Recent Advances in the Excipients Used for Modified Ocular Drug Delivery
Abstract
:1. Introduction
2. Anatomy and Physiology of the Eye
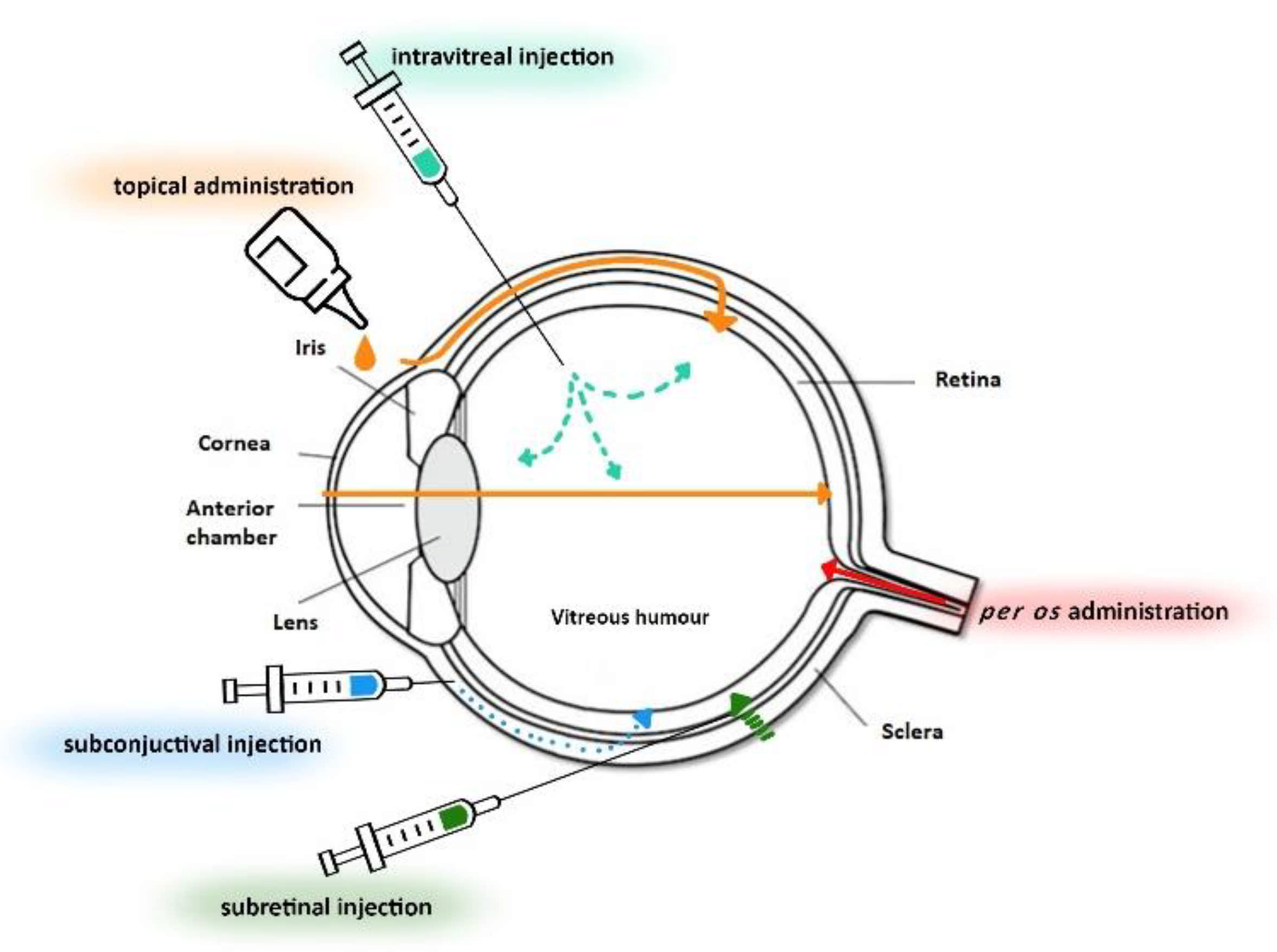
3. Ocular Drug Delivery
4. Factors That Affect Drug Absorption in the Eye
5. Pharmaceutical Dosage Forms for Ocular Administration
6. Excipients Used in Ocular Formulations for Modified Drug Delivery
6.1. Chitosan
6.2. Hyaluronic Acid
6.3. Poloxamer
6.4. PLGA
6.5. PVCL–PVA–PEG
6.6. Cetalkonium Chloride
6.7. Gelatin
6.8. Pharmaceutical Nanotechnology in Ocular Formulations
7. Triggered Release of Drugs and Their Potential Applications
8. Future Development Direction of Ocular Delivery
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sultana, Y.; Jain, R.; Aqil, M.; Ali, A. Review of Ocular Drug Delivery. Curr. Drug Deliv. 2006, 3, 207–217. [Google Scholar] [CrossRef]
- Cholkar, K.; Dasari, S.R.; Pal, D.; Mitra, A.K. Ocular Transporters and Receptors: Their Role in Drug Delivery, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 1–36. [Google Scholar]
- Galloway, N.R.; Amoaku, W.M.K.; Galloway, P.H.; Browning, A.C. Common Eye Diseases and Their Management, 4th ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 7–16. [Google Scholar]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic drug delivery systems for antibiotherapy—A review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, H.J. Anatomy and function of the eye. Chem. Immunol. Allergy 2007, 92, 4–10. [Google Scholar]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Mitra, A.K.; Anand, B.S.; Duvvuri, S. Drug Delivery to the Eye. Adv. Organ Biol. 2005, 10, 307–351. [Google Scholar]
- Prasad, D.; Chauhan, H. Chapter 24: Excipients utilized for ophthalmic drug delivery systems. Nano-Biomaterials For Ophthalmic Drug Delivery, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 555–582. [Google Scholar]
- Bowling, B. Kanski’s Clinical Ophthalmology: A Systematic Approach, 8th ed.; Saunders Ltd.: Philadelphia, PA, USA, 2015; p. 928. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, M. Pharmaceutical Preformulation and Formulation: A Practical Guide from Candidate Drug Selection to Commercial Dosage Form, 2nd ed.; Informa Helathcare: New York, NY, USA, 2009; pp. 432–434. [Google Scholar]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Camins Espuny, A.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 861904. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Maher, S.; Casettari, L.; Illum, L. Transmucosal Absorption Enhancers in the Drug Delivery Field. Pharmaceutics 2019, 11, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D. Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef]
- Morrison, W.J.P.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellepeddi, V.K.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82. [Google Scholar] [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Edelhauser, H.F.; Boatright, J.H.; Nickerson, J.M.; Edelhauser, H.F.; Abrams, G.; Adamis, A.; Aguirre, G.D.; Ambati, J.; Becerra, P.; Behar-Cohen, F.; et al. Drug delivery to posterior intraocular tissues: Third annual ARVO/Pfizer ophthalmics research institute conference. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4712–4720. [Google Scholar] [CrossRef]
- Nettey, H.; Darko, Y.; Bamiro, O.A.; Addo, R.T. Ocular Drug Delivery: Advances, Challenges and Applications, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–36. [Google Scholar]
- Alqawlaq, S.; Huzil, J.T.; Ivanova, M.V.; Foldvari, M. Challenges in neuroprotective nanomedicine development: Progress towards noninvasive gene therapy of glaucoma. Nanomedicine 2012, 7, 1067–1083. [Google Scholar] [CrossRef]
- Bachu, R.; Chowdhury, P.; Al-Saedi, Z.; Karla, P.; Boddu, S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Lee, J.; Pelis, R.M. Drug transport by the blood-aqueous humor barrier of the eye. Drug Metab. Dispos. 2016, 44, 1675–1681. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef]
- Pitkänen, L.; Ranta, V.P.; Moilanen, H.; Urtti, A. Permeability of retinal pigment epithelium: Effects of permeant molecular weight and lipophilicity. Investig. Ophthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Ambati, J.; Adamis, A.P. Transscleral drug delivery to the retina and choroid. Prog. Retin. Eye Res. 2002, 21, 145–151. [Google Scholar] [CrossRef]
- Kato, A.; Kimura, H.; Okabe, K.; Okabe, J.; Kunou, N.; Ogura, Y. Feasibility of Drug Delivery to the Posterior Pole of the Rabbit Eye with an Episcleral Implant. Investig. Ophthalmol. Vis. Sci. 2004, 45, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Gunda, S.; Mitra, A.K. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: Role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J. Pharmacol. Exp. Ther. 2004, 311, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Gonzalez, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Nanomedicines for ocular NSAIDs: Safety on drug delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 394–401. [Google Scholar] [CrossRef]
- Abdelkader, H.G.; Alany, R. Controlled and Continuous Release Ocular Drug Delivery Systems: Pros and Cons. Curr. Drug Deliv. 2012, 9, 421–430. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Kaur, I.P.; Kanwar, M. Ocular Preparations: The Formulation Approach. Drug Dev. Ind. Pharm. 2002, 28, 473–493. [Google Scholar] [CrossRef]
- Schmitt, M. Innovative Dosage Forms: Design and Development at Early Stage, 1st ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2020; pp. 331–365. [Google Scholar]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Tangri, P.; Khurana, S. Basics of Ocular Drug Delivery Systems. Int. Res. J. Pharm. Appl. Sci. 2011, 2, 1541–1552. [Google Scholar]
- Chen, H. Recent developments in ocular drug delivery. J. Drug Target. 2015, 23, 597–604. [Google Scholar] [CrossRef]
- WHO. Opthalmic Preparations—International Pharmacopoeia, 9th ed.; WHO Department of Essential Medicines and Health Products: Geneva, Switzerland, 2019; Available online: https://apps.who.int/phint/pdf/b/6.2.1.3.Ophthalmic-preparations.pdf (accessed on 20 May 2021).
- Rathore, K.S.; Nema, R.K. An Insight into Ophthalmic Drug Delivery System. Int. J. Pharm. 2009, 1, 1–5. [Google Scholar]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Pandey, V.; Tiwari, R.; Asati, S.; Tekade, R.K. Basic Fundamentals of Drug Delivery, 2nd ed.; Academic Press: London, UK, 2019; pp. 473–538. [Google Scholar]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Başaran, E.; Yazan, Y. Ocular application of chitosan. Expert Opin. Drug Deliv. 2012, 9, 701–712. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.; Kay, G. Eye gels for ophthalmic delivery. Expert Rev. Ophthalmol. 2015, 10, 127–133. [Google Scholar] [CrossRef]
- Majeed, A.; Khan, N.A. Ocular in situ gel: An overview. J. Drug Deliv. Ther. 2019, 9, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Jervis, L.P. A Summary of Recent Advances in Ocular Inserts and Implants. J. Bioequiv. Availab. 2017, 9, 320–323. [Google Scholar]
- Dave, V.S. Chapre 8: Formulation approaches for ocular drug delivery. Nano-Biomaterials for Ophthalmic Drug Delivery, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 147–175. [Google Scholar]
- Short, B.G. Safety Evaluation of Ocular Drug Delivery Formulations: Techniques and Practical Considerations. Toxicol. Pathol. 2008, 36, 49–62. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular Inserts-Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan derivatives as promising materials for controlled drug delivery. J. Biomater. Appl. 2008, 23, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Zambito, Y.; Burgalassi, S.; Monti, D.; Tampucci, S.; Terreni, E.; Fabiano, A.; Balzano, F.; Ucello-Barretta, G.; Chetoni, P. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-β-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J. Mater. Sci. Mater. Med. 2018, 29, 42. [Google Scholar] [CrossRef]
- Piras, A.M.; Fabiano, A.; Chiellini, F.; Zambito, Y. Methyl-β-cyclodextrin quaternary ammonium chitosan conjugate: Nanoparticles vs macromolecular soluble complex. Int. J. Nanomed. 2018, 13, 2531–2541. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef] [PubMed]
- Račić, A.; Čalija, B.; Milić, J.; Milašinović, N.; Krajišnik, D. Development of polysaccharide-based mucoadhesive ophthalmic lubricating vehicles: The effect of different polymers on physicochemical properties and functionality. J. Drug Deliv. Sci. Technol. 2019, 49, 50–57. [Google Scholar] [CrossRef]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Jurišić Dukovski, B.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Crespo Moral, M.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar; Imam, S.S.; Bukhari, S.N.A.; Ali, A. Preparation and evaluation of novel chitosan: Gelrite ocular system containing besifloxacin for topical treatment of bacterial conjunctivitis: Scintigraphy, ocular irritation and retention assessment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Ameeduzzafar; Imam, S.S.; Abbas Bukhari, S.N.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C 2018, 88, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Abilova, G.K.; Kaldybekov, D.B.; Ozhmukhametova, E.K.; Saimova, A.Z.; Kazybayeva, D.S.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Chitosan/poly(2-ethyl-2-oxazoline) films for ocular drug delivery: Formulation, miscibility, in vitro and in vivo studies. Eur. Polym. J. 2019, 116, 311–320. [Google Scholar] [CrossRef]
- Franca, J.R.; Fuscaldi, L.L.; Ribeiro, T.G.; Foureaux, G.; Cesar, A.L.A.; Castilho, R.O.; Cronemberger, S.; Ferreira, A.J.; Fernandes, S.O.A.; Cardoso, V.N.; et al. Use of chitosan as pharmaceutical excipient in ocular drug delivery systems: Sterilization and pharmacokinetics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2227–2237. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- Meyer, K.; Palmer, J.W. The Polysaccharide of the Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly (N -isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.A.; Mora, M.J.; Onnainty, R.; Tartara, L.I.; Granero, G.E.; Longhi, M.R.; Diebold, Y.; Vallés, E.M.; Palma, S.D. Cross-linked hyaluronan films loaded with acetazolamide-cyclodextrin-triethanolamine complexes for glaucoma treatment. Ther. Deliv. 2018, 9, 205–219. [Google Scholar] [CrossRef]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Donia, M.; Osman, R.; Awad, G.A.S.; Mortada, N. Polypeptide and glycosaminoglycan polysaccharide as stabilizing polymers in nanocrystals for a safe ocular hypotensive effect. Int. J. Biol. Macromol. 2020, 162, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmolka, I.R. A review of block polymer surfactants. J. Am. Oil Chem. Soc. 1977, 54, 110–116. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ahmadi, Z.; Reza Saeb, M.; Mozafari, M. Poloxamer-based stimuli-responsive biomaterials. Mater. Today Proc. 2018, 5, 15516–15523. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Wang, T.; Markham, A.; Thomas, S.J.; Wang, N.; Huang, L.; Clemens, M.; Rajagopalan, N. Solution Stability of Poloxamer 188 Under Stress Conditions. J. Pharm. Sci. 2019, 108, 1264–1271. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Gupta, C.; Juyal, V.; Nagaich, U. Formulation and optimization of thermosensitive in-situ gel of moxifloxacin hydrochloride for ocular drug delivery. Int. J. Appl. Pharm. 2018, 10, 123–130. [Google Scholar]
- Üstündağ Okur, N.; Çağlar, E.Ş.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef]
- Wen, Y.; Jia, H.; Mo, Z.; Zheng, K.; Chen, S.; Ding, Y.; Zhang, Y.; Wen, Y.; Xie, Q.; Qiu, J.; et al. Cross-linked thermosensitive nanohydrogels for ocular drug delivery with a prolonged residence time and enhanced bioavailability. Mater. Sci. Eng. C 2021, 119, 111445. [Google Scholar] [CrossRef]
- Krtalić, I.; Radošević, S.; Hafner, A.; Grassi, M.; Nenadić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. D-Optimal Design in the Development of Rheologically Improved In Situ Forming Ophthalmic Gel. J. Pharm. Sci. 2018, 107, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs 2011, 25, 79–85. [Google Scholar]
- Mir, M.; Ahmed, N.; Rehman, A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Silva-Abreu, M.; Calpena, A.C.; Egea, M.A.; Espina, M.; García, M.L. Development of fluorometholone-loaded PLGA nanoparticles for treatment of inflammatory disorders of anterior and posterior segments of the eye. Int. J. Pharm. 2018, 547, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Fang, A.; Wang, F.; Li, H.; Jin, Q.; Huang, L.; Fu, C.; Zeng, J.; Jin, Z.; Song, X. Core-shell lipid-polymer nanoparticles as a promising ocular drug delivery system to treat glaucoma. Chin. Chem. Lett. 2020, 31, 494–500. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Rong, X.; Yang, J.; Ji, Y.; Zhu, X.; Lu, Y.; Mo, X. Biocompatibility and safety of insulin-loaded chitosan nanoparticles/ PLGA-PEG-PLGA hydrogel (ICNPH) delivered by subconjunctival injection in rats. J. Drug Deliv. Sci. Technol. 2019, 49, 556–562. [Google Scholar] [CrossRef]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.S.Y.; To, K.K.W. Biodegradable Thermosensitive PLGA-PEG-PLGA Polymer for Non-irritating and Sustained Ophthalmic Drug Delivery. AAPS J. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cespi, M.; Casettari, L.; Palmieri, G.F.; Perinelli, D.R.; Bonacucina, G. Rheological characterization of polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®) water dispersions. Colloid Polym. Sci. 2014, 292, 235–241. [Google Scholar] [CrossRef]
- Ogawa, N.; Hiramatsu, T.; Suzuki, R.; Okamoto, R.; Shibagaki, K.; Fujita, K.; Takahashi, C.; Kawashima, Y.; Yamamoto, H. Improvement in the water solubility of drugs with a solid dispersion system by spray drying and hot-melt extrusion with using the amphiphilic polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer and D-mannitol. Eur. J. Pharm. Sci. 2018, 111, 205–214. [Google Scholar] [CrossRef]
- Lakshman, D.; Chegireddy, M.; Hanegave, G.K.; Sree, K.N.; Kumar, N.; Lewis, S.A.; Dengale, S.J. Investigation of drug-polymer miscibility, biorelevant dissolution, and bioavailability improvement of Dolutegravir-polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer solid dispersions. Eur. J. Pharm. Sci. 2020, 142, 105137. [Google Scholar] [CrossRef]
- Tousif Ayyub, K.; Moravkar, K.; Maniruzzaman, M.; Amin, P. Effect of melt extrudability and melt binding efficiency of polyvinyl caprolactam polyvinyl acetate polyethylene glycol graft copolymer (Soluplus®) on release pattern of hydrophilic and high dose drugs. Mater. Sci. Eng. C 2019, 99, 563–574. [Google Scholar] [CrossRef]
- Sun, F.; Zheng, Z.; Lan, J.; Li, X.; Li, M.; Song, K.; Wu, X. New micelle myricetin formulation for ocular delivery: Improved stability, solubility, and ocular anti-inflammatory treatment. Drug Deliv. 2019, 26, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Amangeldykyzy, S.; Nurlybek, A.N.; Nurlan, A.N.; Juszkiewicz, K.T.; Polski, A.; Seisembay, U.B.; Mukasheva, A.M.; Urumbaeva, K.U.; Poleszak, E. The effect of a combined choline salicylate and cetalkonium chloride gel on particular strains of Pseudomonas aeruginosa, Staphylococcus spp. and Streptococcus spp. Curr. Issues Pharm. Med. Sci. 2015, 28, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Brignole-Baudouin, F.; Rabinovich-Guilatt, L.; Mao, Z.; Riancho, L.; Faure, M.O.; Warnet, J.M.; Lambert, G.; Baudouin, C. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: An in vivo study in rabbits. Mol. Vis. 2008, 14, 204–216. [Google Scholar]
- Daull, P.; Lallemand, F.; Garrigue, J.-S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrigue, J.S.; Amrane, M.; Faure, M.O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domszy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug. Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef]
- Mhd Sarbon, N.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Hathout, R.M.; Gad, H.A.; Abdel-Hafez, S.M.; Nasser, N.; Khalil, N.; Ateyya, T.; Amr, A.; Yasser, N.; Nasr, S.; Metwally, A.A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199. [Google Scholar] [CrossRef]
- Shokry, M.; Hathout, R.M.; Mansour, S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. Int. J. Pharm. 2018, 545, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalu, L.; Tambe, V.; Pradhan, D.; Nayak, K.; Bagchi, S.; Maheshwari, R.; Kalia, K.; Tekade, R.K. Novel nanosystems for the treatment of ocular inflammation: Current paradigms and future research directions. J. Control. Release 2017, 268, 19–39. [Google Scholar] [CrossRef]
- Naziris, N.; Pippa, N.; Demetzos, C. A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles. Materials 2020, 13, 5035. [Google Scholar] [CrossRef]
- Lallemand, F.; Schmitt, M.; Bourges, J.L.; Gurny, R.; Benita, S.; Garrigue, J.S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur. J. Pharm. Biopharm. 2017, 117, 14–28. [Google Scholar] [CrossRef]
- Rodriguez Villanueva, J.; Navarro, M.G.; Rodríguez Villanueva, L. Dendrimers as a promising tool in ocular therapeutics: Latest advances and perspectives. Int. J. Pharm. 2016, 511, 359–366. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Demetzos, C.; Pippa, N. Advanced drugdelivery nanosystems (aDDnSs): A mini-review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Stellas, D.; Skandalis, A.; Pispas, S.; Demetzos, C. Chimeric lipid/block copolymer nanovesicles: Physico-chemical and bio-compatibility evaluation. Eur. J. Pharm. Biopharm. 2016, 107, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Gai, X.; Cheng, L.; Li, T.; Liu, D.; Wang, Y.; Wang, T.; Pan, W.; Yang, X. In vitro and In vivo Studies on a Novel Bioadhesive Colloidal System: Cationic Liposomes of Ibuprofen. AAPS Pharm. Sci. Tech. 2018, 19, 700–709. [Google Scholar] [CrossRef]
- Hassan, D.; Abdelmonem, R.; Abdellatif, M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tian, S.; Tao, Q.; Zhao, Y.; Gui, R.; Yang, F.; Zang, L.; Chen, Y.; Ping, Q.; Hou, D. Montmorillonite/chitosan nanoparticles as a novel controlled-release topical ophthalmic delivery system for the treatment of glaucoma. Int. J. Nanomed. 2018, 10, 3975–3987. [Google Scholar] [CrossRef] [Green Version]
- Conde Penedo, A.; Díaz Tomé, V.; Fernández Ferreiro, A.; González Barcia, M.; Otero Espinar, F.J. Enhancement in corneal permeability of riboflavin using cyclodextrin derivates complexes as a previous step to transepithelial cross-linking. Eur. J. Pharm. Biopharm. 2021, 162, 12–22. [Google Scholar] [CrossRef]
- Budai-Szűcs, M.; Kiss, E.L.; Szilágyi, B.Á.; Szilágyi, A.; Gyarmati, B.; Berkó, S.; Kovács, A.; Horvát, G.; Aigner, Z.; Soós, J.; et al. Mucoadhesive cyclodextrin-modified thiolated poly(aspartic acid) as a potential ophthalmic drug delivery system. Polymers 2018, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Willem de Vries, J.; Schnichels, S.; Hurst, J.; Strudel, L.; Gruszka, A.; Kwak, M.; Bartz-Schmidt, K.U.; Spitzer, M.S.; Herrmann, A. DNA nanoparticles for ophthalmic drug delivery. Biomaterials 2018, 157, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of transgelosomes for enhancing the ocular delivery of acetazolamide: Statistical optimization, in vitro characterization, and in vivo study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Elbahwy, I.A.; Lupo, N.; Ibrahim, H.M.; Ismael, H.R.; Kasem, A.A.; Caliskan, C.; Matuszczak, B.; Bernkop-Schnürch, A. Mucoadhesive self-emulsifying delivery systems for ocular administration of econazole. Int. J. Pharm. 2018, 541, 72–80. [Google Scholar] [CrossRef]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Benzoic acid derivative-modified chitosan-g-poly(N-isopropylacrylamide): Methoxylation effects and pharmacological treatments of Glaucoma-related neurodegeneration. J. Control. Release 2020, 317, 246–258. [Google Scholar] [CrossRef]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of dorzolamide-HCL via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef]
- Hamcerencu, M.; Desbrieres, J.; Popa, M.; Riess, G. Thermo-sensitive gellan maleate/N-isopropylacrylamide hydrogels: Initial “in vitro” and “in vivo” evaluation as ocular inserts. Polym. Bull. 2020, 77, 741–755. [Google Scholar] [CrossRef]
- Nanda, A.; Sahoo, R.N.; Pramanik, A.; Mohapatra, R.; Pradhan, S.K.; Thirumurugan, A.; Das, D.; Mallick, S. Drug-in-mucoadhesive type film for ocular anti-inflammatory potential of amlodipine: Effect of sulphobutyl-ether-beta-cyclodextrin on permeation and molecular docking characterization. Colloids Surf. B 2018, 172, 555–564. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Kaur, G. Leucaena leucocephala (Lam.) galactomannan nanoparticles: Optimization and characterization for ocular delivery in glaucoma treatment. Int. J. Biol. Macromol. 2019, 139, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Craig, J.P.; Krösser, S.; Eickhoff, K.; Swift, S.; Rupenthal, I.D. Topical semifluorinated alkane-based azithromycin suspension for the management of ocular infections. Eur. J. Pharm. Biopharm. 2019, 142, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ Okur, N.; Yozgatlı, V.; Evren Okur, M.; Yoltaş, A.; Siafaka, P.I. Improving therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 323–333. [Google Scholar] [CrossRef]
- Wadetwar, R.N.; Agrawal, A.R.; Kanojiya, P.S. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: In-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101575. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- El Hoffy, N.M.; Abdel Azim, E.A.; Hathout, R.M.; Fouly, M.A.; Elkheshen, S.A. Glaucoma: Management and Future Perspectives for Nanotechnology-Based Treatment Modalities. Eur. J. Pharm. Sci. 2021, 158, 105648. [Google Scholar] [CrossRef]


| Release Rate/Mucoadhesive Properties * | API(s) | Excipients Used in the Formulation | Reference |
|---|---|---|---|
| maintenance of mucoadhesive properties | dexamethasone | QA-Ch60-MCD => water soluble CS derivative 2-diethylaminoethyl chloride, DMSO, 1, 6-hexamethylene diisocyanate, triethylamine | [65] |
| gradual release in the initial 4 h | dexamethasone | QA-Ch-MCD vs. NPs | [66] |
| sustained | dexamethazone | CS oligosaccharide-va-lylvaline-stearic acid (CSO-VV-SA) nanomicelles | [67] |
| increased mucoadhesive properties | lubricant | CS, hydroxypropyl guar gum, NaCl, Na2HPO4, BZC | [68] |
| sustained (up to 12 h) | dorzolamideHCl | CS, Polycaprolactone, PVA | [69] |
| quick initial following sustained (<48 h) | dexamethasone | Succinated Dex, Glycol CS, N-hydro-xysuccinimide, N-(3-dimethylaminopropyl)-N′-ethylcarbodii- mide hydrochloride | [70] |
| relevant reservoir effect | cyclosporine | poloxamer 407, D-α-Tocopheryl, PEG 1000, succinate (Kolliphor® TPGS), kolliphor® TPGS, Trifluoroacetic acid, vit E, vit E succinate | [99] |
| sustained | fluorometholone | PLGA, poloxamer 188 (P188), Transcutol P® | [109] |
| sustained | brinzolamide | PLGA—SPC, soybean phosphatidylcholine | [112] |
| sustained | myricetin | PVCL-PVA-PEG | [118] |
| sustained (over 150 h) | diclofenac sodium | ethyl butyrate, 2-hydroxyethyl methacrylate, ethylene glycol dimethacrylate, azobis-iso-butrylonitrile, Tween 80, Brij 97, CKC | [124] |
| sustained | timolol maleate | cholesterol, gelatin (A), soya bean phosphatidylcholine, glycerol, Na2HPO4, KH2PO4, NaCl | [130] |
| burst effect following sustained | timolol maleate | gelatin (A) bloom 300 glutaraldehyde, glycine | [131] |
| extended release (4 days) | timolol maleate | gelatin (B) glyoxal solution 40%, glycine, HPMC | [132] |
| controlled | betaxolol HCl | montmorillonite/CS 1-(4, 5-dimethylthiazol-2-yl)-3, 5-diphenylformazan | [146] |
| constant over time | riboflavin | CS HCl, Arginine L HCl PBS, soy lecithin/polysorbate 80, poloxamer 407 | [147] |
| modified release for up to 24 h | prednisolone | PASP-CEA-CD, CD-modified thiolated poly(aspartic acid), L-aspartic acid, cysteamine | [148] |
| increased adherence time | neomycin B, kanamycin B | DNA block copolymers | [149] |
| prolonged | acerazolamide | Cremophore RH40, Span 60, Tween 80, Brij 35, L-alpha-phosphatidylcholine | [150] |
| sustained | econazole nitrate | L-Cysteine linked to 6-mercaptonicotinamide => attached to Eudragit® L100-55DMSO/glycerol/thiourea/6-chloronicotinamide/H2O2/PG/PEG 400/cremphor EL/5,5′-dithiobis (2-nitrobenzoic acid)/1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/N-hydroxysuccinimide/BZC/methoxyphenylazo-2-naphthol/Triton® X 100 | [151] |
| Release Rate * | API(s) | Excipients Used in the Formulation | Reference |
|---|---|---|---|
| prolonged | ibuprofen | Miglyol® 812, lecithin, Kolliphor® EL, glycerol | [71] |
| sustained | besifloxacin | CS, PVA, Gellan gum (GelriteTM) | [73] |
| prolonged/sustained | levofloxacin | CS, Tripolyphos-phate, sodium alginate, HPMC | [74] |
| sustained | levofloxacin | CS | [75] |
| prolonged/sustained | timolole malate | CS—gelatin crosslinked to β-CDs lysozyme, and β-glycerophosphate disodium salt hydrate, PBS, sodium fluorensic | [76] |
| sustained | latanoprost, curcumin | CS, PLGA, PVA | [77] |
| sustained | ketoconazole | Poly(N-isopropylacrylamide)/HA | [89] |
| prolonged | moxifloxacin hydrochloride | poloxamer F-127, gellan-gum, carbopol | [101] |
| sustained (8 h) | voriconazole | poloxamers P407, P188, CMC, BZC, NaCl | [102] |
| sustained | dexamethasone | poloxamer 407/188, Soy lecithine, glycerol, polycarbophil | [103] |
| prolonged | timolol maleate, dexamethasone, dorzolamide HCl | poloxamers P407, P188, CS gel | [104] |
| sustained | insuline | PLGA-PEG-PLGA CS, Sodium tripolyphosphate (TPP) (solution to form NPs) | [112] |
| prolonged (4–7 weeks) | rhodamine B, coumarin 6 | PLGA-PEG-PLGA, PBS powder, Sulfo-cyanine 7 NHS ester (Cy7), cyanine 7.5 alkyne (Cy7.5) | [113] |
| extended (for 6 months) | ranibizumab | (PEG-PLLA-DA/NIPAAm) PLGA microspheres, PVA, Bovine serum albumin (BSA), PEG, Mg(OH)2 | [152] |
| extended (for 6 months) | aflibercept | (PEG-PLLA-DA/NIPAAm) PLGA microspheres, PVA, Bovine serum albumin (BSA), PEG, Mg(OH)2 | [153] |
| sustained | pilocarpine RGFP966 | 4-hydroxy-3,5-dimethoxybenzoic acid CS-g-poly(N-isopropylacrylamide) | [154] |
| sustained | dorzolamide-HCl | chol/Span 40 L-a-lecithin, chtiosan | [155] |
| sustained for 3 h | olopatadine HCl | gellan gum, carbopol 934P, benzododecenium bromide | [156] |
| Release Rate * | API(s) | Excipients Used in the Formulation | Reference |
|---|---|---|---|
| sustained | fluorescein sodium salt | CS, poly(2-ethyl-2-oxazoline), PBS | [78] |
| maintance of mucoadhesiveness | CS, acetic acid | [79] | |
| immediate (lev), sustained (dex) | dexamethazone, levofloxasin | HA, Glycol CS | [90] |
| sustained | acetazolamide | HA sodium salt, HP-β-CD, TEA, PEG, diglycidylether, 2,3-bis(2-methoxy-4-nitro-5-sulphophenyl)-2H-tetrazolium-5-carboxanilide inner salt | [91] |
| sustained | ketorolac tromethamine diclofenac sodium salt, vit E | CKC, sterylamine Dulbecco’s PBS, stearylamine, flur-biprofen sodium | [125] |
| graduated | chloramphenicol hemisuccinate, adrenaline | Gellan maleate/N-isopropylacrylamide Gellan gum => gelan maleate, BIS, TEMED/APS | [157] |
| sustained for more than 6 h | amlodipine | sulphobutyl-ether-β-CD, β-CD, hydro-xypropyl-β-CD | [158] |
| sustained | doxycyclin, glial-cell derived neurotrophic factor | alginate-collagen | [159] |
| Release Rate * | API(s) | Excipients Used in the Formulation | Reference |
|---|---|---|---|
| sustained | acetazolamide | poly-γ-glutamic acid—HA, PVA, soya bean lecithin, L-α-soya bean phosphatidyl choline, sodium hyaluronate, Leucine/Mannitol/PEG/glycerol, betamethasone sodium phosphate, betamethasone dipropionate ampoule, PEG 400 | [142] |
| sustained | ibuprofen | soybean phospholipids, cholesterol, octadecylamine | [143] |
| prolonged | carvedilol | soy phosphatidylcholine, cetyltrimethylammonium/dimethyldidodecylammonium bromide | [92] |
| prolonged | moxifloxacin HCl | 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine, cholesterol, egg phospholipid, CS, Tripolyphosphate sodium, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, N-Hydroxysuccinimide | [144] |
| sustained | dorzolamide HCl | Leucaena leucocephala galactomannan (CMLLG/AMLLG)-NaOH | [160] |
| Excipients | Key Characteristics | Potential Advantages | Potential Disadvantages |
|---|---|---|---|
| chitosan | Deacetylation Molecular weight Solubility Contains hydroxyl and amino groups/polycationic in H+ env. | Interacts with negatively charged => delivery to mucosa Biocompatible Biodegradable Nontoxic Antimicrobial Antioxidant Limits growth of cancer cells | Final products of remarkable variability |
| hyaluronic acid | Anionic in physiological pH Has groups suitable for modification | Improves tissue hydration, lubricates Improves tissue resistance Widely available Fully resorbable Biocompatible Removes free radicals | Marginal side effects |
| poloxamer | Nonionic Amphiphilic Liquid/paste/flakes | Thermoreactive | Unstable from 20–25 °C Concentration needed to achieve gelation is high, affecting osmolarity |
| PLGA | Linear Soluble in various solvents Nontoxic | Biocompatible Highly crystalline (PLLA) Completely amorphous (PDLA) Can encapsulate molecules of virtually any size | |
| PVCL–PVA–PEG | Amphiphilic Soluble both in water and in organic solvents | Increases solubility of poorly soluble drugs/increases bioavailability through the formed micelles Does not absorb moisture from air | |
| cetalkonium chloride | Positively charged High lipophilicity Low toxicity | Antiseptic properties | |
| gelatin | High dissolution in water | Gelling emulsifying and foaming properties Widely available Not expensive Not as antigenic as collagen Groups can be chemically modified | None for long-term drug delivery systems Low rate of degradation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsoviti, M.; Siamidi, A.; Pavlou, P.; Vlachou, M. Recent Advances in the Excipients Used for Modified Ocular Drug Delivery. Materials 2021, 14, 4290. https://doi.org/10.3390/ma14154290
Koutsoviti M, Siamidi A, Pavlou P, Vlachou M. Recent Advances in the Excipients Used for Modified Ocular Drug Delivery. Materials. 2021; 14(15):4290. https://doi.org/10.3390/ma14154290
Chicago/Turabian StyleKoutsoviti, Melitini, Angeliki Siamidi, Panagoula Pavlou, and Marilena Vlachou. 2021. "Recent Advances in the Excipients Used for Modified Ocular Drug Delivery" Materials 14, no. 15: 4290. https://doi.org/10.3390/ma14154290
APA StyleKoutsoviti, M., Siamidi, A., Pavlou, P., & Vlachou, M. (2021). Recent Advances in the Excipients Used for Modified Ocular Drug Delivery. Materials, 14(15), 4290. https://doi.org/10.3390/ma14154290








