Ionic Liquids Separating Rubber Latex from Guayule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sarkar, M.; Mukunda, P.G.; De, P.P.; Bhowmick, A.K. Degradation of hydrogenated styrene-butadiene rubber at high temperature. Rubber Chem. Technol. 1997, 70, 855–870. [Google Scholar] [CrossRef]
- Cornish, K.; Williams, J.; Hall, J.L.; McCoy, R.G. Production and properties of Yulex® - The natural solution to latex allergy. Rubber Chem. Technol. 2008, 81, 709–722. [Google Scholar] [CrossRef]
- Moore, M. Leaf Disease Threatens Natural Rubber Production. Available online: https://www.tirebusiness.com/news/leaf-disease-threatens-natural-rubber-production (accessed on 7 August 2020).
- Cornish, K.; Brichta, J.L. Purification of Hypoallergenic Latex from Guayule; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Hamilton, R.G.; Cornish, K. Immunogenicity studies of guayule and guayule latex in occupationally exposed workers. Ind. Crop. Prod. 2010, 31, 197–201. [Google Scholar] [CrossRef]
- Kreuzberger, M.; Hahn, T.; Zibek, S.; Schiemann, J.; Thiele, K. Seasonal pattern of biomass and rubber and inulin of wild Russian dandelion (Taraxacum koksaghyz L. Rodin) under experimental field conditions. Eur. J. Agron. 2016, 80, 66–77. [Google Scholar] [CrossRef]
- Rousset, A.; Amor, A.; Punvichai, T.; Perino, S.; Palu, S.; Dorget, M.; Pioch, D.; Chemat, F. Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications. Molecules 2021, 26, 664. [Google Scholar] [CrossRef]
- Rasutis, D.; Soratana, K.; McMahan, C.; Landis, A.E. A sustainability review of domestic rubber from the guayule plant. Ind. Crop. Prod. 2015, 70, 383–394. [Google Scholar] [CrossRef]
- Coffelt, T.A.; Williams, C.F. Characterization and recycling of waste water from guayule latex extraction. Ind. Crop. Prod. 2009, 29, 648–653. [Google Scholar] [CrossRef]
- Huang, Y.; Mouri, H.; Beaulieu, M. Processes for the Removal of Rubber from TKS Plant Matter. U.S. Patent 9,567,457, 14 February 2017. [Google Scholar]
- Mariani, A.; Bonomo, M.; Gao, X.; Centrella, B.; Nucara, A.; Buscaino, R.; Barge, A.; Barbero, N.; Gontrani, L.; Passerini, S. The unseen evidence of Reduced Ionicity: The elephant in (the) room temperature ionic liquids. J. Mol. Liq. 2021, 324. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Vasquez, V.R.; Coronella, C.J. Pretreatment of rice hulls by ionic liquid dissolution. Bioresour. Technol. 2012, 114, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Lynam, J.G. Loblolly pine pretreatment by ionic liquid-glycerol mixtures. Biomass Convers. Biorefinery 2015. [Google Scholar] [CrossRef]
- Maciel, V.G.; Wales, D.J.; Seferin, M.; Ugaya, C.M.L.; Sans, V. State-of-the-art and limitations in the life cycle assessment of ionic liquids. J. Clean. Prod. 2019, 217, 844–858. [Google Scholar] [CrossRef]
- Isci, A.; Erdem, G.M.; Elmaci, S.B.; Sakiyan, O.; Lamp, A.; Kaltschmitt, M. Effect of microwave-assisted deep eutectic solvent pretreatment on lignocellulosic structure and bioconversion of wheat straw. Cellulose 2020. [Google Scholar] [CrossRef]
- Available online: http://www.iolitec-usa.com (accessed on 29 July 2021).
- Lynam, J.G.; Chow, G.I.; Coronella, C.J.; Hiibel, S.R. Ionic liquid and water separation by membrane distillation. Chem. Eng. J. 2016, 288, 557–561. [Google Scholar] [CrossRef]
- Gao, J.; Chen, C.; Wang, L.; Lei, Y.; Ji, H.; Liu, S. Utilization of inorganic salts as adjuvants for ionic liquid–water pretreatment of lignocellulosic biomass: Enzymatic hydrolysis and ionic liquid recycle. 3 Biotech 2019, 9. [Google Scholar] [CrossRef]
- Banigan, T.F.; Verbiscar, A.J.; Oda, T.A. Infrared spectrophotometric analysis for natural rubber in guayule shrubs. Rubber Chem. Technol. 1982, 55, 407–415. [Google Scholar] [CrossRef]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Choi, I.S.; Roland, C.M. Strain-crystallization of guayule and hevea rubbers. Rubber Chem. Technol. 1997, 70, 202–210. [Google Scholar] [CrossRef]
- Kishore, K.; Pandey, H.K. Spectral studies on plant rubbers. Prog. Polym. Sci. 1986, 12, 155–178. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Ventura, S.P.M.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.M.; Gonçalves, F.; Coutinho, J.A.P. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef] [PubMed]

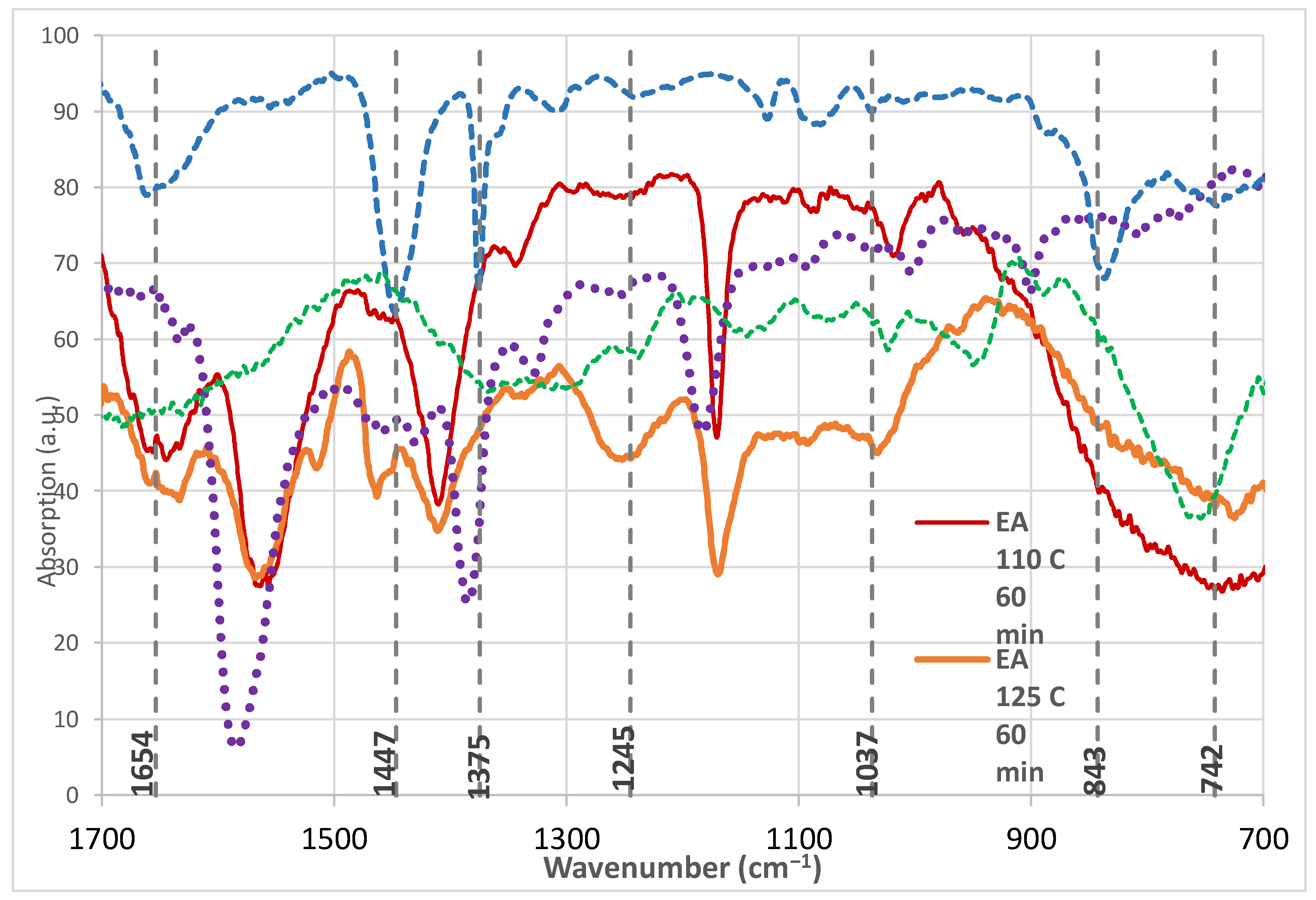
| Latex Wave-Number (cm−1) | 1654 | 1447 | 1375 | 1245 | 1037 | 843 | 742 |
|---|---|---|---|---|---|---|---|
| Bond Vibration | C=C stretching | –CH2– deformation/rocking –CH3 | –CH3 deformation | twisting –CH2– | rocking –CH3 group | backbone C–H out-of-plane mode | rocking –CH2– |
| 110 °C/30 min | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 110 °C/60 min | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 110 °C/90 min | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 110 °C/120 min | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 125 °C/30 min | ✓ | ✓ | ✓ | ||||
| 125 °C/60 min | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 125 °C/90 min | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 125 °C/120 min | ✓ | ✓ | ✓ | ✓ | |||
| 140 °C/30 min | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 140 °C/60 min | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 140 °C/90 min | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 140 °C/120 min | ✓ | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynam, J.G.; Zugger, H.T.; Amedee, E.T. Ionic Liquids Separating Rubber Latex from Guayule. Materials 2021, 14, 4255. https://doi.org/10.3390/ma14154255
Lynam JG, Zugger HT, Amedee ET. Ionic Liquids Separating Rubber Latex from Guayule. Materials. 2021; 14(15):4255. https://doi.org/10.3390/ma14154255
Chicago/Turabian StyleLynam, Joan G., Holden T. Zugger, and Elizabeth T. Amedee. 2021. "Ionic Liquids Separating Rubber Latex from Guayule" Materials 14, no. 15: 4255. https://doi.org/10.3390/ma14154255







