Synthesis, Characterization and Filtration Properties of Ecofriendly Fe3O4 Nanoparticles Derived from Olive Leaves Extract
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.1.1. Olive Leaves Extract Preparation
2.1.2. Synthesis of Fe3O4-NPs
2.1.3. WBDF Preparation Using Different Concentrations of Fe3O4-NPs
2.2. Characterization
2.2.1. Fe3O4-NPs Analysis
2.2.2. Rheological Properties Investigation
2.2.3. Filtration Properties
3. Results and Discussion
3.1. XRD Analysis
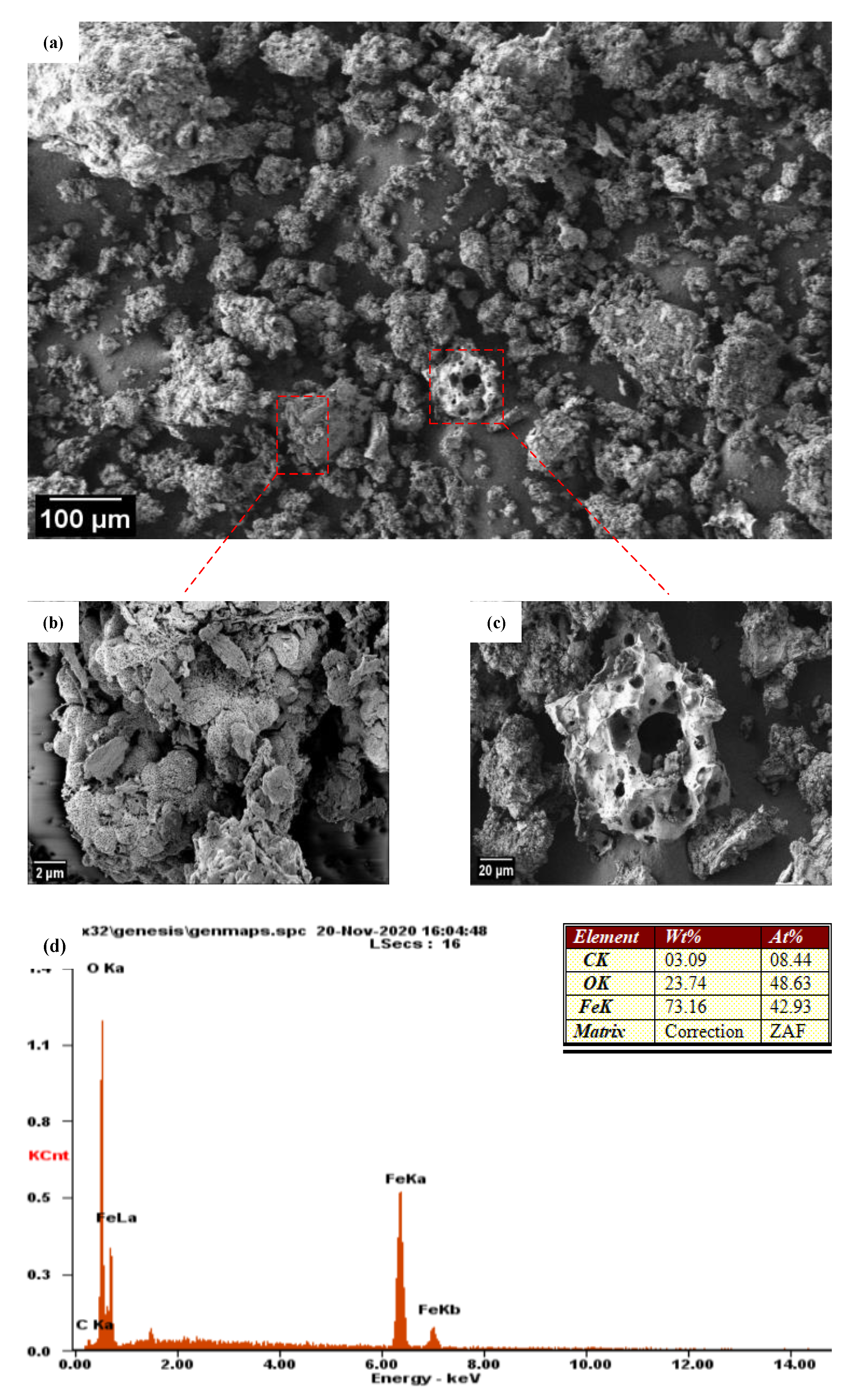
3.2. FESEM Analysis
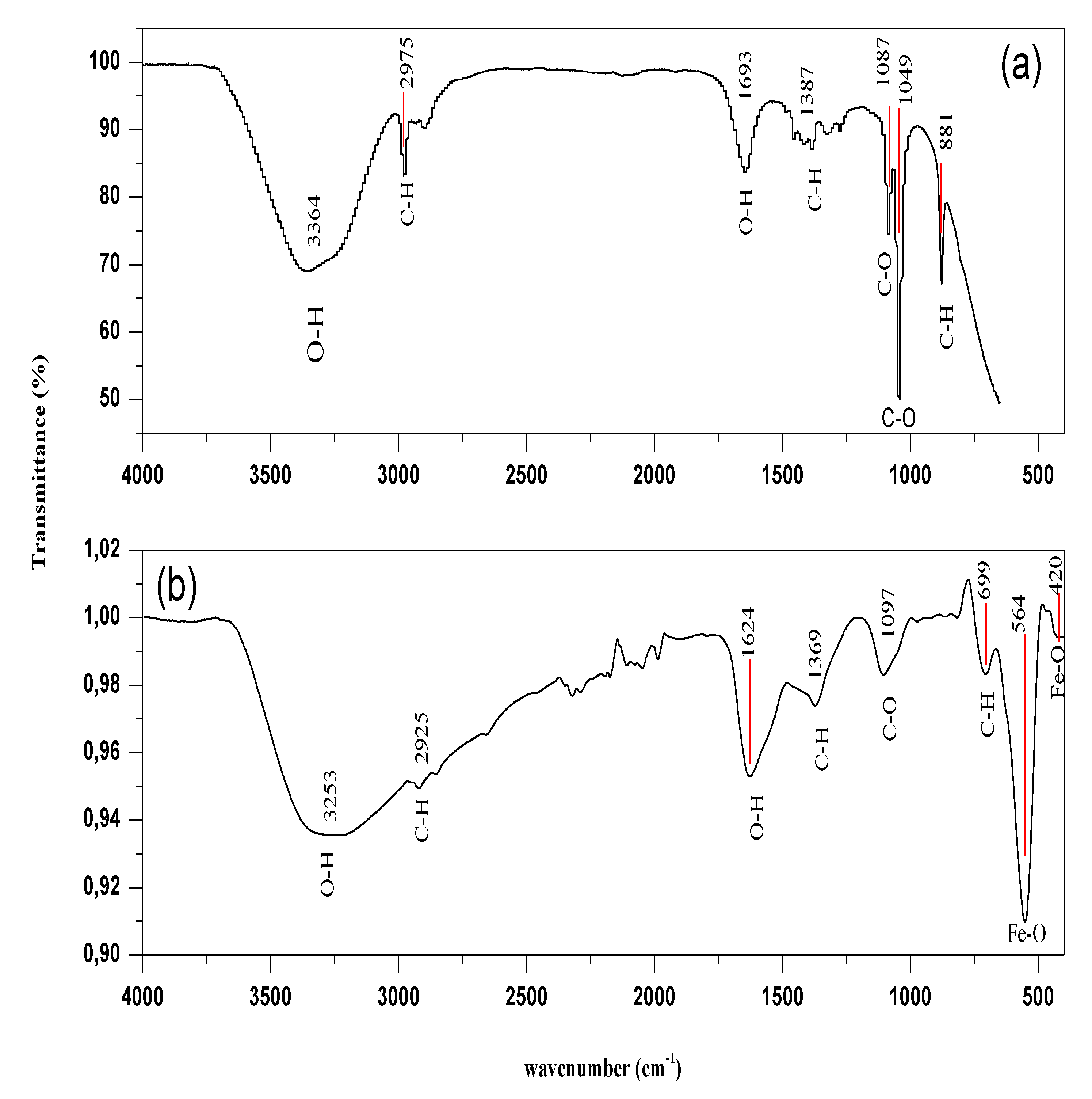
3.3. FTIR Study
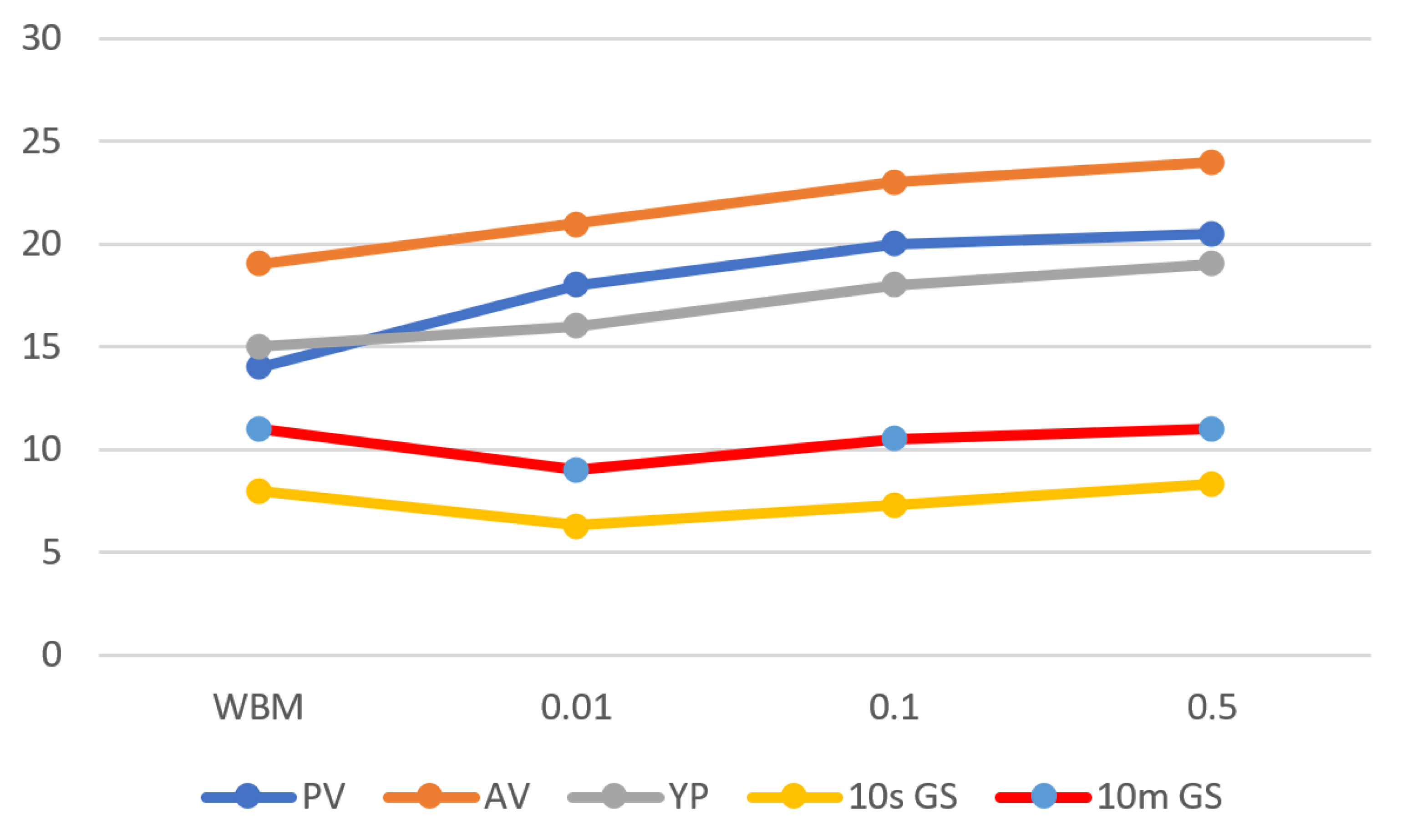
3.4. Effect of Fe3O4-NPs Concentration on Drilling Fluid: Rheological Properties
3.4.1. Plastic Viscosity
3.4.2. Yield Point
3.4.3. Gel Strength
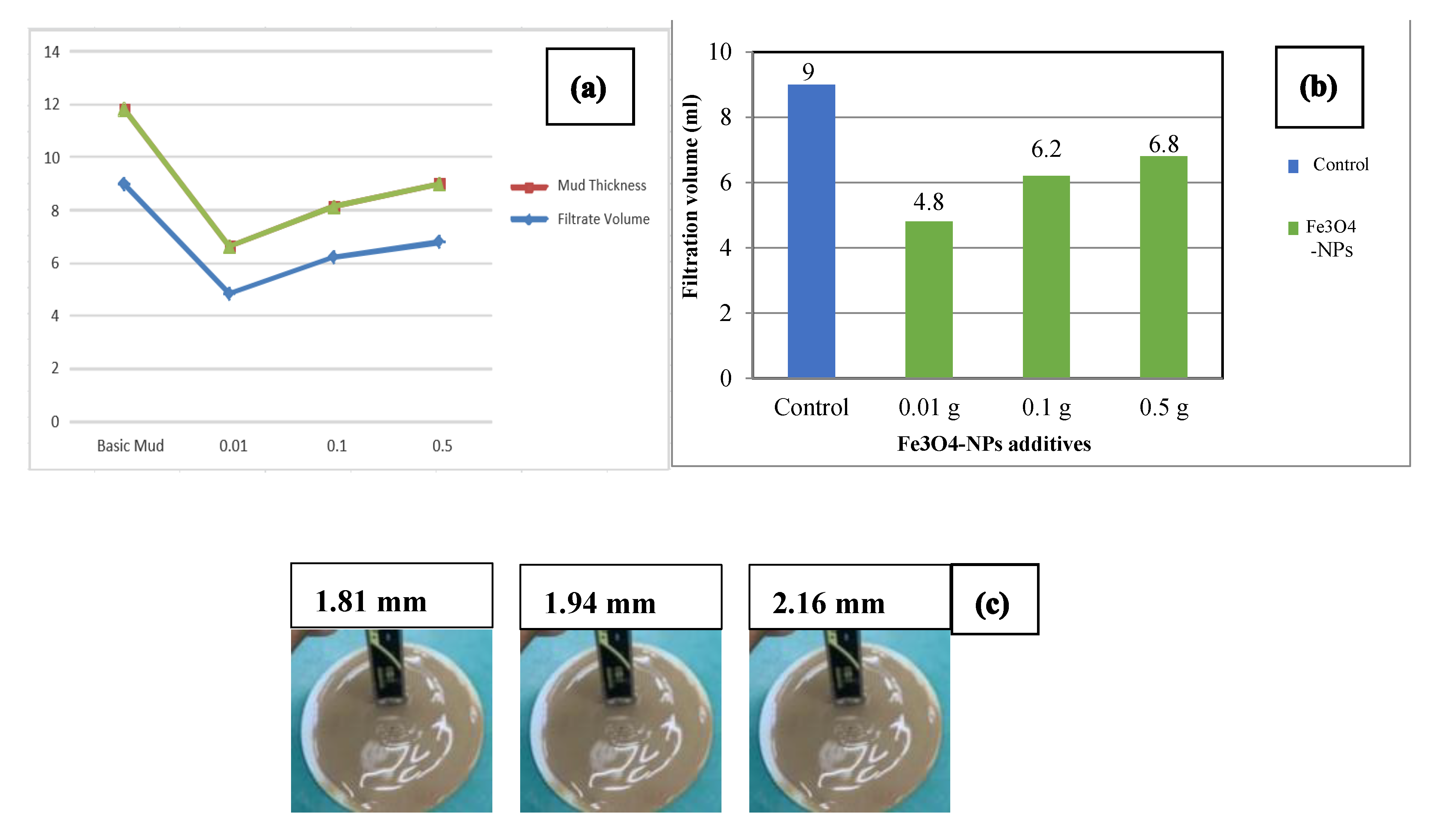
3.5. Effect of Fe3O4-NPs Concentration on Drilling Fluid: Fluid Filtration Loss
4. Conclusions
- ➢
- The effect of Fe3O4-NPs concentration on the rheological property and fluid filtration was investigated.
- ➢
- The best performance in both mud cake thickness and filtrate loss enhancement was obtained with the lowest concentration of Fe3O4-NPs.
- ➢
- In an aqueous setting, the Fe3O4-NPs exhibit positive charges due to the phenolic compounds coated the nanoparticles, which would attract the bentonite clay platelets’ negatively charged surfaces.
- ➢
- Coagulation was induced by the addition of Fe3O4-NPs to the WBM; the collective behavior of various types of clay particles were induced by the addition of an electrolyte to the clay solution, promoting a linked structure.
- ➢
- The linked structure allows more water to be trapped between the layers. This causes an increase in viscosity and yield stress while reducing fluid filtration.
- ➢
- The addition of Fe3O4-NPs reduced fluid loss volume, resulting in a thinner filter cake.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoveidavianpoor, M.; Samsuri, A. The use of nano-sized Tapioca starch as a natural water-soluble polymer for filtration control in water-based drilling muds. J. Nat. Gas Sci. Eng. 2016, 34, 832–840. [Google Scholar] [CrossRef]
- Peng, B.; Tang, J.; Luo, J.; Wang, P.; Ding, B.; Tam, K.C. Applications of nanotechnology in oil and gas industry: Progress and perspective. Can. J. Chem. Eng. 2018, 96, 91–100. [Google Scholar] [CrossRef]
- Mitter, N.; Hussey, K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat. Nanotechnol. 2019, 14, 508–510. [Google Scholar] [CrossRef]
- Lekomtsev, A.; Keykhosravi, A.; Moghaddam, M.B.; Daneshfar, R.; Rezvanjou, O. On the prediction of filtration volume of drilling fluids containing different types of nanoparticles by ELM and PSO-LSSVM based models. Petroleum 2021. [Google Scholar] [CrossRef]
- Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Cortés, F.B. Development of composite materials based on the interaction between nanoparticles and surfactants for application in chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2018, 57, 12367–12377. [Google Scholar] [CrossRef]
- Qi, S.; Geng, Z.; Lu, Z.; Zhang, G.; Wu, Z. Synergistic lubricating behaviors of 3D graphene and 2D hexagonal boron nitride dispersed in PAO4 for steel/steel contact. Adv. Mater. Interfaces 2020, 7, 1901893. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, J.; Qing, Y.; Wu, Y.; Lee, S. Rheology, curing temperature and mechanical performance of oil well cement: Combined effect of cellulose nanofibers and graphene nano-platelets. Mater. Des. 2017, 114, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Cheraghian, G. Nanoparticles in drilling fluid: A review of the state-of-the-art. J. Mater. Res. Technol. 2021, 13, 737–753. [Google Scholar] [CrossRef]
- Zhong, H.; Shen, G.; Yang, P.; Qiu, Z.; Jin, J.; Xing, X. Mitigation of lost circulation in oil-based drilling fluids using oil absorbent polymers. Materials 2018, 11, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, J.A.; Marques, N.D.N.; Villetti, M.A.; Balaban, R.D.C. Polymer-Decorated Cellulose Nanocrystals as Environmentally Friendly Additives for Olefin-Based Drilling Fluids. Int. J. Mol. Sci. 2021, 22, 352. [Google Scholar] [CrossRef]
- Mohamed, A.; Basfar, S.; Elkatatny, S.; Al-Majed, A. Enhancement of Static and Dynamic Sag Performance of Water-Based Mud Using a Synthetic Clay. ACS Omega 2021, 6, 8179–8188. [Google Scholar] [CrossRef]
- Vryzas, Z.; Zaspalis, V.; Nalbandian, L.; Terzidou, A.; Kelessidis, V.C. Rheological and HP/HT fluid loss behavior of nano-based drilling fluids utilizing Fe3O4 nanoparticles. Mater. Today Proc. 2018, 5, 27387–27396. [Google Scholar] [CrossRef]
- Bayat, A.E.; Shams, R. Appraising the impacts of SiO2, ZnO and TiO2 nanoparticles on rheological properties and shale inhibition of water-based drilling muds. Colloids Surf. A 2019, 581, 123792. [Google Scholar] [CrossRef]
- Ali, M.; Jarni, H.H.; Aftab, A.; Ismail, A.R.; Saady, N.M.C.; Sahito, M.F.; Keshavarz, A.; Iglauer, S.; Sarmadivaleh, M. Nanomaterial-based drilling fluids for exploitation of unconventional reservoirs: A review. Energies 2020, 13, 3417. [Google Scholar] [CrossRef]
- He, S.; Liang, L.; Zeng, Y.; Ding, Y.; Lin, Y.; Liu, X. The influence of water-based drilling fluid on mechanical property of shale and the wellbore stability. Petroleum 2016, 2, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.R.; Aftab, A.; Ibupoto, Z.H.; Zolkifile, N. The novel approach for the enhancement of rheological properties of water-based drilling fluids by using multi-walled carbon nanotube, nanosilica and glass beads. J. Pet. Sci. Eng. 2016, 139, 264–275. [Google Scholar] [CrossRef]
- Amanullah, M.; Yu, L. Environment friendly fluid loss additives to protect the marine environment from the detrimental effect of mud additives. J. Pet. Sci. Eng. 2005, 48, 199–208. [Google Scholar] [CrossRef]
- Seetharaman, G.R.; Sangwai, J.S. Effect of nanoparticles on the performance of drilling fluids. In Nanotechnology for Energy and Environmental Engineering; Sangwai, J.S., Ledwani, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 279–297. [Google Scholar]
- Li, X.; Jiang, G.; He, Y.; Chen, G. Novel starch composite fluid loss additives and their applications in environmentally friendly water-based drilling fluids. Energy Fuels 2021, 35, 2506–2513. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-based drilling fluids: A review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Boudouh, D.; Hamana, D.; Metselaar, H.S.C.; Achour, S.; Chetibi, L.; Akhiani, A.R. Low-temperature green route synthesis of Fe3O4-C nanocomposite using Olive Leaves Extract. Mater. Sci. Eng. B 2021, 271, 115276. [Google Scholar] [CrossRef]
- Aftab, A.; Ali, M.; Sahito, M.F.; Mohanty, U.S.; Jha, N.K.; Akhondzadeh, H.; Azhar, M.R.; Ismail, A.R.; Keshavarz, A.; Iglauer, S. Environmental friendliness and high performance of multifunctional tween 80/ZnO-nanoparticles-added water-based drilling fluid: An experimental approach. ACS Sustain. Chem. Eng. 2020, 8, 11224–11243. [Google Scholar] [CrossRef]
- Khattak, A.; Ahmad, B.; Rauf, A.; Bawazeer, S.; Farooq, U.; Ali, J.; Patel, S.; El-Sharkawy, E.R.; Ikram, R.; Linfang, H. Green synthesis, characterisation and biological evaluation of plant-based silver nanoparticles using Quercus semecarpifolia Smith aqueous leaf extract. IET Nanobiotechnol. 2019, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Minakov, A.V.; Mikhienkova, E.I.; Voronenkova, Y.O.; Neverov, A.L.; Zeer, G.M.; Zharkov, S.M. Systematic experimental investigation of filtration losses of drilling fluids containing silicon oxide nanoparticles. J. Nat. Gas Sci. Eng. 2019, 71, 102984. [Google Scholar] [CrossRef] [Green Version]
- Al-Anssari, S.; Nwidee, L.; Ali, M.; Sangwai, J.S.; Wang, S.; Barifcani, A.; Iglauer, S. Retention of silica nanoparticles in limestone porous media. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Saboori, R.; Sabbaghi, S.; Kalantariasl, A. Improvement of rheological, filtration and thermal conductivity of bentonite drilling fluid using copper oxide/polyacrylamide nanocomposite. Powder Technol. 2019, 353, 257–266. [Google Scholar] [CrossRef]
- López-López, M.T.; Gómez-Ramírez, A.; Rodríguez-Arco, L.; Durán, J.D.; Iskakova, L.; Zubarev, A. Colloids on the frontier of ferrofluids. Rheological properties. Langmuir 2012, 28, 6232–6245. [Google Scholar] [CrossRef]
- Vryzas, Z.; Zaspalis, V.; Nalbantian, L.; Mahmoud, O.; Nasr-El-Din, H.A.; Kelessidis, V.C. A comprehensive approach for the development of new magnetite nanoparticles giving smart drilling fluids with superior properties for HP/HT applications. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 14–16 November 2016. [Google Scholar]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Perween, S.; Beg, M.; Shankar, R.; Sharma, S.; Ranjan, A. Effect of zinc titanate nanoparticles on rheological and filtration properties of water based drilling fluids. J. Pet. Sci. Eng. 2018, 170, 844–857. [Google Scholar] [CrossRef]
- Medhi, S.; Chowdhury, S.; Kumar, A.; Gupta, D.K.; Aswal, Z.; Sangwai, J.S. Zirconium oxide nanoparticle as an effective additive for non-damaging drilling fluid: A study through rheology and computational fluid dynamics investigation. J. Pet. Sci. Eng. 2020, 187, 106826. [Google Scholar] [CrossRef]
- Ali, J.A.; Kalhury, A.M.; Sabir, A.N.; Ahmed, R.N.; Ali, N.H.; Abdullah, A.D. A state-of-the-art review of the application of nanotechnology in the oil and gas industry with a focus on drilling engineering. J. Pet. Sci. Eng. 2020, 191, 107118. [Google Scholar] [CrossRef]
- Jafari, A.; Shayesteh, S.F.; Salouti, M.; Boustani, K. Effect of annealing temperature on magnetic phase transition in Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2015, 379, 305–312. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe (III) precursor by a sucrose bifunctional hydrothermal method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Bayat, A.E.; Moghanloo, P.J.; Piroozian, A.; Rafati, R. Experimental investigation of rheological and filtration properties of water-based drilling fluids in presence of various nanoparticles. Colloids Surf. A 2018, 555, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Sundar, L.S.; Sharma, K.V.; Naik, M.T.; Singh, M.K. Empirical and theoretical correlations on viscosity of nanofluids: A review. Renew. Sustain. Energy Rev. 2013, 25, 670–686. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2016, 53, 779–791. [Google Scholar] [CrossRef]
- Rafati, R.; Smith, S.R.; Haddad, A.S.; Novara, R.; Hamidi, H. Effect of nanoparticles on the modifications of drilling fluids properties: A review of recent advances. J. Pet. Sci. Eng. 2018, 161, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.M.; Jung, Y.; Lee, J.K.; Phuoc, T.X.; Chyu, M.K. Fluid filtration and rheological properties of nanoparticle additive and intercalated clay hybrid bentonite drilling fluids. J. Pet. Sci. Eng. 2015, 127, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Son, Y.H.; Lee, J.K.; Phuoc, T.X.; Soong, Y.; Chyu, M.K. Rheological behavior of clay–nanoparticle hybrid-added bentonite suspensions: Specific role of hybrid additives on the gelation of clay-based fluids. ACS Appl. Mater. Interfaces 2011, 3, 3515–3522. [Google Scholar] [CrossRef]
- Ikram, R.; Mohamed Jan, B.; Vejpravova, J.; Choudhary, M.I.; Zaman Chowdhury, Z. Recent Advances of Graphene-Derived Nanocomposites in Water-Based Drilling Fluids. Nanomaterials 2020, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.R.; Hakim, A.R.A.; Norddin, M.N.A.M. Potential of Nano-Fluid Application in Deep Well Drilling Operation Challenges. IOP Conf. Ser. Mater. Sci. Eng. 2018, 429, 012058. [Google Scholar] [CrossRef]






| Components | Amount (Concentration) |
|---|---|
| Distilled water (mL) | 259.65 |
| Pre-hydrated bentonite slurry (g) | 25.0 |
| NaOH (g) | 0.15 |
| CMC (g) | 1.60 |
| KCl (g) | 25.0 |
| Barite (g) | 65.41 |
| Fe3O4-NPs (g) | 0.01, 0.1, 0.5 |
| Properties | WBDF | 1% Fe3O4 NPs | 10% Fe3O4 NPs | 50% Fe3O4 NPs |
|---|---|---|---|---|
| PV (mpa.s) | 14 | 18 | 20 | 20.5 |
| AV (mpa.s) | 19 | 21 | 23 | 24 |
| YP (pa) | 15 | 16 | 18 | 19 |
| 10s GS (pa) | 8 | 6.3 | 7.3 | 8.3 |
| 10m GS (pa) | 11 | 9 | 10.5 | 11 |
| 30 mn filtrate (mL) | 9 | 4.8 | 6.2 | 6.8 |
| Filter cake thickness (mm) | 2.31 | 1.81 | 1.94 | 2.16 |
| Filter cake thickness (inch) | 0.0787 | 0.0393 | 0.3937 | 0.7874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudouh, D.; Ikram, R.; Mohamed Jan, B.; Simon Cornelis Metselaar, H.; Hamana, D.; Kenanakis, G. Synthesis, Characterization and Filtration Properties of Ecofriendly Fe3O4 Nanoparticles Derived from Olive Leaves Extract. Materials 2021, 14, 4306. https://doi.org/10.3390/ma14154306
Boudouh D, Ikram R, Mohamed Jan B, Simon Cornelis Metselaar H, Hamana D, Kenanakis G. Synthesis, Characterization and Filtration Properties of Ecofriendly Fe3O4 Nanoparticles Derived from Olive Leaves Extract. Materials. 2021; 14(15):4306. https://doi.org/10.3390/ma14154306
Chicago/Turabian StyleBoudouh, Djahida, Rabia Ikram, Badrul Mohamed Jan, Hendrik Simon Cornelis Metselaar, Djamel Hamana, and George Kenanakis. 2021. "Synthesis, Characterization and Filtration Properties of Ecofriendly Fe3O4 Nanoparticles Derived from Olive Leaves Extract" Materials 14, no. 15: 4306. https://doi.org/10.3390/ma14154306






