Nakamura, M.C. Basic Aspects of Osteoclast Differentiation and Function. In Osteoporosis: Pathophysiology and Clinical Management, 3rd ed.; Leder, B.Z., Wein, M.N., Eds.; Humana: Cham, Switzerland, 2020; pp. 17–41. [Google Scholar]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef]
- Park, K.H.; Gu, D.R.; Kim, M.S.; Lee, S.H. Inhibitory Effect of Rosae Multiflorae Fructus Extracts on the Receptor Activator of NF-κB Ligand-Induced Osteoclastogenesis through Modulation of P38- and Ca(2+)-Mediated Nuclear Factor of Activated T-Cells Cytoplasmic 1 Expression. J. Bone Metab. 2020, 27, 53–63. [Google Scholar] [CrossRef] [PubMed]

| Gene | Gene Name | Assay ID |
|---|---|---|
| Reference | Glyceraldehyde-3-phosphate dehydrogenase (GADPH) | qHsaCED0038674 |
| Osteoblastic | Runt-related transcription factor 2 (Runx2) | qHsaCED0044067 |
| SP7 transcription factor (SP7) | qHsaCED0003759 | |
| Collagen type I alpha I chain (Col1α1) | qHsaCED0043248 | |
| Alkaline phosphatase (ALP) | qHsaCED0045991 | |
| Secreted protein acidic and rich in cysteine (SPARC), aka osteonectin | qHsaCID0010332 | |
| Tumor necrosis factor receptor superfamily member 11b (TNFRSF11B), aka osteoprotegerin | qHsaCED0046251 | |
| Osteoclastic | Spi-1 proto-oncogene (SPI1) | qHsaCID0022097 |
| Nuclear factor of activated T cells 1 (NFATC1) | qHsaCED0044370 | |
| Acid phosphatase 5, tartrate-resistant (ACP5) | qHsaCED0056724 | |
| Carbonic anhydrase II (CA2) | qHsaCID0021039 | |
| Cathepsin K (CTSK) | qHsaCID0016934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, L.C.; Garbieri, T.F.; Grenho, L.; Alves, M.M.; Sousa Gomes, P.; Santos, C.F.; Fernandes, M.H.; Santos, C.; Colaço, B. Rosehip Extract-Functionalized Magnesium Hydroxide Nanoparticles and Its Effect on Osteoblastic and Osteoclastic Cells. Materials 2021, 14, 4172. https://doi.org/10.3390/ma14154172
Pinho LC, Garbieri TF, Grenho L, Alves MM, Sousa Gomes P, Santos CF, Fernandes MH, Santos C, Colaço B. Rosehip Extract-Functionalized Magnesium Hydroxide Nanoparticles and Its Effect on Osteoblastic and Osteoclastic Cells. Materials. 2021; 14(15):4172. https://doi.org/10.3390/ma14154172
Chicago/Turabian StylePinho, Laura Costa, Thais Francini Garbieri, Liliana Grenho, Marta M. Alves, Pedro Sousa Gomes, Carlos Ferreira Santos, Maria Helena Fernandes, Catarina Santos, and Bruno Colaço. 2021. "Rosehip Extract-Functionalized Magnesium Hydroxide Nanoparticles and Its Effect on Osteoblastic and Osteoclastic Cells" Materials 14, no. 15: 4172. https://doi.org/10.3390/ma14154172
APA StylePinho, L. C., Garbieri, T. F., Grenho, L., Alves, M. M., Sousa Gomes, P., Santos, C. F., Fernandes, M. H., Santos, C., & Colaço, B. (2021). Rosehip Extract-Functionalized Magnesium Hydroxide Nanoparticles and Its Effect on Osteoblastic and Osteoclastic Cells. Materials, 14(15), 4172. https://doi.org/10.3390/ma14154172










