Experimental Study of the Resistance to Influence of Aggressive Liquids on Lightweight Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Characterization
2.2. Mix Proportion and Mixtures
2.3. Tests
2.3.1. The Penetration Depth of Aggressive Liquids
2.3.2. LWC Absorption after Exposure to Corrosive Environments
2.3.3. Corrosion Resistance as a Change in the Density of LWC
2.3.4. Corrosion Resistance as a Change in the Compressive Strength of LWC
2.3.5. Microstructure (SEM)
3. Results and Discussion
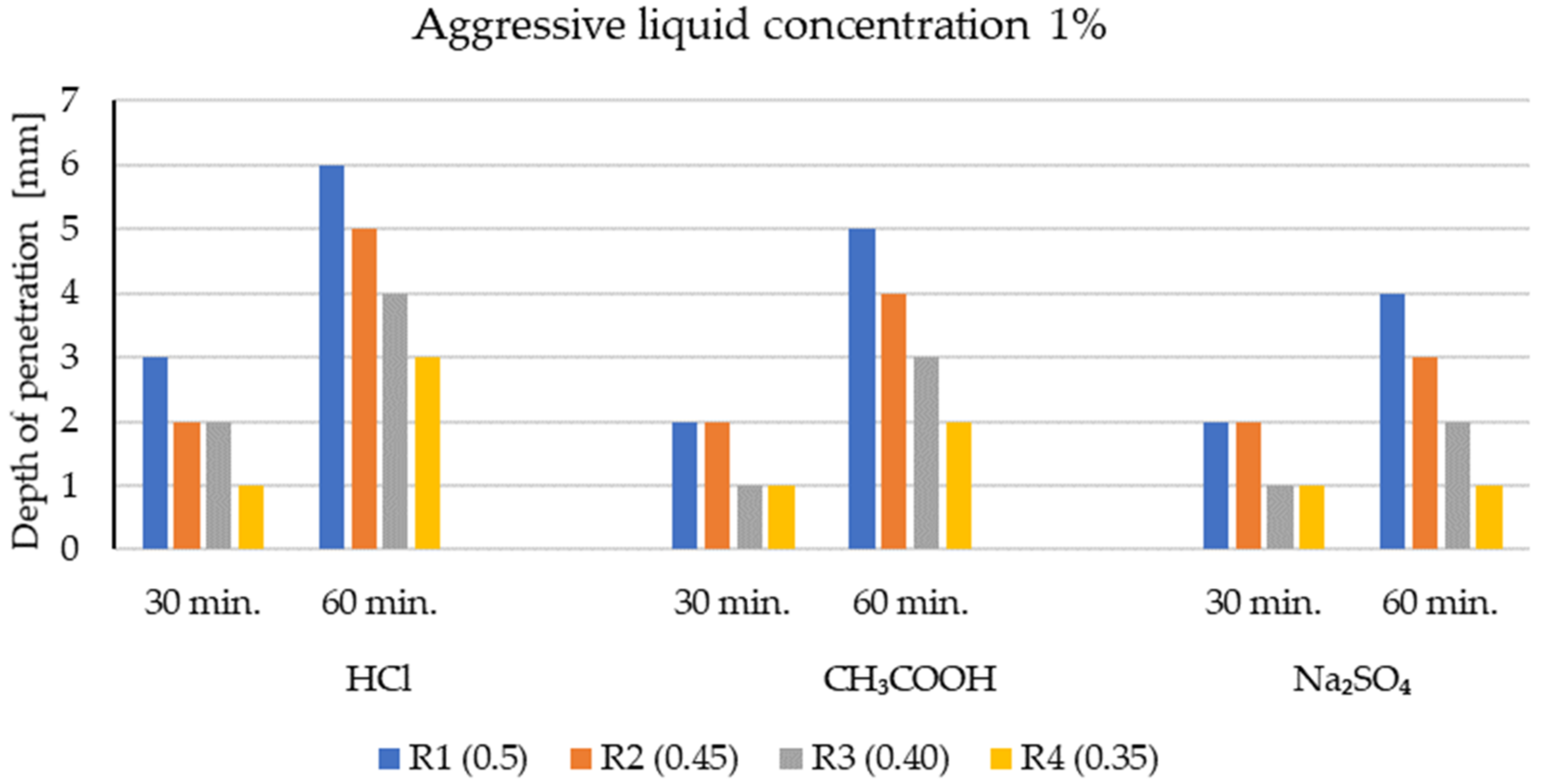
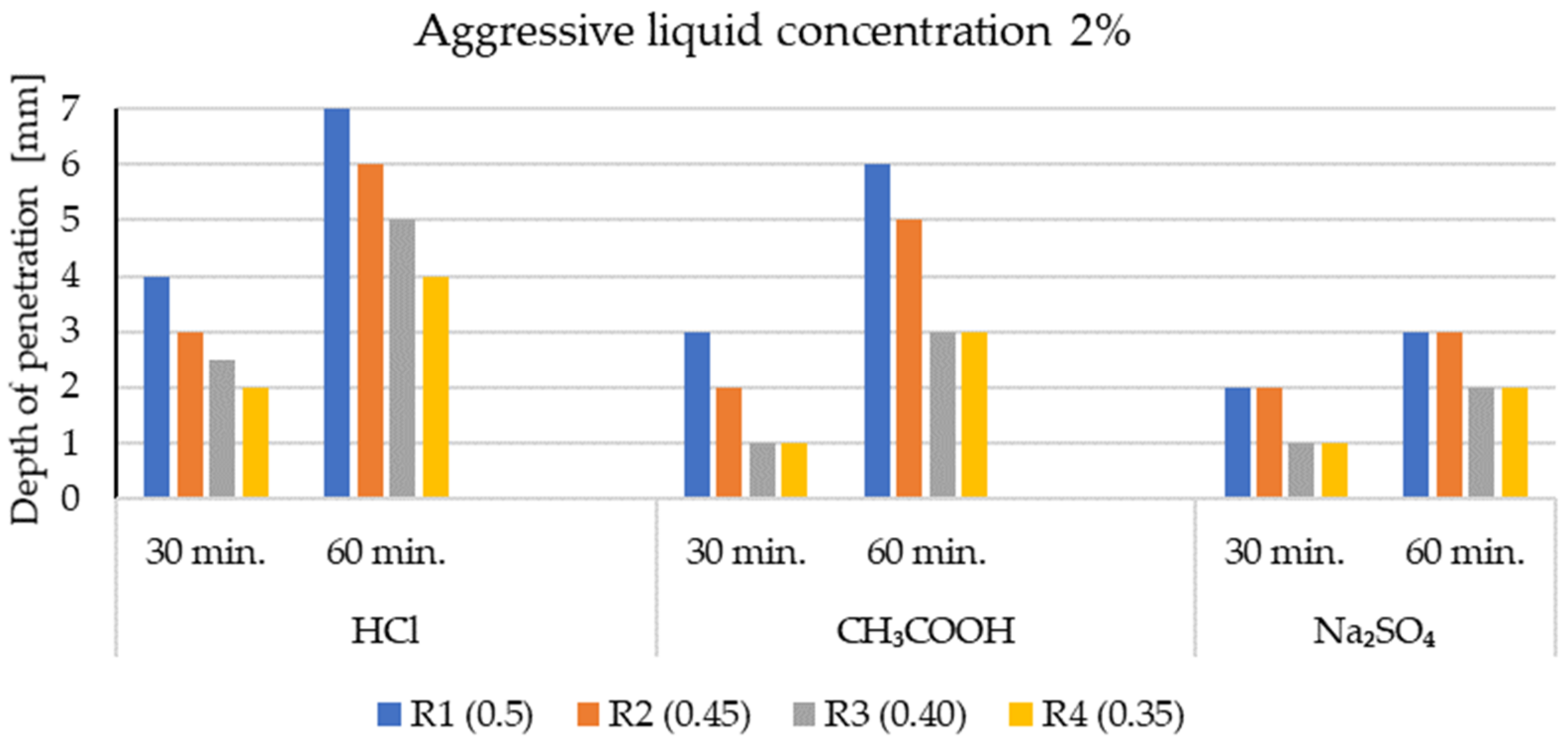
3.1. Penetration Depths of Aggressive Liquids in LWC after Exposure to Corrosive Environments
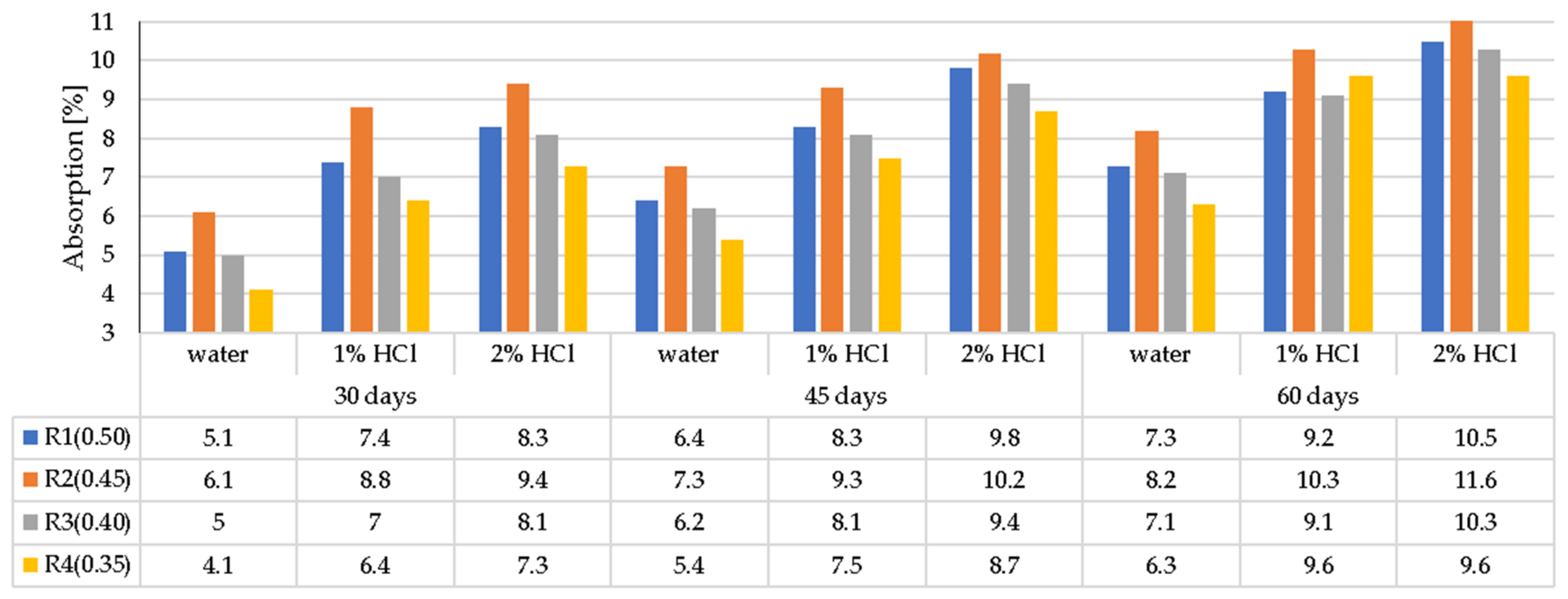
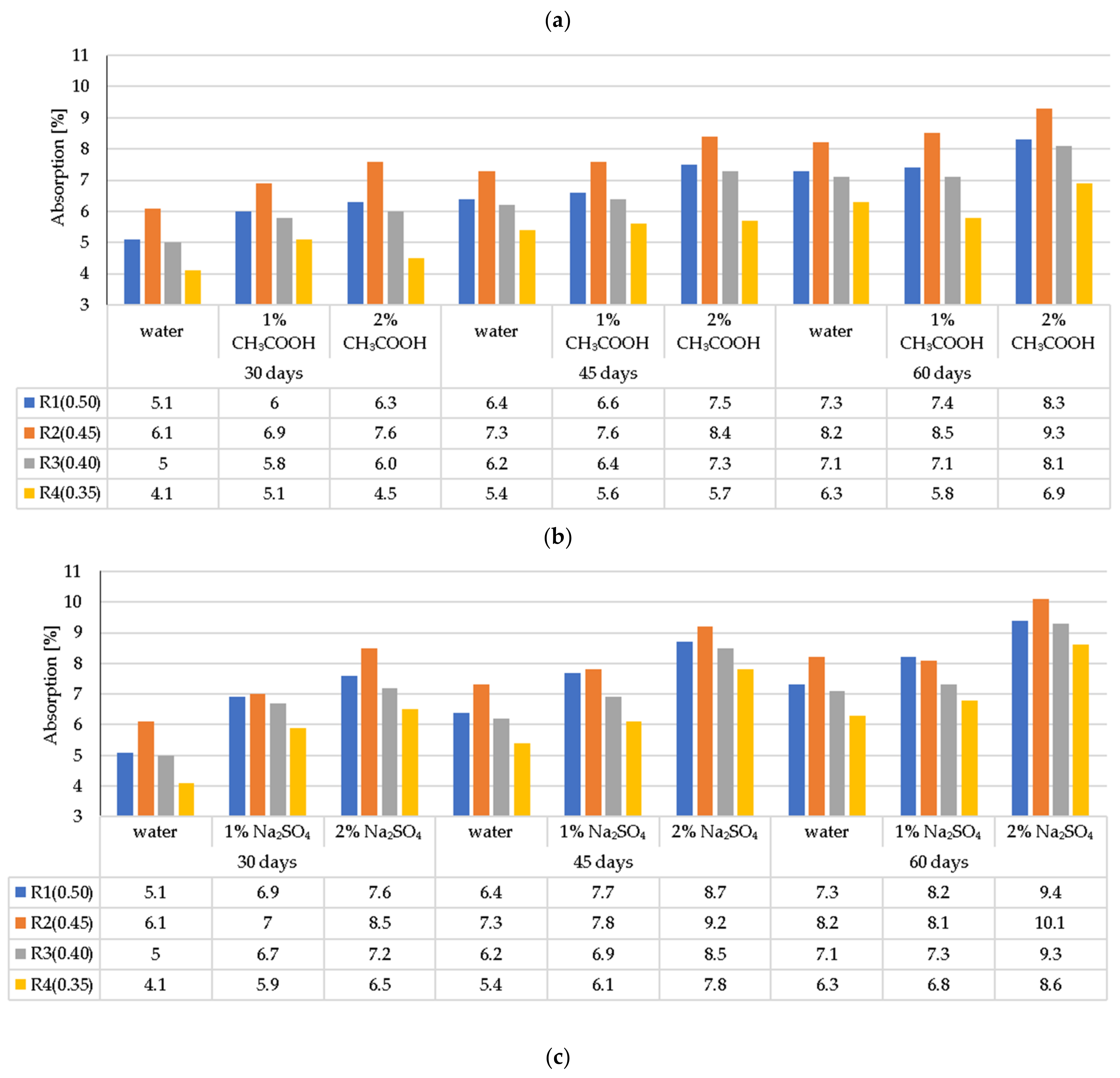
3.2. LWC Absorption after Exposure to Corrosive Environments
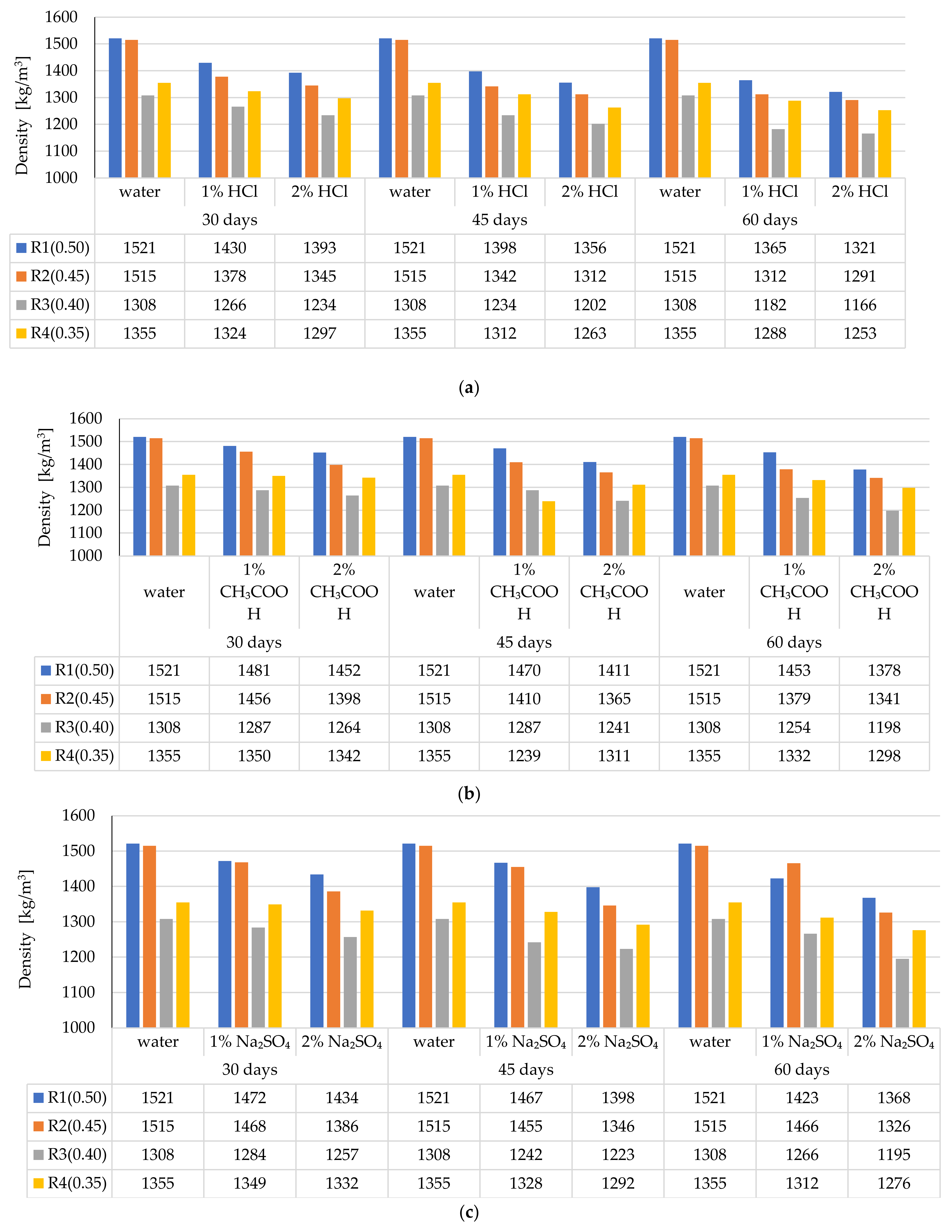
3.3. Changes in the LWC Density after Exposure to Corrosive Environments
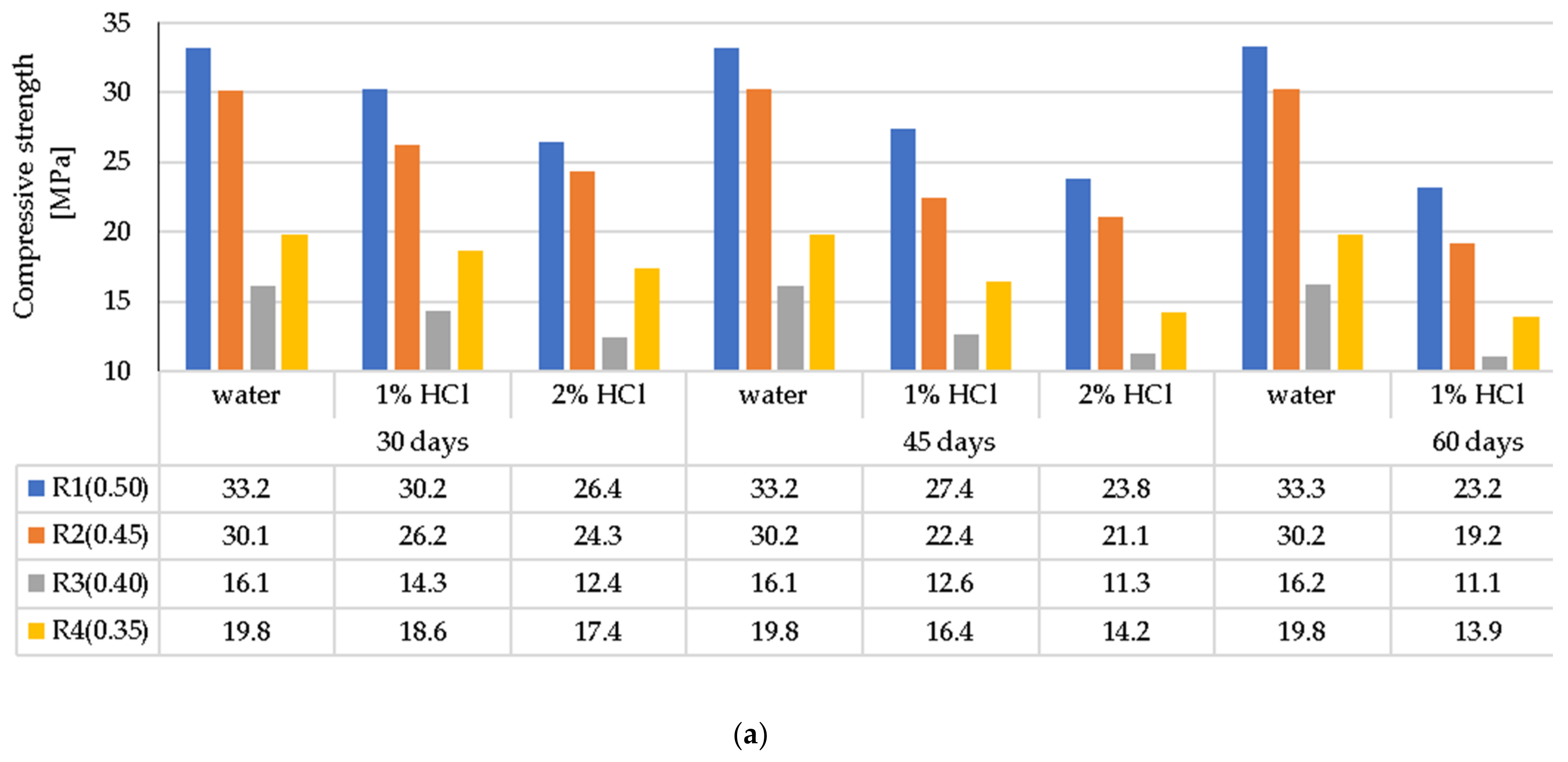
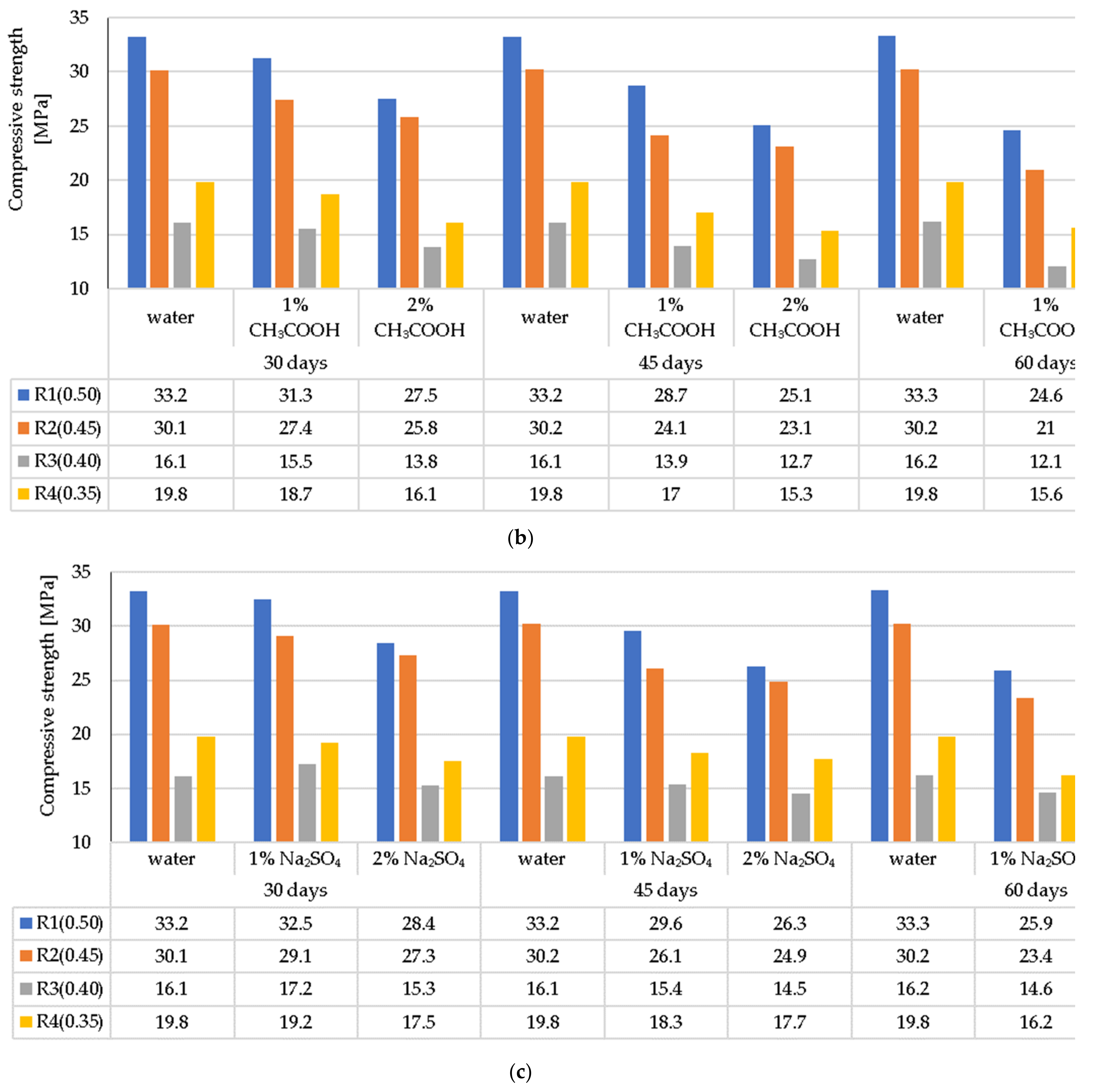
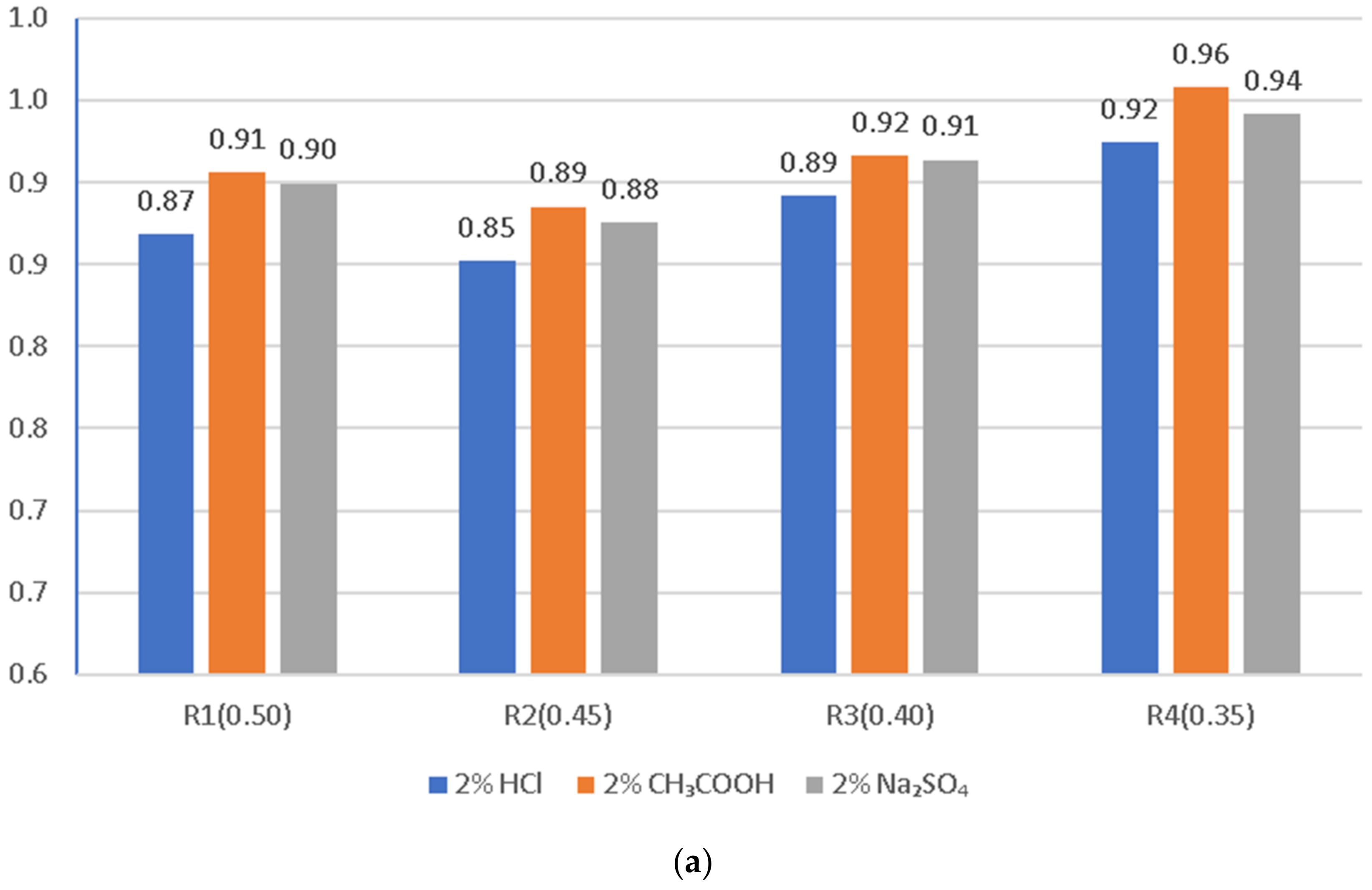
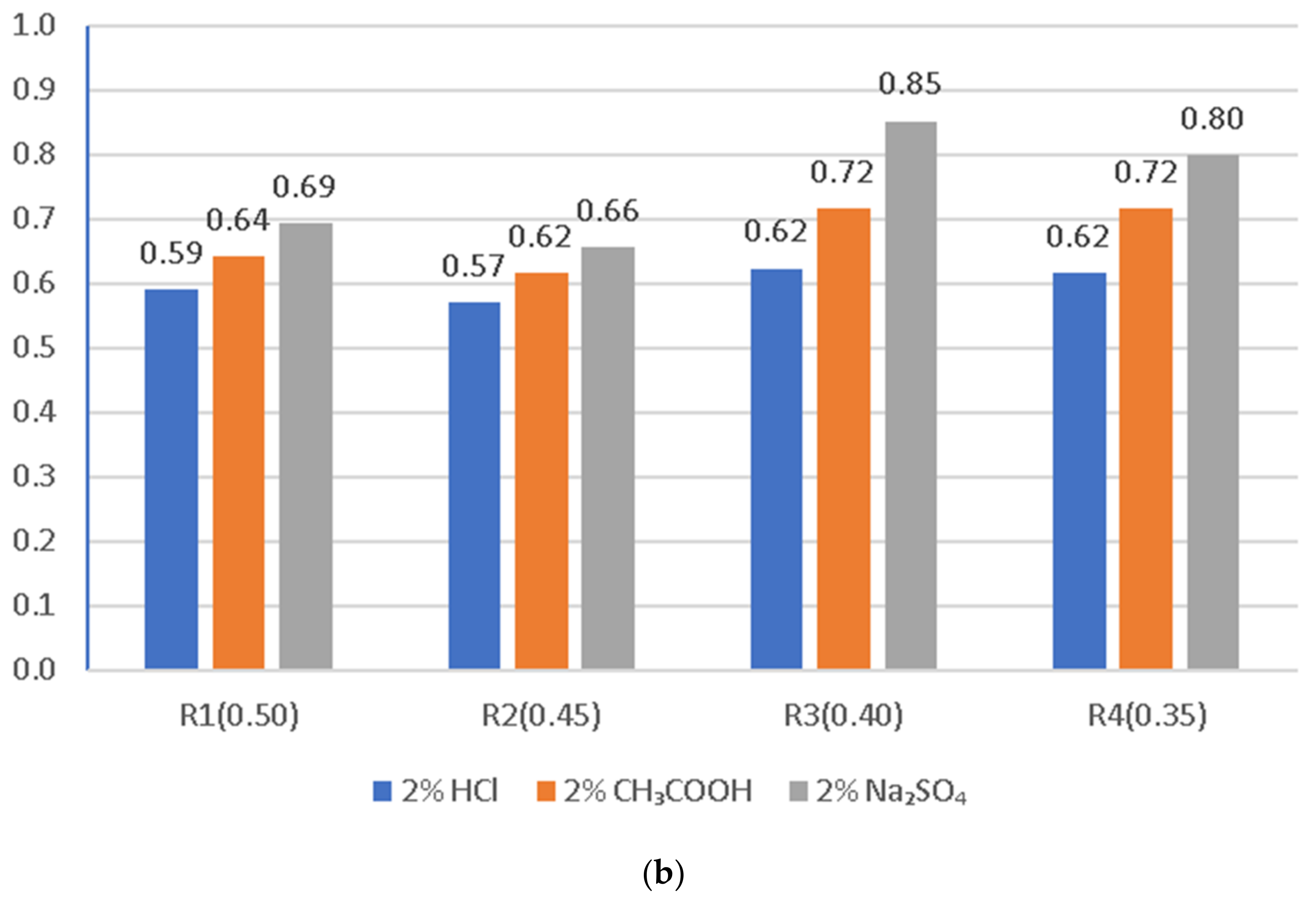
3.4. Compressive Strength of the LWC after Exposure to Corrosive Environments
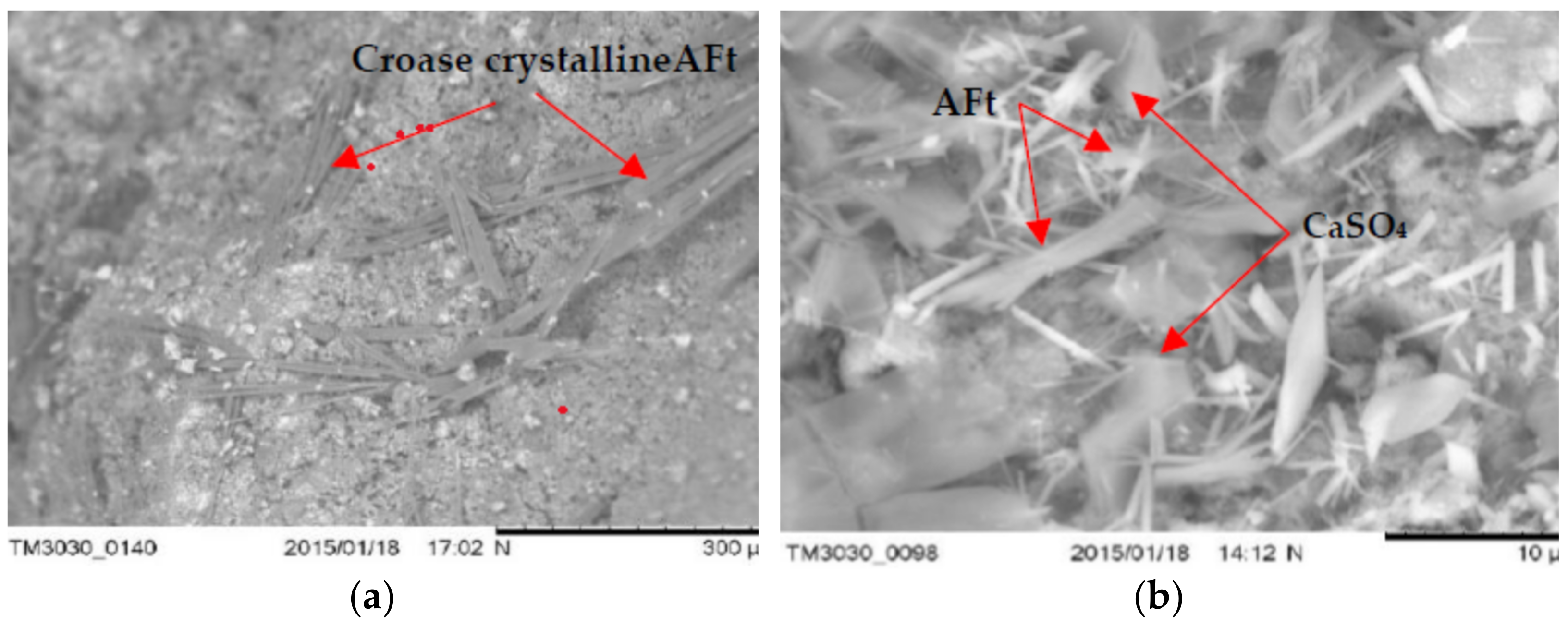
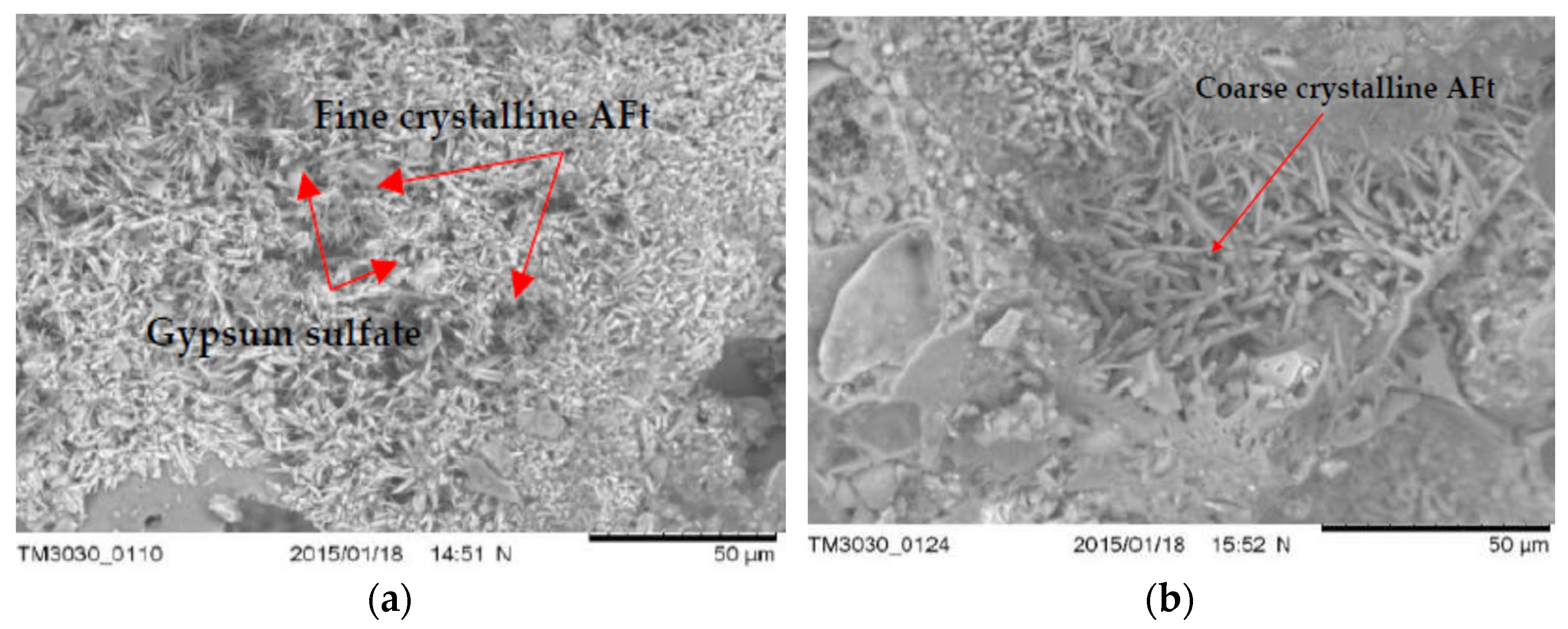
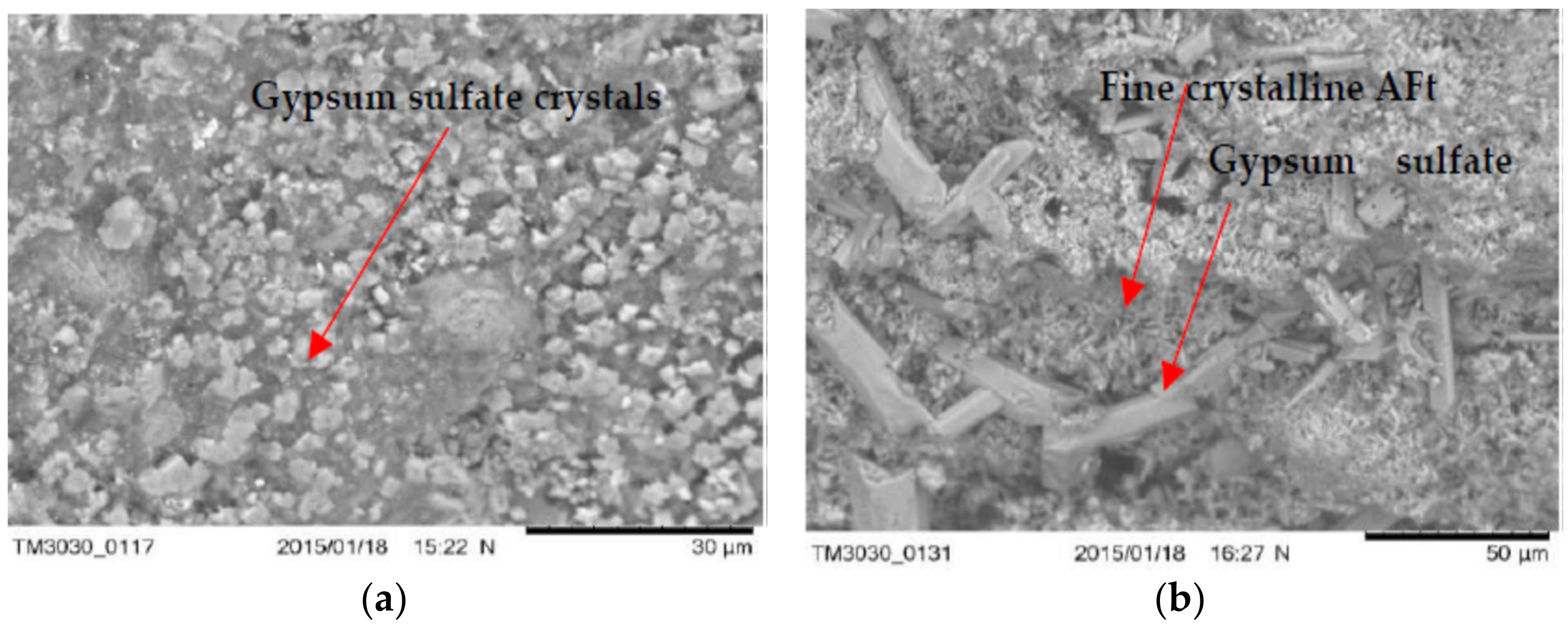
3.5. The Microstructure of Concrete with LWA (GEGA and GAA) after Exposure to Corrosive Environments
4. Conclusions
- The use of a mixture of lightweight aggregate (GEGA and GAA) with cement and mineral additive such as fly ash allows a higher compressive strength of LWC over a long-term period (365 days) to be obtained compared to concrete with cement alone.
- The level of penetration of aggressive corrosive solutions depends on the open porosity of lightweight aggregates and the type of mineral additives.
- The presented test results for resistance to aggressive environments for LWC (R1 ÷ R4) with an aggregate (GEGA and GAA), modified with the addition of fly ash (R1, R4), indicate the influence of the LWC composition on the average compressive strength, even though the samples had been previously cured for a year (365 days) under standard conditions (temperature 20 ± 2 °C and humidity ≤95%). After a one year period, the samples were exposed to aggressive environments.
- The use of GAA aggregate in the amount of 50% of the total aggregate volume in LWC increased the strength of concrete by approx. 50%.
- LWC containing 100% of the total aggregate volume in the concrete is characterized by a higher resistance to corrosive environments by 8%.
- In general, the corrosion resistance decreases as the water/binder ratio increases.
- The introduction of silica fly ash or a mixture of fly ash as a partial replacement for cement causes significant changes in the chemical composition of the pore solution phase in the concrete structure with the use of lightweight aggregates.
- Microstructure tests (SEM) show that the type of mineral additive affects the degree of corrosion resistance of LWC. The highest corrosion resistance, considering the change in the average compressive strength after 60 days of exposure to a corrosive environment, is demonstrated by the concrete with a mixture of lightweight aggregate (GEGA and GAA) and fly ash.
- Concrete with lightweight aggregate (GEGA) and fly ash has a higher effect of corrosion resistance. The observed phenomenon may be the result of a slow pozzolana reaction as well as a slow process of filling the voids in the concrete structure with the products of the binding reaction.
- 10.
- The use of lightweight aggregates (GEGA and GAA), regardless of their mixing ratio and grain size, enables the migration of aggressive corrosive solutions, resulting in the precipitation of corrosive products such as ettringite. Its amount probably depends on the degree of sealing of the interfacial transition zone and the matrix as well as the lightweight aggregate absorption mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Dieb, A.S.; As, E.-D. Cementless concrete for sustainable construction. MOJ Civ. Eng. 2016, 1, 1. [Google Scholar] [CrossRef]
- Obe, R.K.D.; Lye, C.Q.; Lynn, C.J.; Elgalhud, A.A. Sustainable construction materials for concrete: A question of responsible use. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 442, p. 012001. [Google Scholar] [CrossRef]
- Davraz, M.; Koru, M.; Akdağ, A.E. The Effect of Physical Properties on Thermal Conductivity of Lightweight Aggregate. Procedia Earth Planet. Sci. 2015, 15, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Cavalline, T.L.; Castrodale, R.W.; Freeman, C.; Wall, J. Impact of Lightweight Aggregate on Concrete Thermal Properties. ACI Mater. J. 2017, 114, 945–956. [Google Scholar] [CrossRef]
- Shannag, M.J.; Charif, A.; Dghaither, S. Developing Structural Lightweight Concrete Using Volcanic Scoria Available in Saudi Arabia. Arab. J. Sci. Eng. 2014, 39, 3525–3534. [Google Scholar] [CrossRef]
- Bessonov, I.V.; Bulgakov, B.I.; Zhukov, A.D.; Gradov, V.A.; Ivanova, N.A.; Kodzoev, M.-B.K. Lightweight concrete based on crushed foam glass aggregate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1083, p. 012038. [Google Scholar] [CrossRef]
- Divyah, N.; Thenmozhi, R.; Neelamegam, M. Strength properties and durability aspects of sintered-fly-ash lightweight aggregate concrete. Mater. Teh. 2020, 54, 301–310. [Google Scholar] [CrossRef]
- Callsen, B.; Thienel, K.-C. Besondere Aspekte bei der Entwicklung und Ausführung eines hochwärme- dämmenden Hochleistungs-Leichtbetons mit sehr niedriger Betonrohdichte. Beton 2017, 67, 128–134. [Google Scholar]
- Habsya, C.; Diharjo, K.; Setyono, P.; Satwiko, P. Physical, mechanical and thermal properties of lightweight foamed concrete with fly ash. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 420, p. 012062. [Google Scholar] [CrossRef] [Green Version]
- Kockal, N.U.; Ozturan, T. Strength and elastic properties of structural lightweight concretes. Mater. Des. 2011, 32, 2396–2403. [Google Scholar] [CrossRef]
- Bumanis, G.; Bajare, D.; Locs, J.; Korjakins, A. Alkali-silica reactivity of expanded glass granules in structure of lightweight concrete. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2013; Volume 47, p. 012022. [Google Scholar] [CrossRef] [Green Version]
- Kerkhoff, B. Effects of Substances on Concrete and Guide to Protective Treatment; Portland Cement Association: Skokie, IL, USA, 2007; p. 36. [Google Scholar]
- Kurdowski, W.; Bochenek, A. Three principles of concrete corrosion prevention|Trzy zasady zapobiegania korozji betonu. Cem. Wapno Bet. 2012, 2012, 434–442. [Google Scholar]
- Bazán, Á.M.; Reyes, E.; Gálvez, J.C. Influence of Pore Networking and Electric Current Density on the Crack Pattern in Reinforced Concrete Test Due to Pressure Rust Layer at Early Ages of an Accelerated Corrosion Test. Materials 2019, 12, 2477. [Google Scholar] [CrossRef] [Green Version]
- Najjar, M.; Nehdi, M.; Soliman, A.; Azabi, T. Damage mechanisms of two-stage concrete exposed to chemical and physical sulfate attack. Constr. Build. Mater. 2017, 137, 141–152. [Google Scholar] [CrossRef]
- Ghorab, H.Y.; Zahran, F.S.; Kamal, M.; Meawad, A.S. On the durability of Portland limestone cement: Effect of pH on the thaumasite formation. HBRC J. 2018, 14, 340–344. [Google Scholar] [CrossRef]
- Fediuk, R.; Uvarova, T.; Zverev, A.; Smoliakov, A.; Cherkasov, A. Natural Effects on Offshore Structures in the Arctic. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 463, p. 032063. [Google Scholar] [CrossRef]
- El Fattah, A.A.; Al-Duais, I.; Riding, K.; Thomas, M.; Al-Dulaijan, S.; Al-Zahrani, A.M. Field Validation of Concrete Transport Property Measurement Methods. Materials 2020, 13, 1166. [Google Scholar] [CrossRef] [Green Version]
- Stawiski, B.; Kania, T. Examining the Distribution of Strength across the Thickness of Reinforced Concrete Elements Subject to Sulphate Corrosion Using the Ultrasonic Method. Materials 2019, 12, 2519. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Shayan, A.; Baburamani, P. Test Methods for Sulfate Resistance of Concrete and Mechanism of Sulfate Attack: A State-of-the-Art Review; Australian Road Research Board: Vermont South, Australia, 1998; p. 38. [Google Scholar]
- Silva, R.V.; De Brito, J.; Neves, R.; Dhir, R. Prediction of Chloride Ion Penetration of Recycled Aggregate Concrete. Mater. Res. 2015, 18, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Leemann, A.; Loser, R. Accelerated sulfate resistance test for concrete—Chemical and microstructural aspects. In Proceedings of the Second International Conference on Microstructural-Related Durability of Cementitious Composites, Amsterdam, The Netherlands, 11–13 April 2012; pp. 1–12. [Google Scholar]
- Wang, Y.; Huang, B.; Mao, Z.; Deng, M.; Cao, H. Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete. Materials 2020, 13, 5036. [Google Scholar] [CrossRef] [PubMed]
- Ukrainczyk, N.; Pecur, I.B.; Bolf, N. Evaluating rebar corrosion damage in RC structures exposed to marine environment using neural network. Civ. Eng. Environ. Syst. 2007, 24, 15–32. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. Chloride Diffusion in Concrete Protected with a Silane-Based Corrosion Inhibitor. Materials 2020, 13, 2001. [Google Scholar] [CrossRef]
- Landa-Sánchez, A.; Bosch, J.; Baltazar-Zamora, M.A.; Croche, R.; Landa-Ruiz, L.; Santiago-Hurtado, G.; Moreno-Landeros, V.M.; Olguín-Coca, J.; López-Léon, L.; Bastidas, J.M.; et al. Corrosion Behavior of Steel-Reinforced Green Concrete Containing Recycled Coarse Aggregate Additions in Sulfate Media. Materials 2020, 13, 4345. [Google Scholar] [CrossRef]
- Joshaghani, A. The Effects of Zeolite as Supplementary Cement Material on Pervious Concrete; ResearchGate: Berlin, Germany, 2016; Volume 43, p. 12. [Google Scholar]
- Bogas, J.; Real, S. A Review on the Carbonation and Chloride Penetration Resistance of Structural Lightweight Aggregate Concrete. Materials 2019, 12, 3456. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.; Tang, W.; Nadeem, A. Comparison of carbonation of lightweight concrete with normal weight concrete at similar strength levels. Constr. Build. Mater. 2008, 22, 1648–1655. [Google Scholar] [CrossRef]
- Wang, J. Steady-State Chloride Diffusion Coefficient and Chloride Migration Coefficient of Cracks in Concrete. J. Mater. Civ. Eng. 2017, 29, 04017117. [Google Scholar] [CrossRef]
- Rumsys, D.; Spudulis, E.; Bacinskas, D.; Kaklauskas, G. Compressive Strength and Durability Properties of Structural Lightweight Concrete with Fine Expanded Glass and/or Clay Aggregates. Materials 2018, 11, 2434. [Google Scholar] [CrossRef] [Green Version]
- Stanaszek-Tomal, E.; Fiertak, M. Biological and chemical corrosion of cement materials modified with polymer. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 591–596. [Google Scholar] [CrossRef]
- Elrahman, M.A.; Chung, S.-Y.; Sikora, P.; Rucinska, T.; Stephan, D. Influence of Nanosilica on Mechanical Properties, Sorptivity, and Microstructure of Lightweight Concrete. Materials 2019, 12, 3078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loser, R.; Leemann, A. An accelerated sulfate resistance test for concrete. Mater. Struct. 2015, 49, 3445–3457. [Google Scholar] [CrossRef]
- Bogas, J.A.; De Brito, J.; Cabaço, J. Long-term behaviour of concrete produced with recycled lightweight expanded clay aggregate concrete. Constr. Build. Mater. 2014, 65, 470–479. [Google Scholar] [CrossRef]
- Wang, X.; Han, P.; Dong, X.; Li, X.; Bai, X.; He, B.; Niu, S.; Sun, F. Influences of High-Sulphur Fly Ash on the Properties of Lightweight Cement-Treated Materials Subjected to Sulphate Corrosion. Appl. Sci. 2020, 10, 5217. [Google Scholar] [CrossRef]
- Lindvall, A.; Espin, O.; Löfgren, I. Properties of concretes with pulverized fly ash and ground granulated blast furnace slag. In Proceedings of the Nordic Mini Seminar: Durability Aspects of Fly Ash and Slag in Concrete, Oslo, Norway, 15–16 February 2012; pp. 15–16. [Google Scholar]
- Vereshagin, V.I.; Sokolova, S.N. Granulated foam glass–ceramic material from zeolitic rocks. Constr. Build. Mater. 2008, 22, 999–1003. [Google Scholar] [CrossRef]
- Bentz, D. Influence of internal curing using lightweight aggregates on interfacial transition zone percolation and chloride ingress in mortars. Cem. Concr. Compos. 2009, 31, 285–289. [Google Scholar] [CrossRef]
- Suchorab, Z.; Barnat-Hunek, D.; Franus, M.; Łagód, G. Mechanical and Physical Properties of Hydrophobized Lightweight Aggregate Concrete with Sewage Sludge. Materials 2016, 9, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.-J.; Wong, H.; Buenfeld, N.R. Assessing the influence of ITZ on the steady-state chloride diffusivity of concrete using a numerical model. Cem. Concr. Res. 2009, 39, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Ghourchian, S.; Wyrzykowski, M.; Lura, P.; Shekarchizadeh, M.; Ahmadi, B. An investigation on the use of zeolite aggregates for internal curing of concrete. Constr. Build. Mater. 2013, 40, 135–144. [Google Scholar] [CrossRef]
- Sturm, R.D.; McAskill, N.; Burg, R.G.; Morgan, D.R. Evaluation of Lightweight Concrete Performance in 55 To 80 Year-Old Ships. ACI Spec. Publ. 1999, 189, 101–120. [Google Scholar]
- EN 197-1:2011 Cement. Composition, Specifications and Conformity Criteria for Common Cements. Available online: http://www.puntofocal.gov.ar/notific_otros_miembros/mwi40_t.pdf (accessed on 26 July 2021).
- EN 12350-1:2019 Testing Fresh Concrete. Sampling and Common Apparatus. Available online: https://civilnode.com/download-standard/10677746504383/bs-en-12350-12019-testing-fresh-concrete-part-1-sampling-and-common-apparatus (accessed on 26 July 2021).
- EN 206:2013+A1: 2016 Concrete. Requirements, Properties, Manufacturing Compatibility. Available online: https://www.techstreet.com/standards/bs-en-206-2013-a1-2016?product_id=1936327 (accessed on 26 July 2021).
- EN 14630:2007 Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Carbonation Depth in Hardened Concrete by the Phenolphthalein Method. Available online: https://standards.iteh.ai/catalog/standards/sist/a90f5a00-08c8-4132-a7fd-646016e40772/sist-en-14630-2007 (accessed on 26 July 2021).
- EN 12390-7:2019 Testing Hardened Concrete. Density of Hardened Concrete. Available online: https://standards.iteh.ai/catalog/standards/cen/811a0cf3-55e3-495a-b06e-5c302d5f2806/en-12390-7-2019 (accessed on 26 July 2021).
- EN 12390-4:2019 Testing Hardened Concrete—Part 4: Compressive Strength—Specification for Testing Machines. Available online: https://standards.iteh.ai/catalog/standards/cen/10b1c613-819b-42d7-8f94-480cd37a666a/en-12390-4-2019 (accessed on 26 July 2021).
- Kayali, O.; Zhu, B. Chloride induced reinforcement corrosion in lightweight aggregate high-strength fly ash concrete. Constr. Build. Mater. 2005, 19, 327–336. [Google Scholar] [CrossRef]

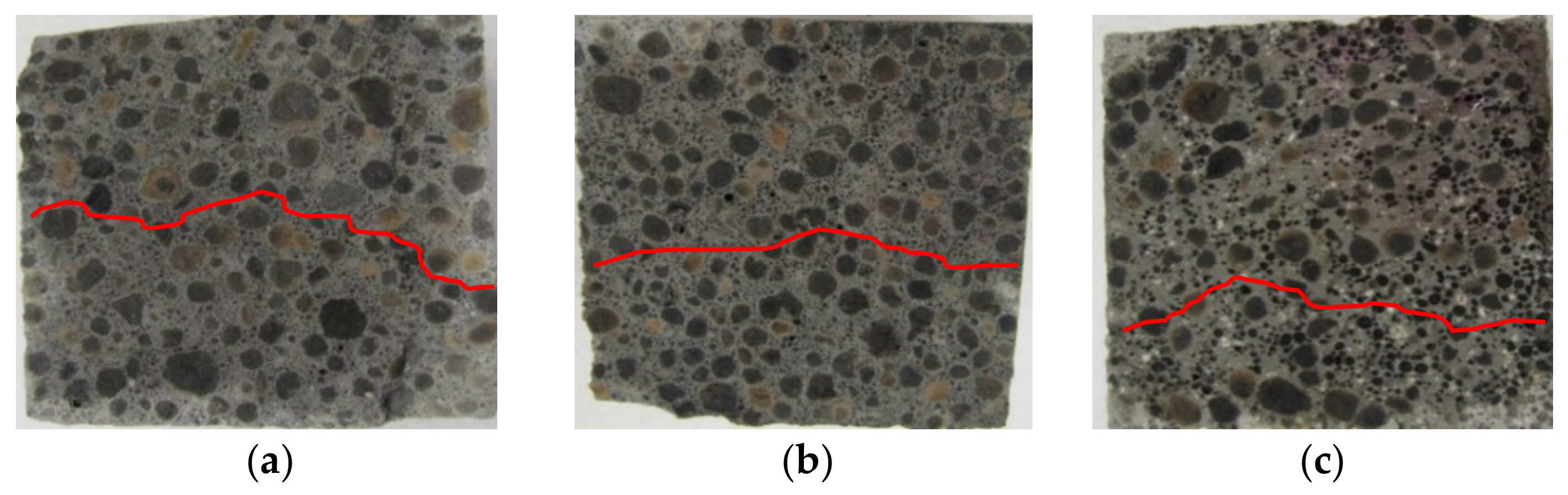
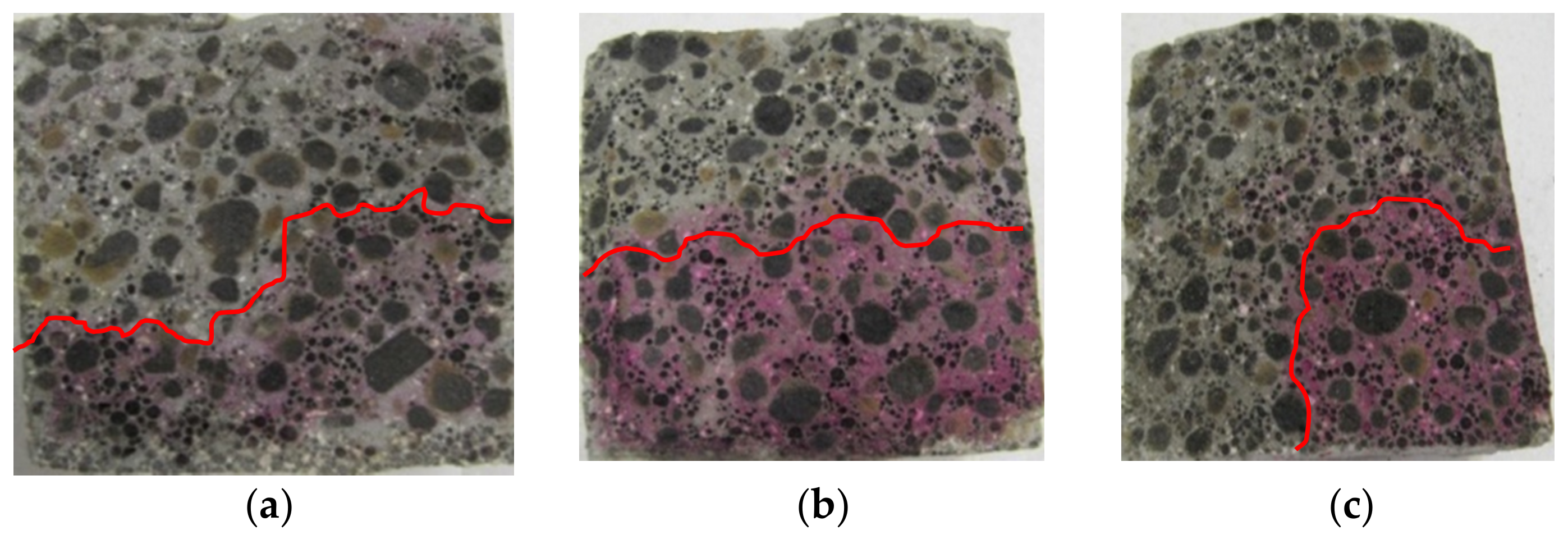
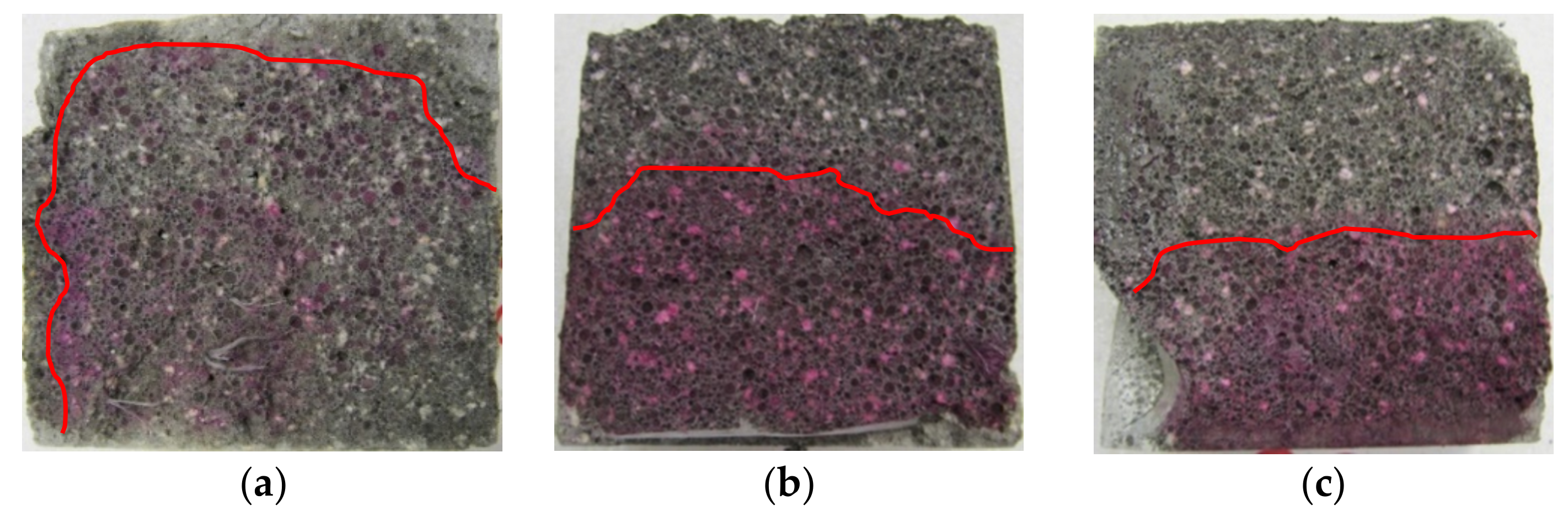
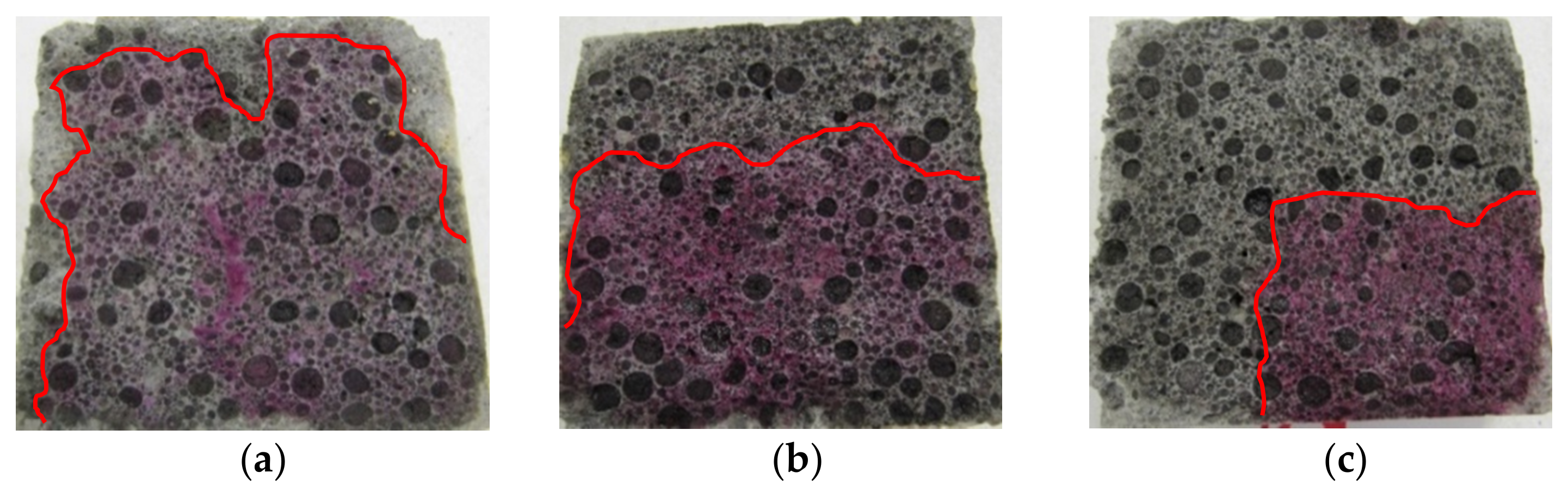
| Binder | Setting Start Time (min) | Setting End Time (min) | Compressive Strength (MPa) | Blaine Fineness (cm2/g) | Loss on Ignition (%) | Water Demand (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 d | 28 d | |||||||||||
| CEM I 42.5R | 165 | 205 | 26.5 | 57.4 | 3384 | 3.5 | 27.0 | |||||
| Content (%) | ||||||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | Cl(−) | |||
| CEM I 42.5R | 21.5 | 6.10 | 3.3 | 63.5 | 1.1 | 2.9 | 0.17 | 0.66 | 0.24 | 0.075 | ||
| Fly ash | 54.8 | 24.4 | 6.6 | 4.2 | 3.0 | 0.5 | 1.10 | 0.36 | 1.20 | 0.055 | ||
| Aggregate Type | Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Loss on Ignition | |
| GEGA | 63.33 | 0.74 | – | 14.19 | 2.98 | 0.32 | 13.35 | 0.57 | 4.53 |
| GAA | 52.82 | 24.28 | 7.5 | 4.5 | 3.19 | 0.43 | – | 0.2 | 7.1 |
| Property | GEGA 2 mm | GEGA 4 mm | GAA 8 mm | |
|---|---|---|---|---|
| Water absorption WA24 | (%) | 15.2 | 17.8 | 16.5 |
| Volume density ρa | (kg/m3) | 380 | 350 | 1350 |
| Open porosity Po | (%) | 37 | 42 | 37 |
| Crumble indicator Xr | (%) | 22.3 | 25.9 | 17.8 |
| pH after 24 h | (–) | 11.9 | 11.9 | 11.1 |
| Pore radius | (nm) | 1.55–3.71 | 1.69–3.70 | 1.32–2.83 |
| Pore volume | (cm3/g) | 1.02–7.56 × 10−3 | 1.25–8.54 × 10−3 | 0.99–6.67 × 10−3 |
| Materials | Density (kg/dm3) | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| Mass (kg/m3) | |||||
| CEM I 42.5 R | 3.10 | 350 | 400 | 450 | 450 |
| Fly ash | 2.05 | 70 | 0 | 0 | 70 |
| GEGA 2 mm | 3.80 | 60 | 64 | 125 | 116 |
| GEGA 4 mm | 3.50 | 53 | 60 | 120 | 116 |
| GAA 8 mm | 1.35 | 450 | 450 | 0 | 0 |
| Water | 1.00 | 194 | 180 | 180 | 180 |
| Superplasticizer | 1.07 | 2.8 | 3.2 | 3.6 | 3.6 |
| (w/c) * | (–) | 0.55 | 0.45 | 0.40 | 0.40 |
| (w/b) ** | (–) | 0.50 | 0.45 | 0.40 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpińska, M.; Haustein, E. Experimental Study of the Resistance to Influence of Aggressive Liquids on Lightweight Concrete. Materials 2021, 14, 4185. https://doi.org/10.3390/ma14154185
Kurpińska M, Haustein E. Experimental Study of the Resistance to Influence of Aggressive Liquids on Lightweight Concrete. Materials. 2021; 14(15):4185. https://doi.org/10.3390/ma14154185
Chicago/Turabian StyleKurpińska, Marzena, and Elżbieta Haustein. 2021. "Experimental Study of the Resistance to Influence of Aggressive Liquids on Lightweight Concrete" Materials 14, no. 15: 4185. https://doi.org/10.3390/ma14154185
APA StyleKurpińska, M., & Haustein, E. (2021). Experimental Study of the Resistance to Influence of Aggressive Liquids on Lightweight Concrete. Materials, 14(15), 4185. https://doi.org/10.3390/ma14154185






