Microstructure, Mechanical Properties, and Corrosion Behavior of Boron Carbide Reinforced Aluminum Alloy (Al-Fe-Si-Zn-Cu) Matrix Composites Produced via Powder Metallurgy Route
Abstract
:1. Introduction
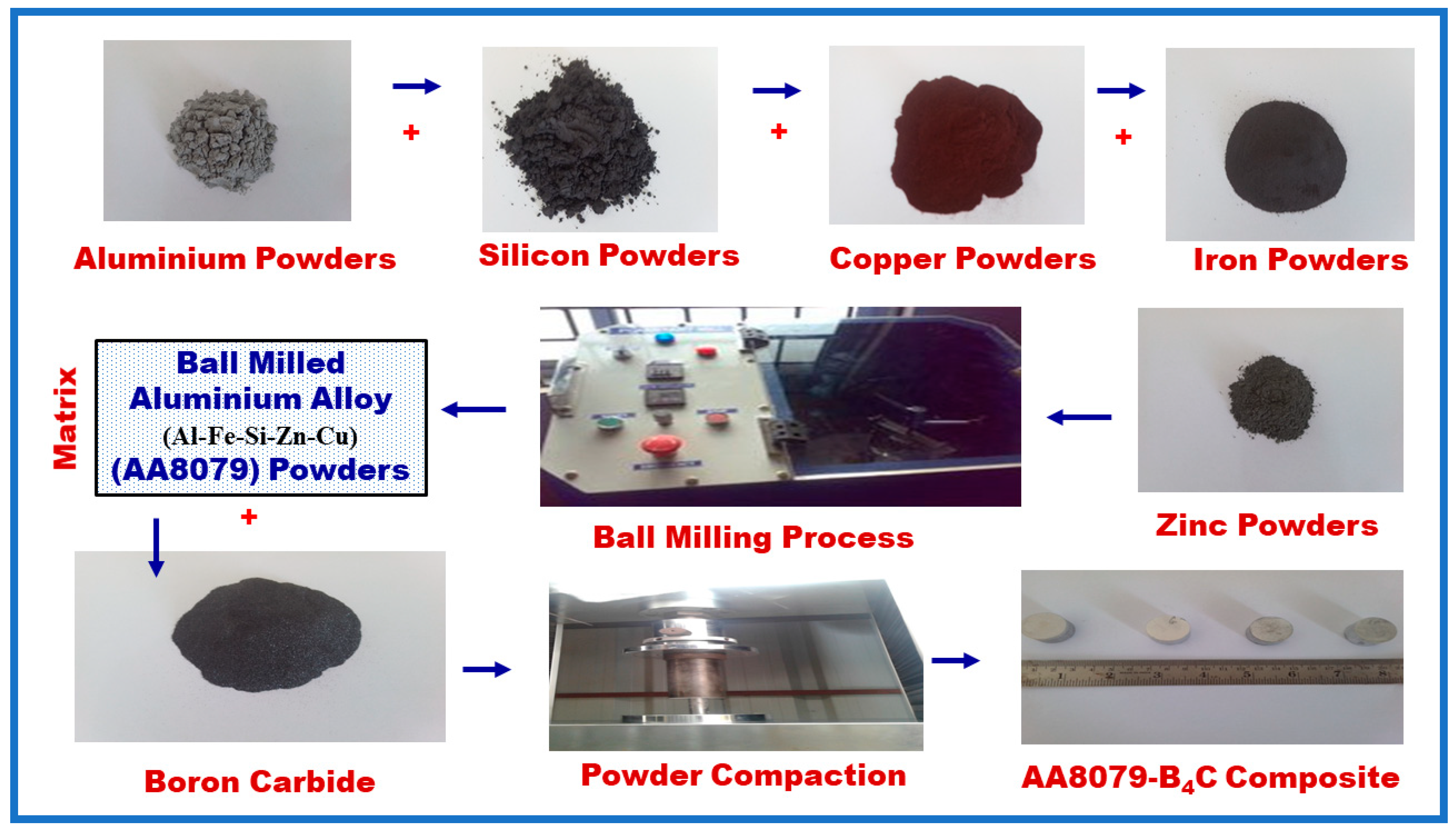
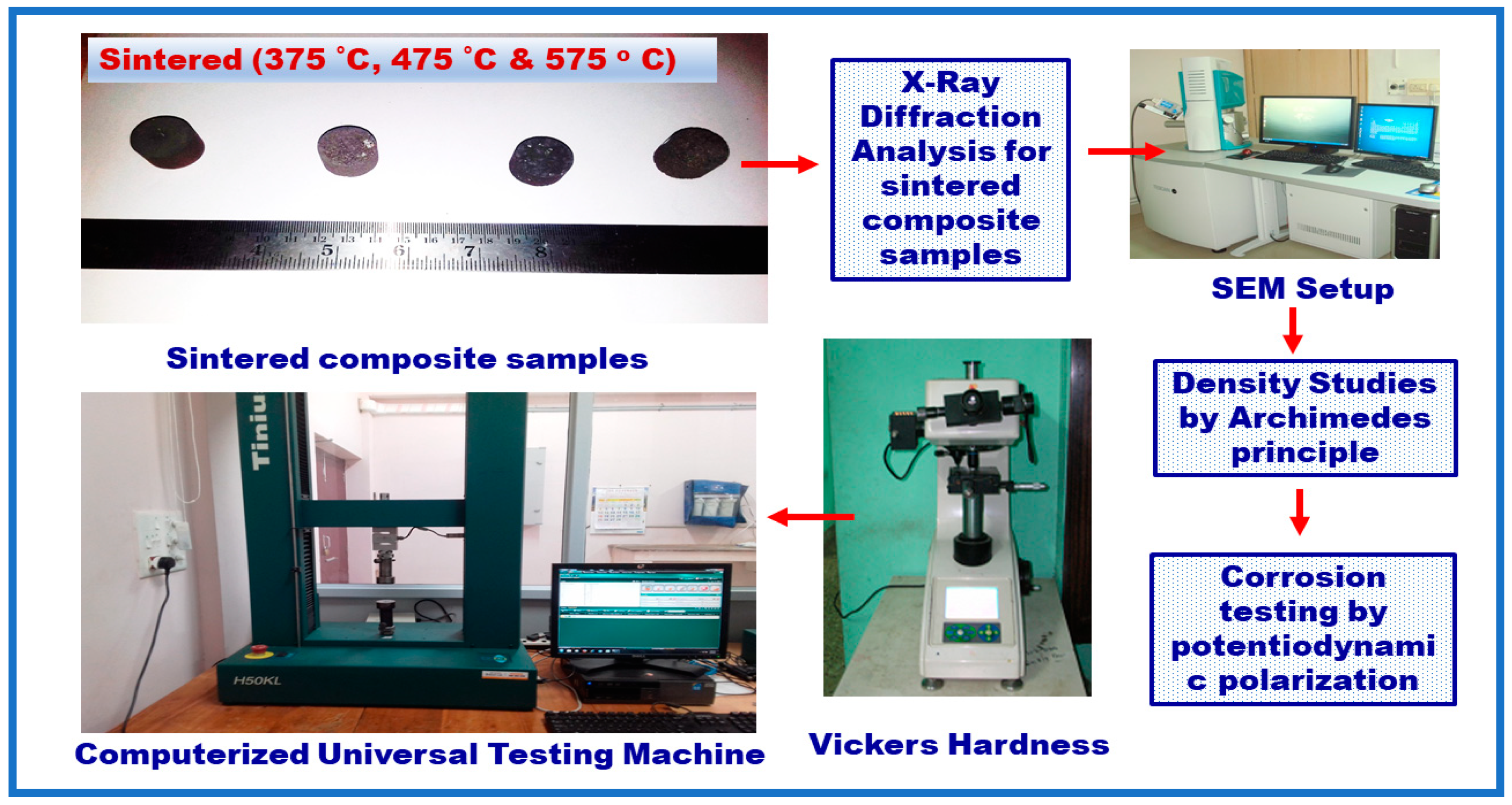
2. Experimental Details
3. Results and Discussions
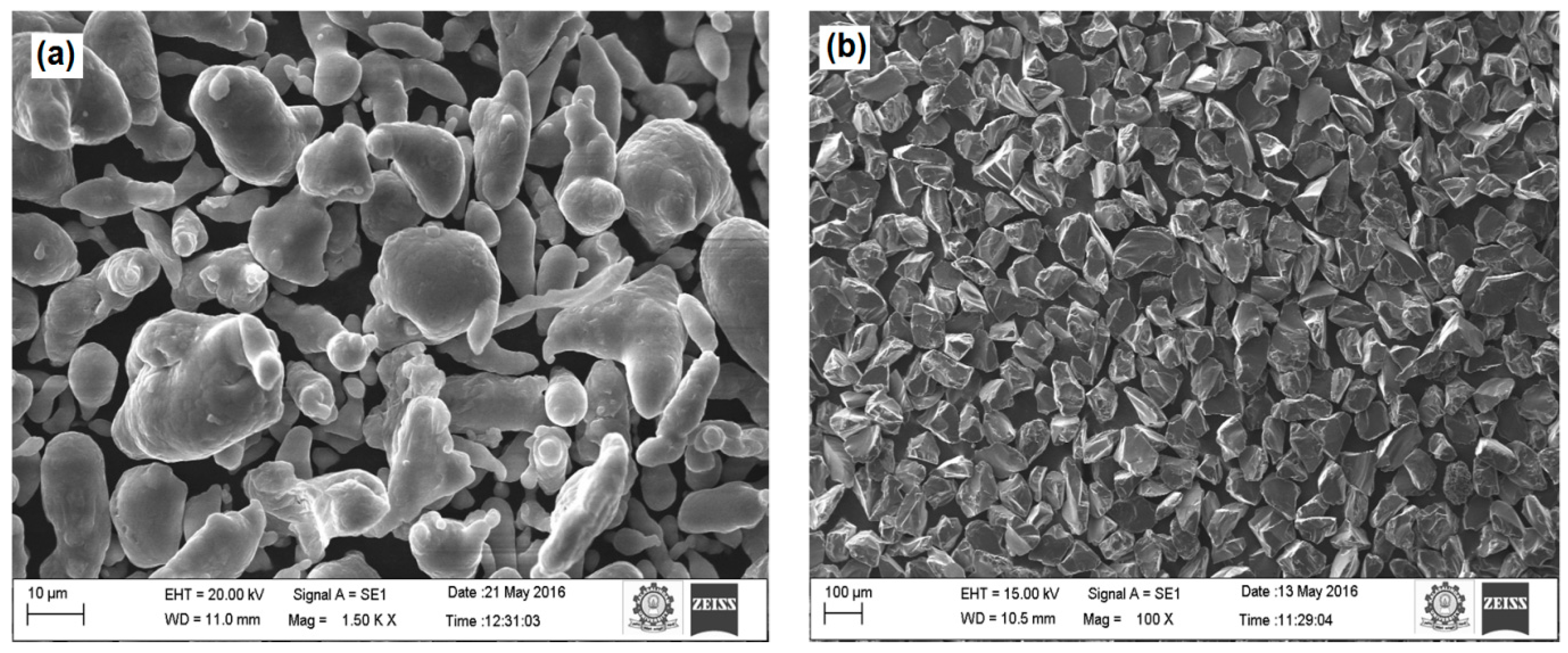
3.1. Characterization Studies on Sintered Preforms
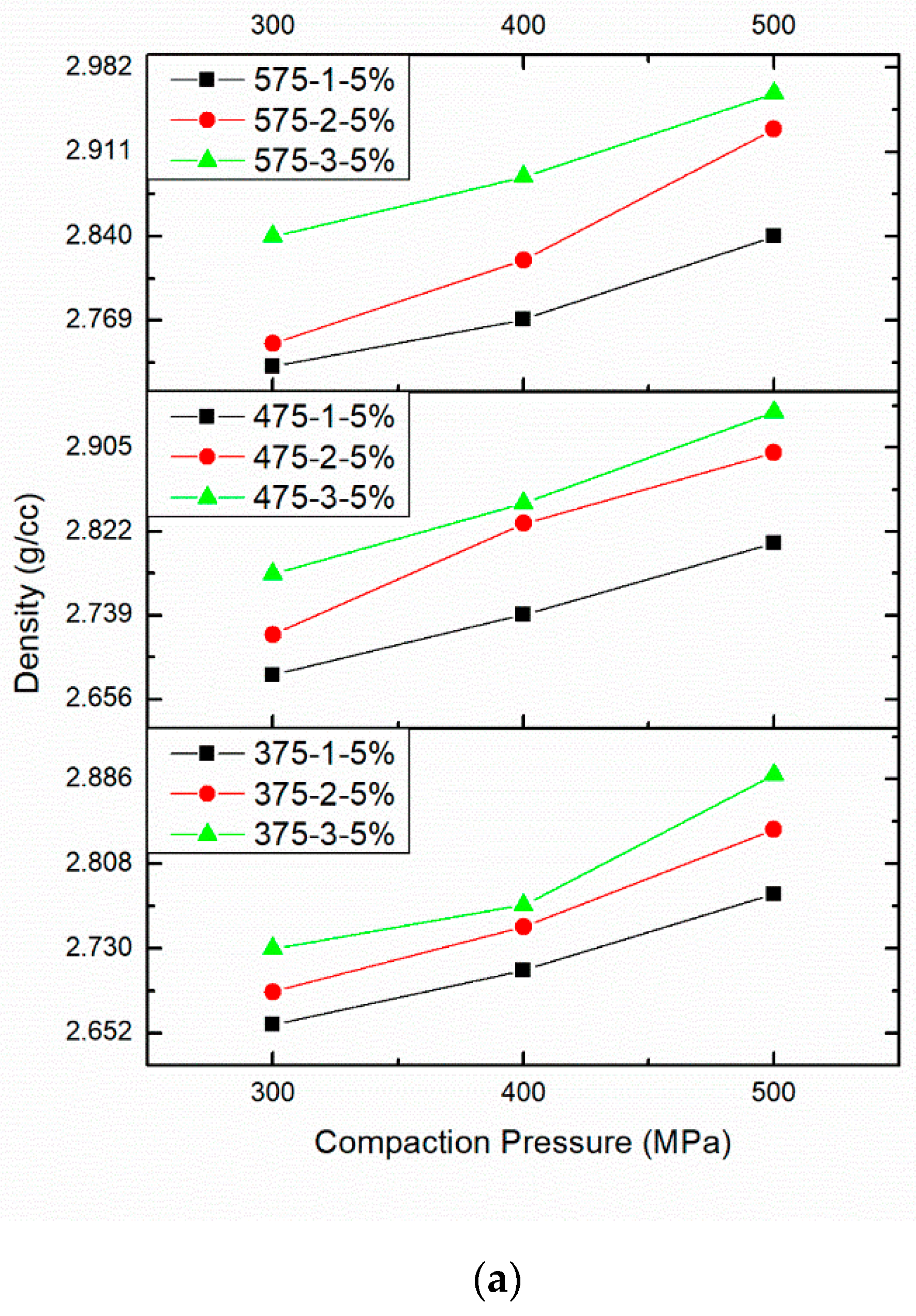
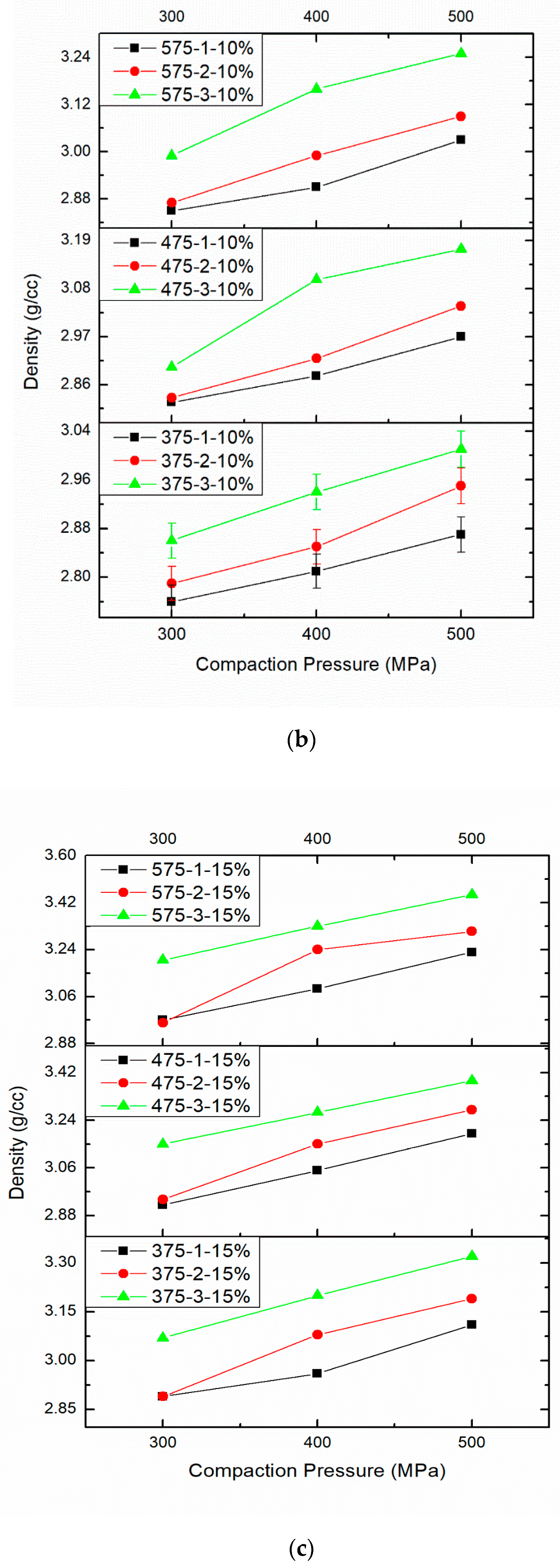
3.2. Effect of PM Parameters on Density
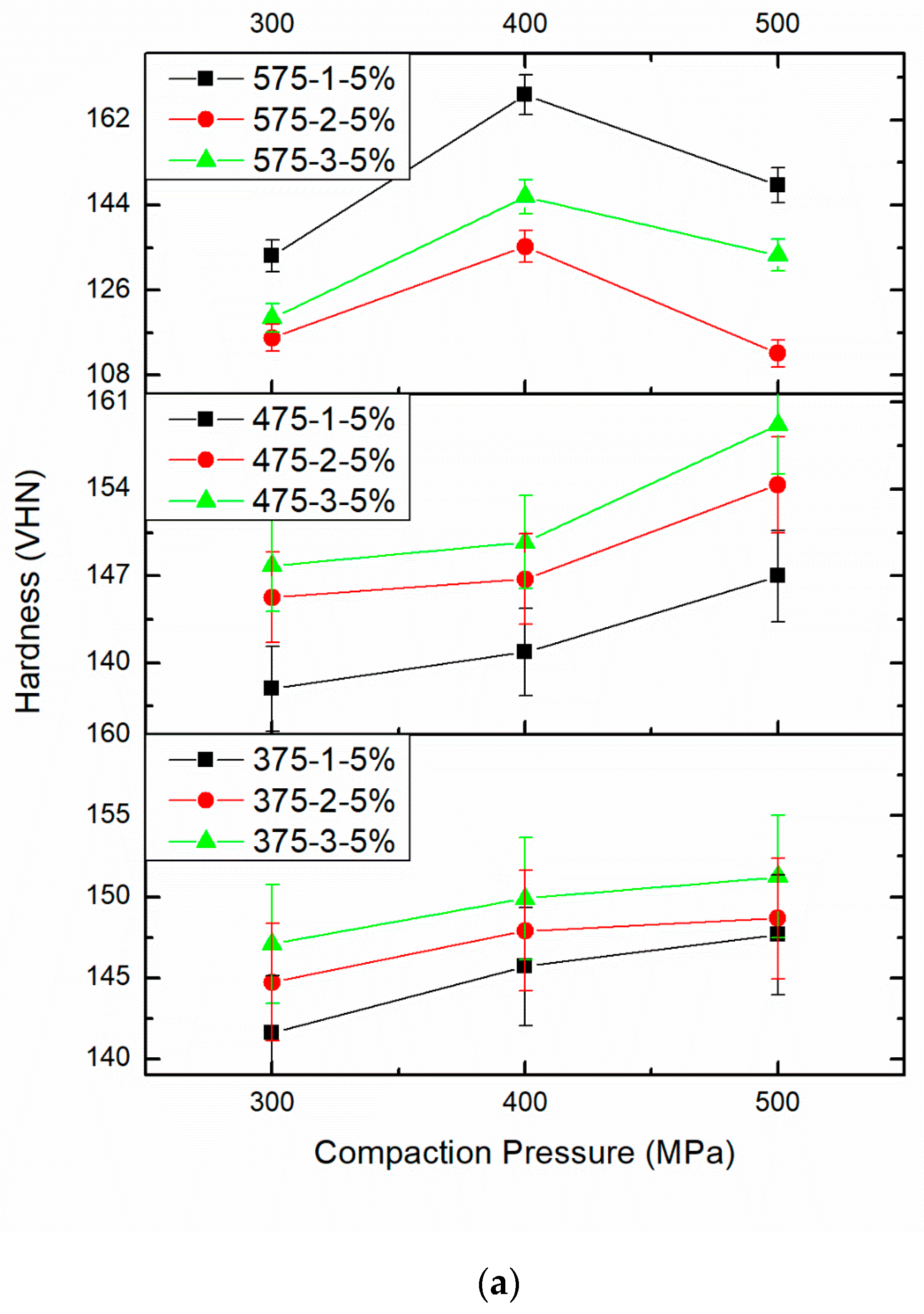
3.3. Effect of PM Parameters on Micro Vickers Hardness
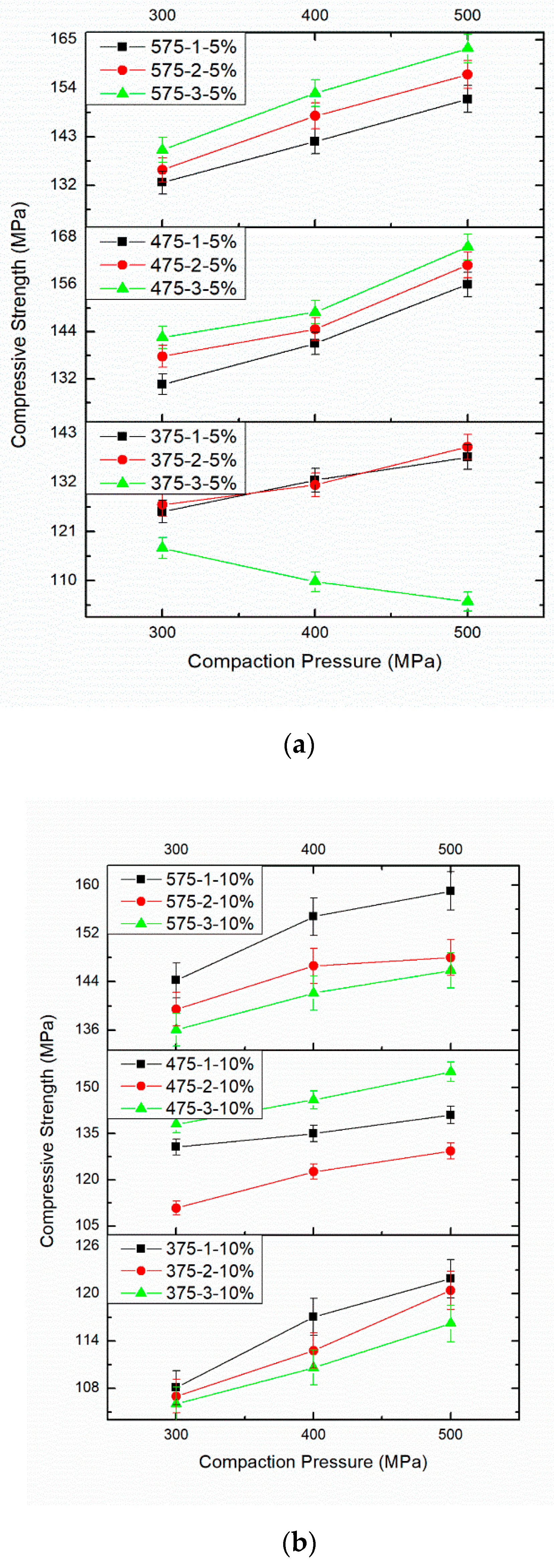
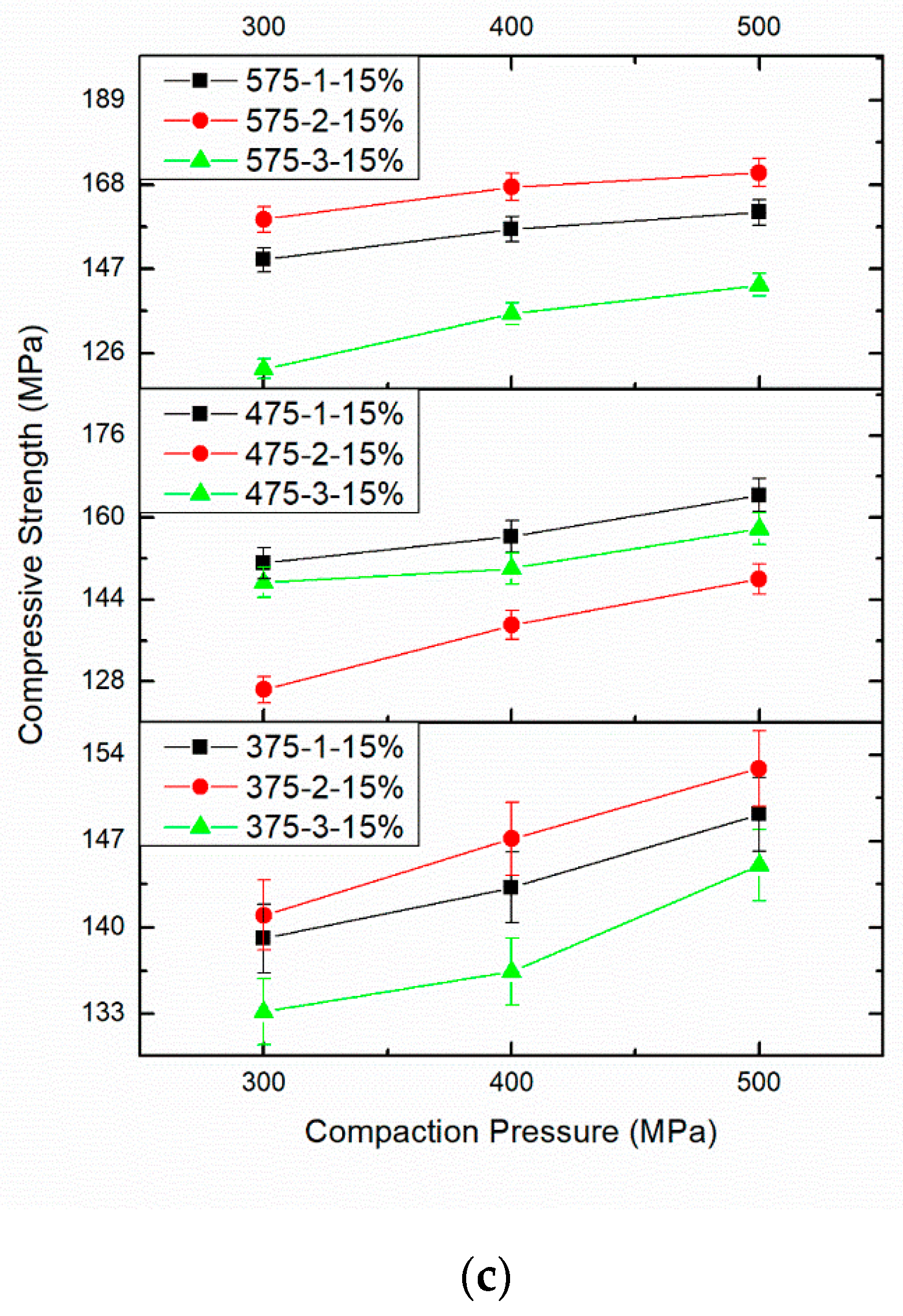
3.4. Effect of PM Parameters on Compressive Strength
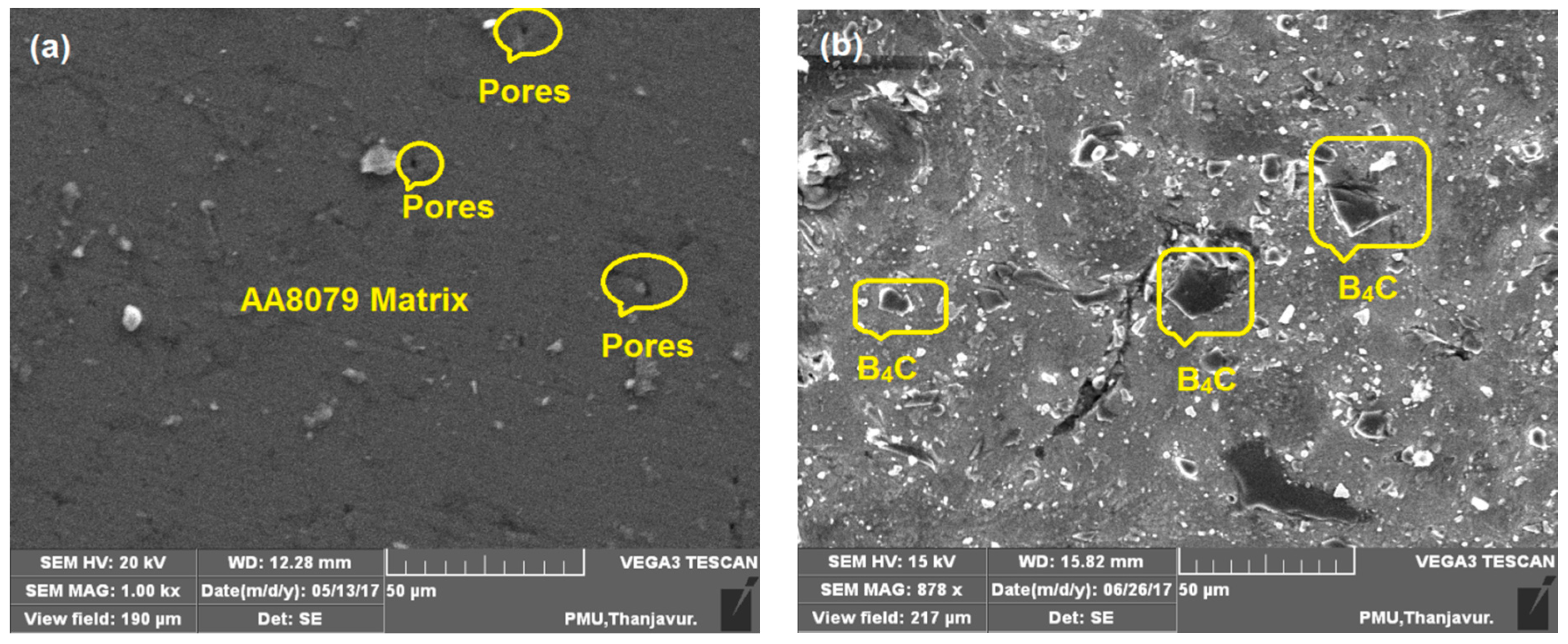
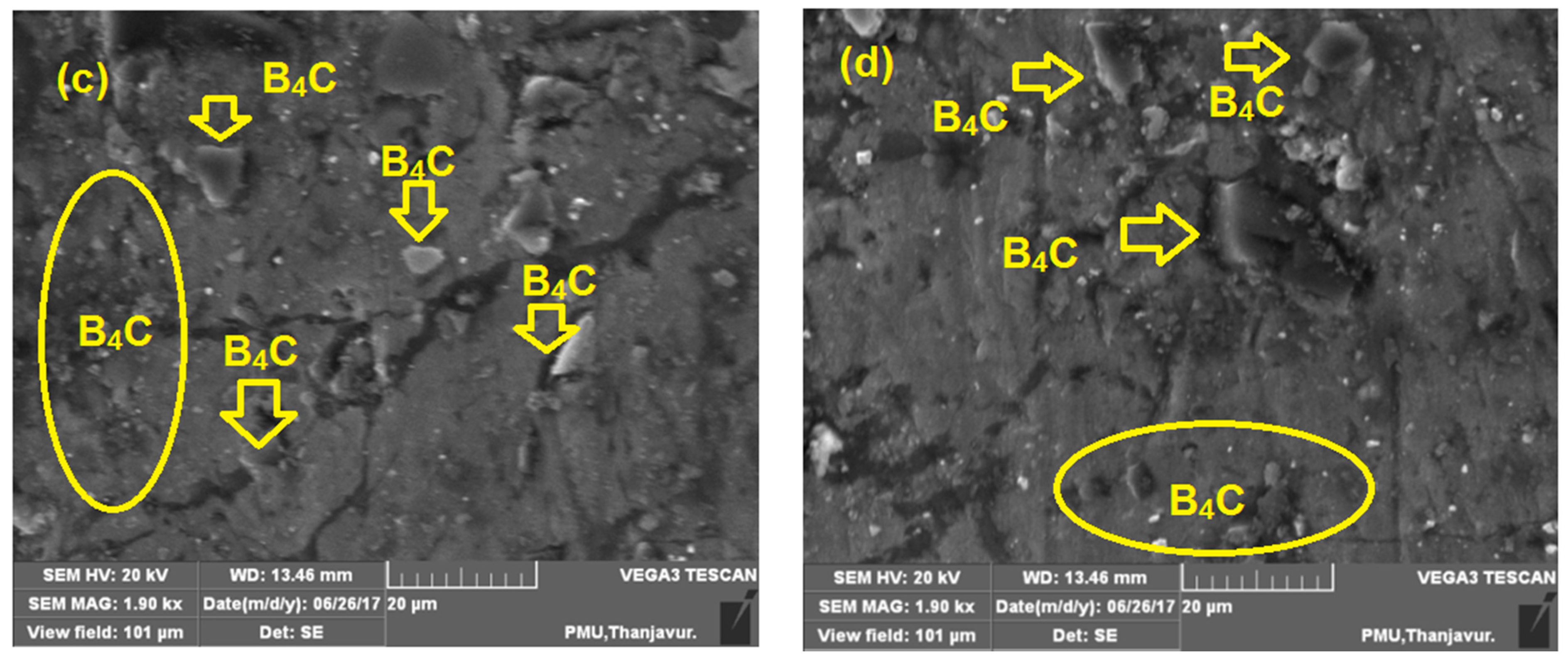
3.5. Microstructure Analysis of Specimens after Compression Test
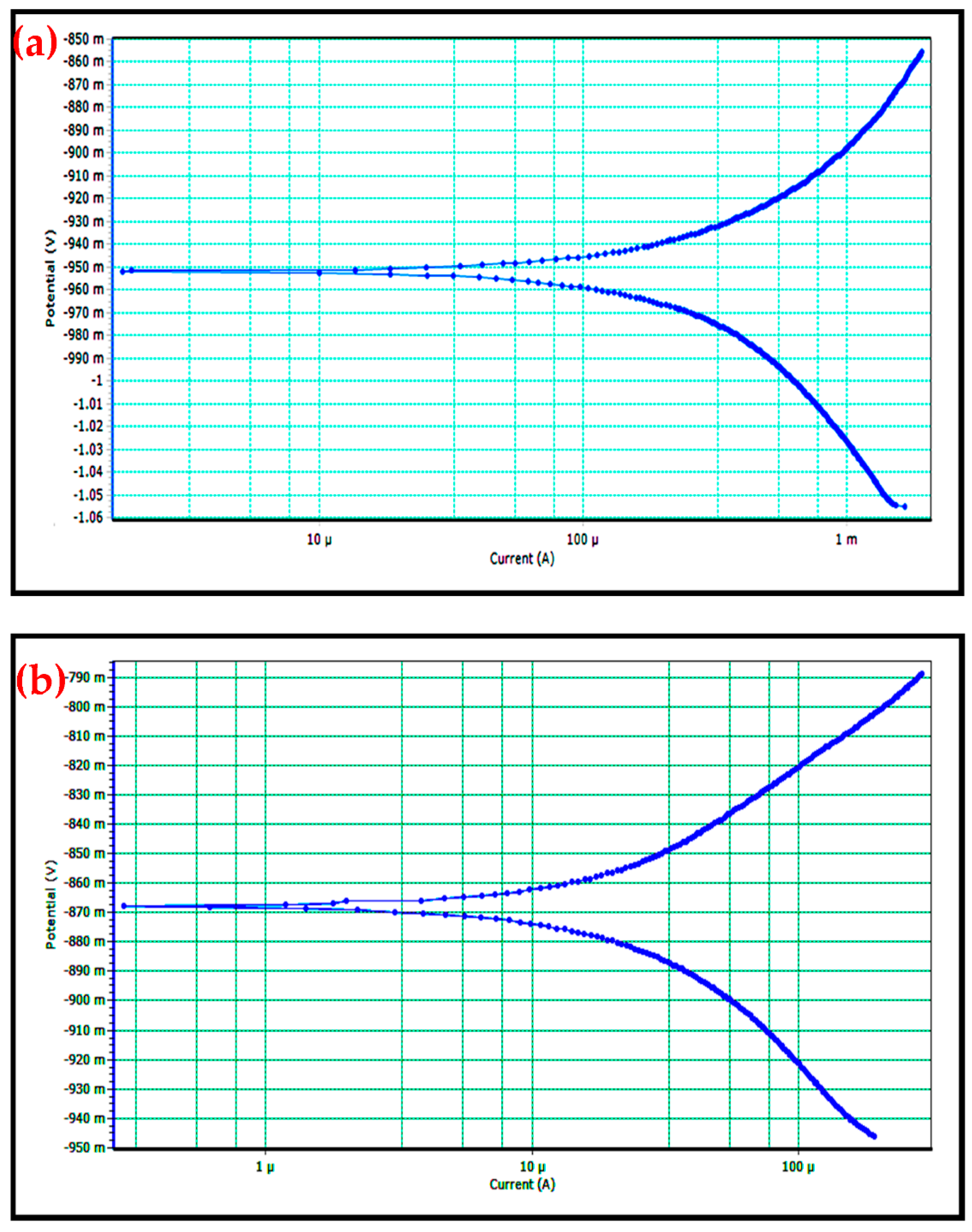
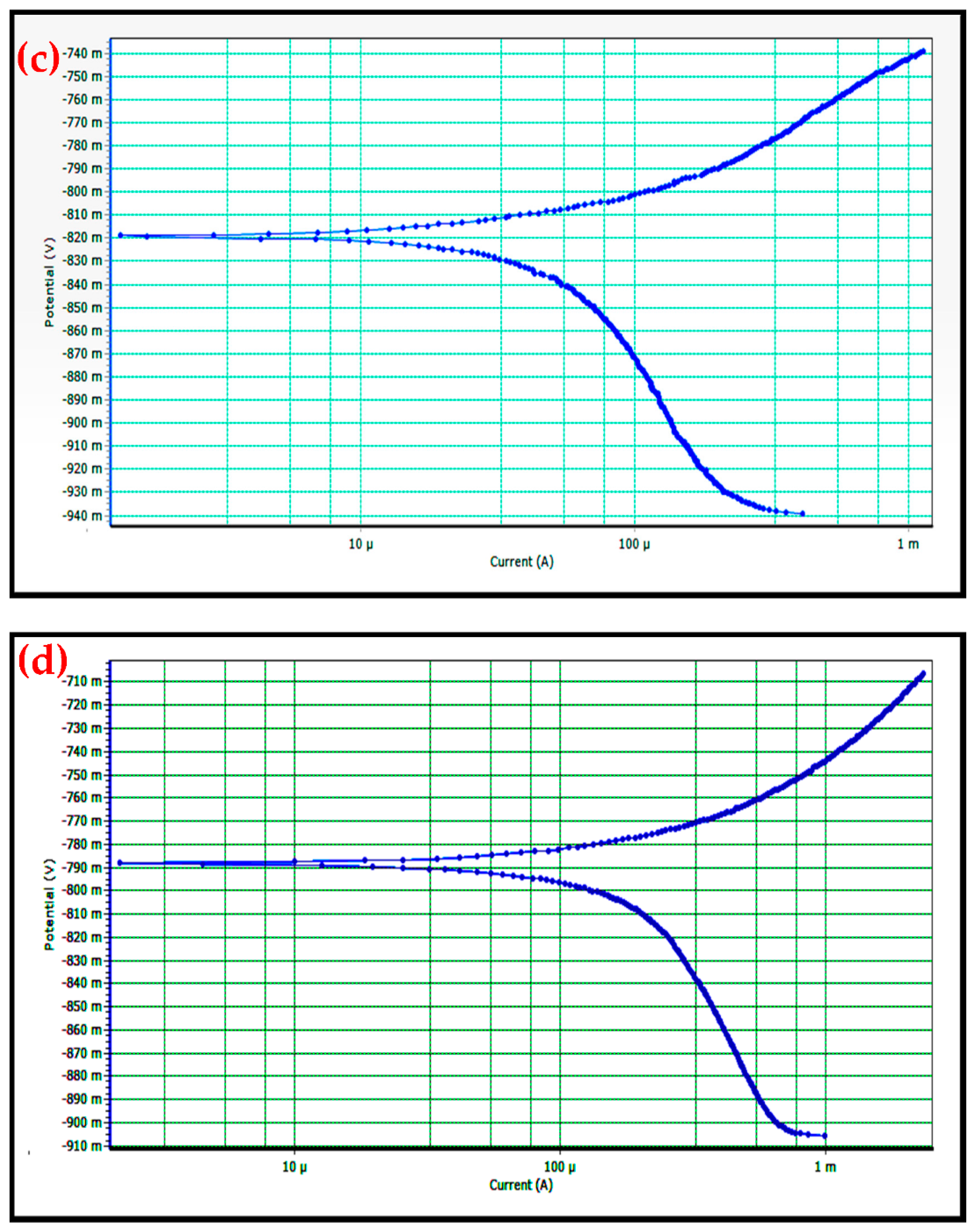
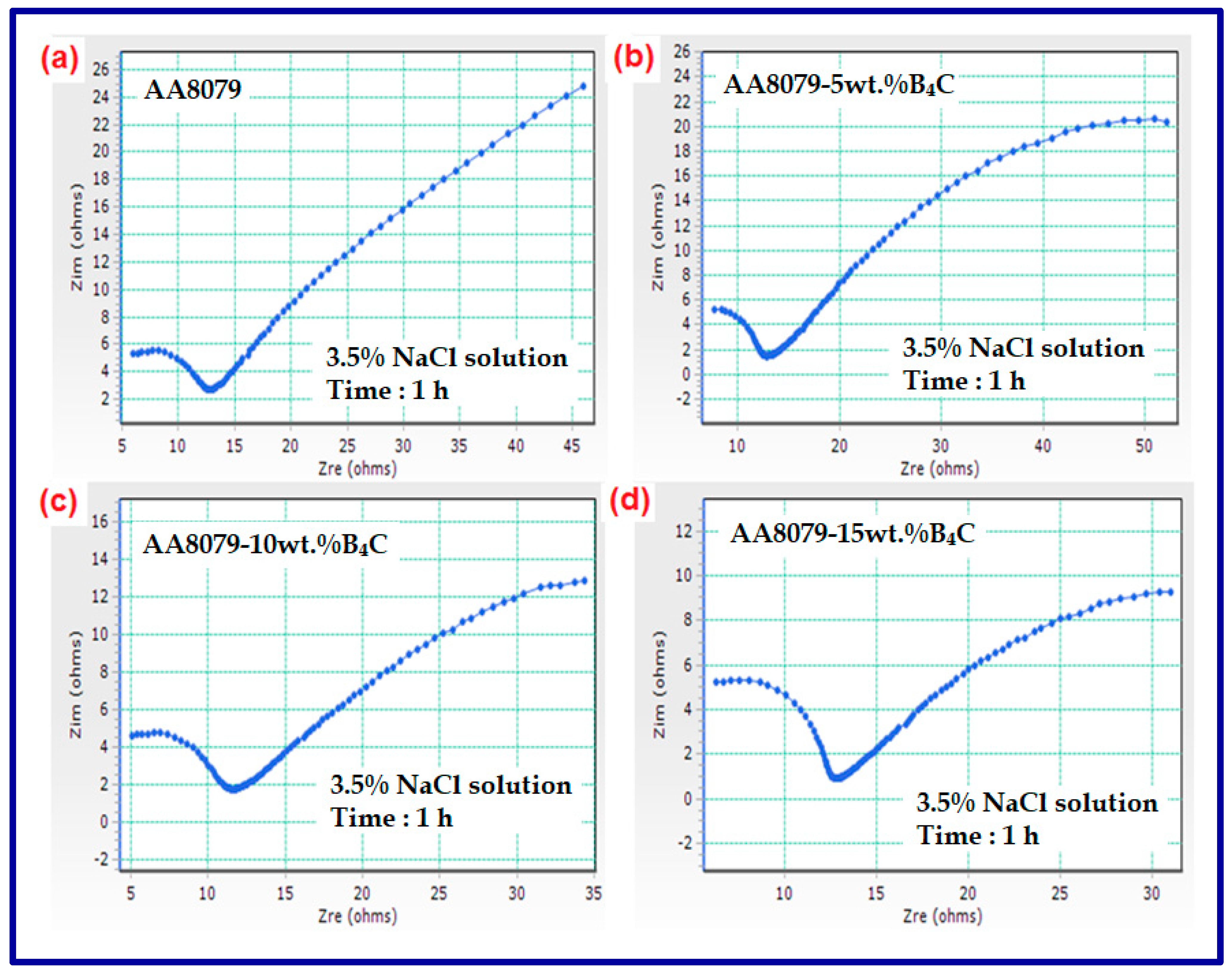
3.6. Corrosion Behavior
4. Conclusions
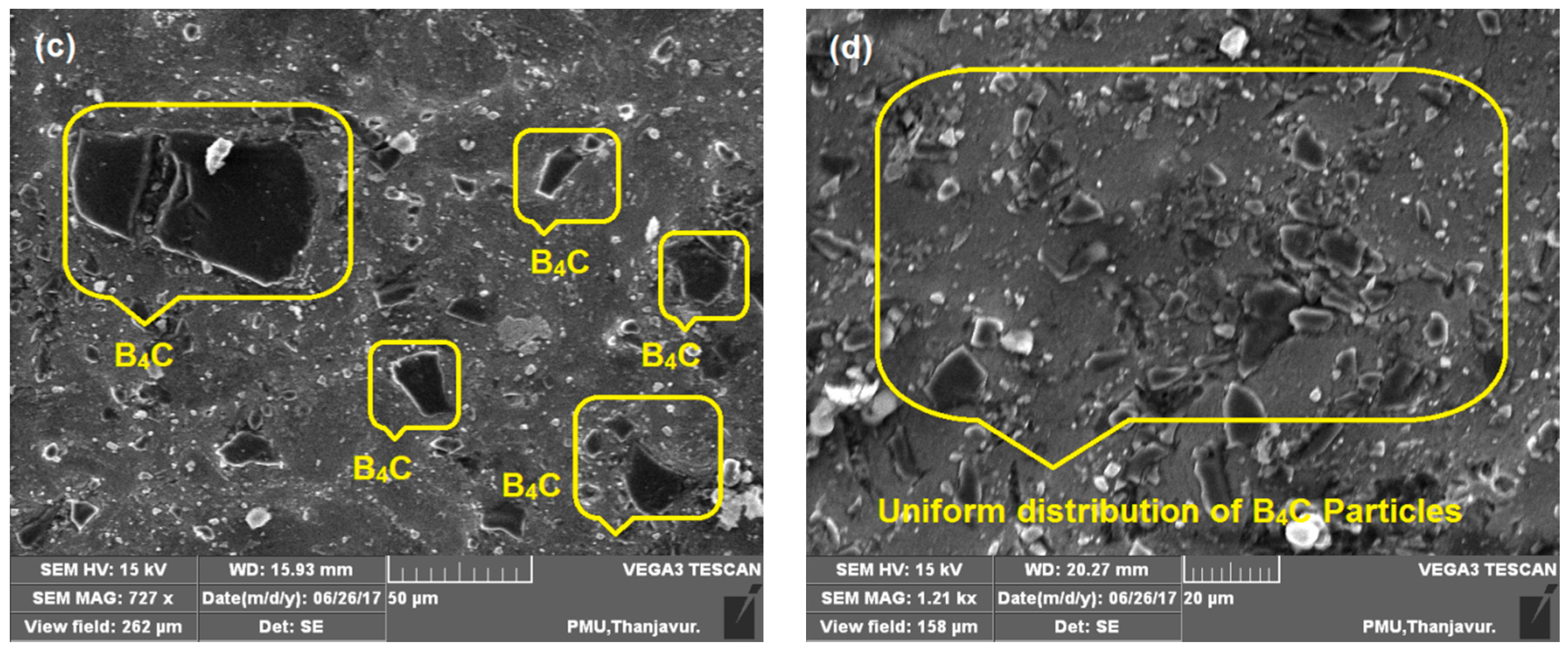
- From the SEM examination, fine dispersal and occurrence of B4C particles with the AA8079 matrix has been observed;
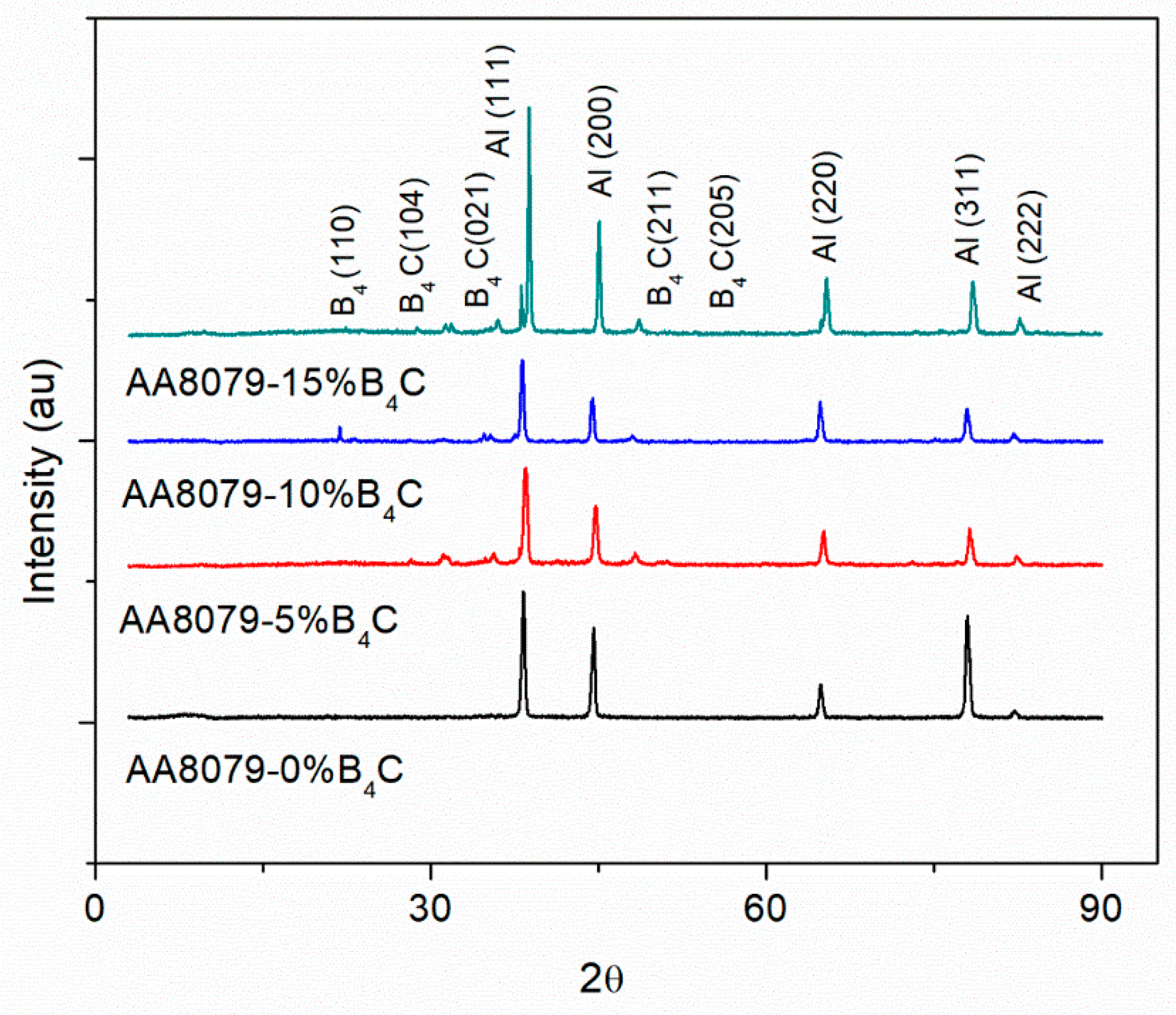
- XRD analysis shows the presence of B4C particles with minor peaks;
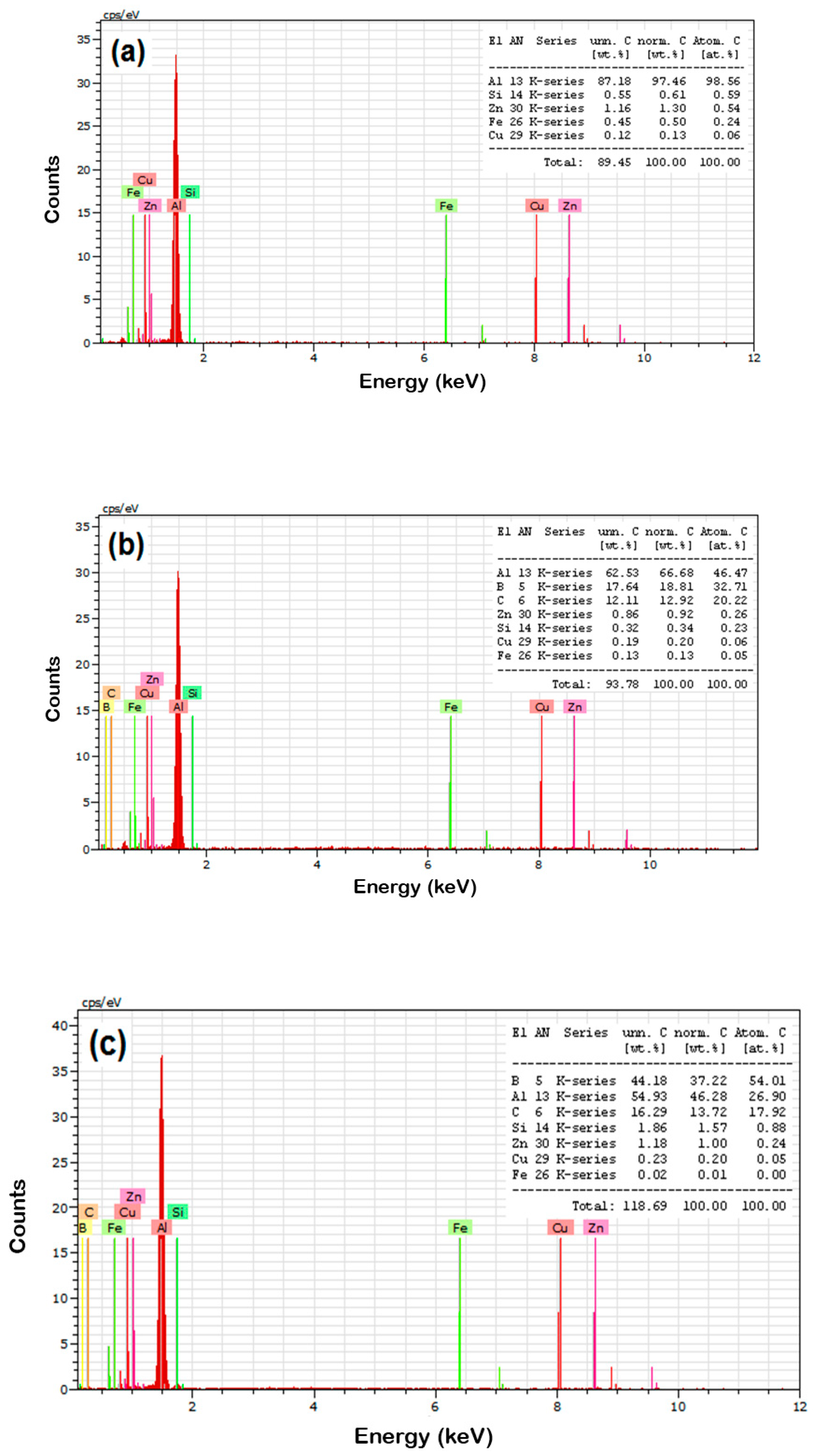
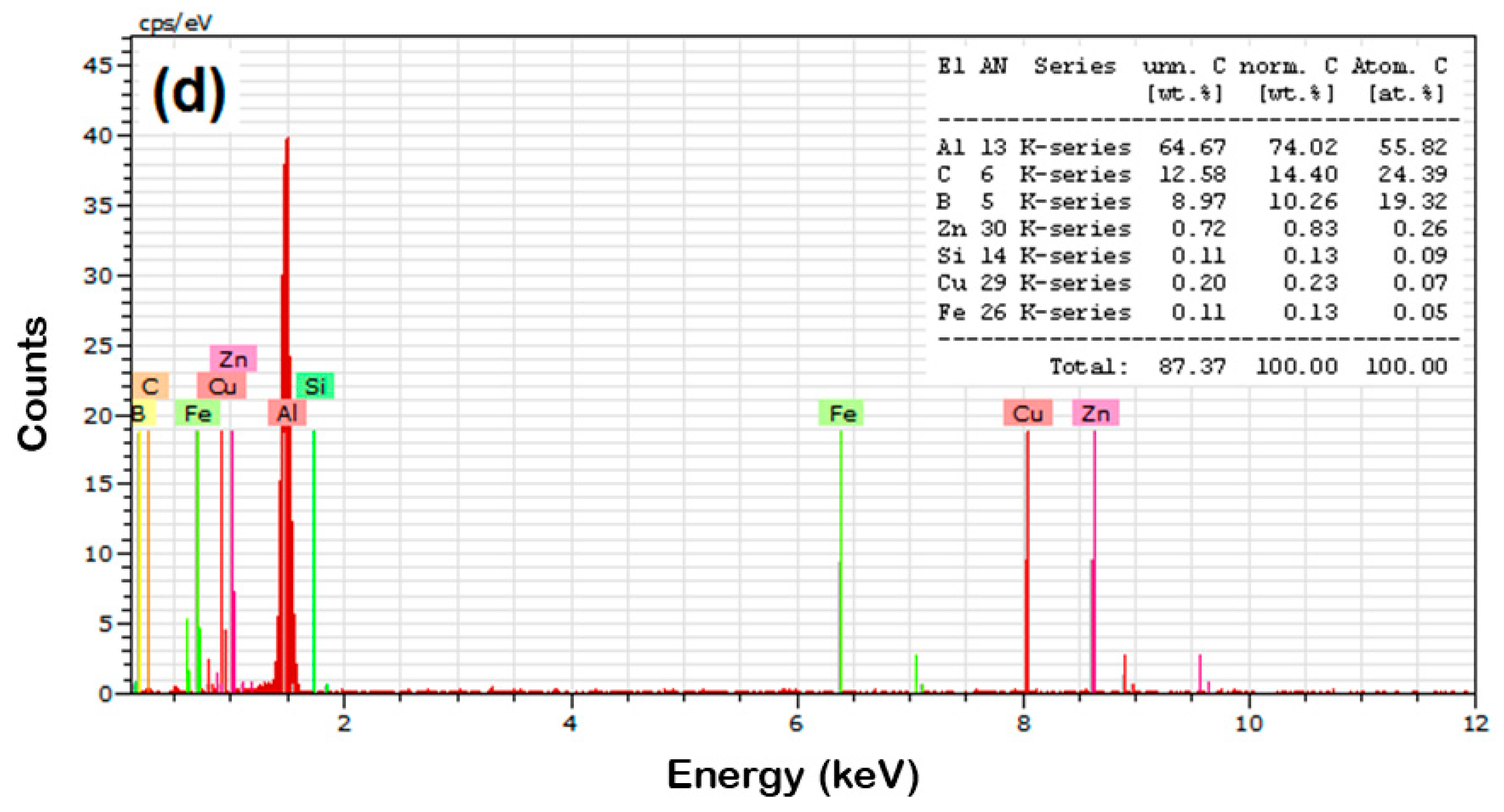
- The EDAX analysis of the sintered samples witnessed the existence of B4C particles with AA8079 matrix and the respective elemental powders of the AA8079 matrix;
- The density, hardness, and compressive strength of the composite was increased while increasing the reinforcement weight percentage from 5 to 15 wt.% with respect to an increase in PM process parameters, compaction pressure, sintering temperature, and time;
- The SEM micrographs, after compression testing, exposed the homogenous dispersal of B4C reinforcement with AA8079 matrix without pores and grain boundaries;
- The AA8079-B4C composites corrosion resistance rose with a rise in weight percentage of B4C reinforcement with AA8079 matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guleryuz, L.F.; Ozan, S.; Uzunsoy, D.; Ipek, R. An investigation of the microstructure and mechanical properties of B4C reinforced PM magnesium matrix composites. Powder Metall. Met. Ceram. 2012, 51, 456–462. [Google Scholar] [CrossRef]
- Pokorska, I. Deformation of powder metallurgy materials in cold and hot forming. J. Mater. Process. Technol. 2008, 196, 15–32. [Google Scholar] [CrossRef]
- Anand, S.; Mohan, B. Effect of particle size, compaction pressure on density and mechanical properties of elemental 6061Al alloy through powder metallurgical process. Int. J. Mater. Eng. Innov. 2012, 3, 259–268. [Google Scholar] [CrossRef]
- Ashwath, P.; Anthony, X.M. Processing methods and property evaluation of Al2O3 and SiC reinforced metal matrix composites based on aluminium 2xxx alloys. J. Mater. Res. 2012, 32, 1201–1219. [Google Scholar] [CrossRef]
- Prabhu, B.; Suryanarayana, C.; An, L.; Vaidyanathan, R. Synthesis and characterization of high volume fraction Al–Al2O3 nanocomposite powders by high-energy milling. Mater. Sci. Eng. A 2009, 425, 192–200. [Google Scholar] [CrossRef]
- Min, K.H.; Kang, S.P.; Kim, D.G.; Do Kim, Y. Sintering characteristic of Al2O3-reinforced 2xxx series Al composite powders. J. Alloys Compd. 2005, 400, 150–153. [Google Scholar] [CrossRef]
- Alizadeh, A.; Taheri-Nassaj, E.; Hajizamani, M. Hot extrusion process effect on mechanical behavior of stir cast Al based composites reinforced with mechanically milled B4C nanoparticles. J. Mater. Sci. Technol. 2011, 27, 1113–1119. [Google Scholar] [CrossRef]
- Abdullah, Y.; Kamarudin, N. Al/B4C composites with 5 and 10 wt% reinforcement content prepared by powder metallurgy. J. Nucl. Relat. Technol. 2012, 9, 42–47. [Google Scholar]
- Nie, C.Z.; Gu, J.J.; Liu, J.L.; Zhang, D. Production of boron carbide reinforced 2024 aluminum matrix composites by mechanical alloying. Mater. Trans. 2007, 48, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Alihosseini, H.; Dehghani, K. Microstructure characterization, mechanical properties, compressibility and sintering behavior of Al-B4Cnanocomposite powders. Adv. Powder Technol. 2017, 28, 2126–2134. [Google Scholar] [CrossRef]
- Jeyasimman, D.; Sivaprasad, K.; Sivasankaran, S.; Ponalagusamy, R.; Narayanasamy, R. Microstructural observation, consolidation and mechanical behavior of AA 6061 nanocomposites reinforced by γ-Al2O3 nanoparticles. Adv. Powder Technol. 2014, 57, 394–404. [Google Scholar]
- Bodukuri, A.K.; Eswaraiah, K.; Rajendar, K.; Sampath, V. Fabrication of Al-SiC-B4C metal matrix composite by powder metallurgy technique and evaluating mechanical properties. Perspect. Sci. 2016, 8, 8428–8431. [Google Scholar] [CrossRef] [Green Version]
- Sivasankaran, S.; Sivaprasad, K.; Narayanasamy, R.; Satyanarayana, P.V. X-ray peak broadening analysis of AA 6061100−x−x wt.% Al2O3 nano composite prepared by mechanical alloying. Mater. Charact. 2011, 62, 661–672. [Google Scholar] [CrossRef]
- Ravichandran, M.; Anandakrishnan, V. Hot upset studies on sintered (Al–TiO2–Gr) powder metallurgy hybrid composite. Strength Mater. 2016, 48, 450–459. [Google Scholar] [CrossRef]
- Almomani, M.A.; Tayfour, W.R. Effect of silicon carbide addition on the corrosion behavior of powder metallurgy cu-30zn brass in a 3.5 wt.% NaCl solution. J. Alloys Compd. 2016, 679, 101–114. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Zamani, N.A.B.N.; Iqbal, A.A.; Nuruzzaman, D.M. Tribo-mechanical characterisation of self-lubricating aluminium based hybrid metal matrix composite fabricated via powder metallurgy. Materialia 2020, 14, 100936. [Google Scholar] [CrossRef]
- Akcamli, N.; Senyurt, B. B4C particulate-reinforced Al-8.5wt% Si-3.5 wt%C matrix composites: Powder metallurgical fabrication, age hardening, and characterization. Ceram. Int. 2021, 47, 6813–6826. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, V. Development of rare-earth oxides based hybrid AMCs reinforced with SiC/Al2O3: Mechanical & metallurgical characterization. J. Mater. Res. Technol. 2019, 8, 1971–1981. [Google Scholar]
- Shaikh, M.B.N.; Arif, S.; Aziz, T.; Waseem, A.; Shaikh, M.A.N.; Ali, M. Microstructural, mechanical and tribological behaviour of powder metallurgy processed SiC and RHA reinforced Al-based composites. Surf. Interface 2019, 15, 166–179. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Ostovan, F.; Jamaludin, K.R.; Amrin, A.; Matori, K.; Shafiei, E. Process microstructure properties relationship in Al−CNTs−Al2O3 nanocomposites manufactured by hybrid powder metallurgy and microwave sintering process. Trans. Nonferrous Met. Soc. China 2020, 30, 2339–2354. [Google Scholar] [CrossRef]
- Erdemir, F.C. Microstructural characterization and mechanical properties of functionally graded Al2024/SiC composites prepared by powder metallurgy techniques. Trans. Nonferrous Met. Soc. China 2015, 25, 3569–3577. [Google Scholar] [CrossRef]
- Karakoc, H.; Ovali, I.; Dundar, S.; Citak, R. Wear and mechanical properties of Al6061/SiC/B4C hybrid composites produced with powder metallurgy. J. Mater. Res. Technol. 2019, 8, 5348–5361. [Google Scholar]
- Fathy, A.; Omyma, E.K.; Mohammed, M.M. Effect of iron addition on microstructure, mechanical and magnetic properties of Al-matrix composite produced by powder metallurgy route. Trans. Nonferrous Met. Soc. China 2015, 25, 46–53. [Google Scholar] [CrossRef]
- Iacob, G.; Ghica, V.G.; Buzatu, M.; Buzatu, T.; Petrescu, M. Studies on wear rate and micro-hardness of the Al/Al2O3/Gr hybrid composites produced via powder metallurgy. Compos. Part B 2015, 69, 603–611. [Google Scholar] [CrossRef]
- Stalin, B.; Sudha, G.T.; Kailasanathan, C.; Ravichandran, M. Effect of MoO3 ceramic oxide reinforcement particulates on the microstructure and corrosion behaviour of Al alloy composites processed by P/M route. Mater. Today Commun. 2020, 25, 101655. [Google Scholar] [CrossRef]
- Patel, J.; Morsi, K. Effect of mechanical alloying on the microstructure and properties of Al–Sn–Mg alloy. J. Alloys Compd. 2012, 540, 100–106. [Google Scholar] [CrossRef]
- Jailani, S.; Rajadurai, A.; Mohan, B.; Kumar, S.A.; Sornakumar, T. Development and properties of aluminium silicon alloy fly ash composites. Powder Metall. 2011, 54, 474–479. [Google Scholar] [CrossRef]
- Jeyasimman, D.; Sivasankaran, S.; Sivaprasad, K.; Narayanasamy, R.; Kambali, R.S. An investigation of the synthesis, consolidation and mechanical behaviour of Al 6061 nanocomposites reinforced by TiC via mechanical alloying. Mater. Des. 2014, 57, 394–404. [Google Scholar] [CrossRef]
- Saravanan, S.; Senthilkumar, P.; Ravichandran, M.; Anandakrishnan, V. Mechanical, electrical, and corrosion behavior of AA6063/TiC composites synthesized via stir casting route. J. Mater. Res. 2017, 32, 606–614. [Google Scholar] [CrossRef]
- Murthy, H.A.; Singh, S.K. Influence of TiC particulate reinforcement on the corrosion behaviour of Al 6061 metal matrix composites. Adv. Mater. Lett. 2015, 6, 633–640. [Google Scholar] [CrossRef]
- Mohanty, R.M.; Balasubramaniam, K.; Seshadri, S.K. Boron-Carbide reinforced aluminium 1100 matrix composites: Fabrications and properties. Mater. Sci. Eng. A 2008, 498, 42–52. [Google Scholar] [CrossRef]
- Ravichandran, M.; Manikandan, A. Investigations on properties of Al-B4C composites synthesized through powder metallurgy rout. Appl. Mech. Mater. 2016, 852, 93. [Google Scholar] [CrossRef]
- Celik, Y.H.; Secilmis, K. Investigation of wear behaviors of Al matrix composites reinforced with different B4C rate produced by powder metallurgy method. Adv. Powder Technol. 2017, 28, 2218–2224. [Google Scholar] [CrossRef]
- Ghasali, E.; Alizadeh, M.; Ebadzadeh, T.; Pakseresht, A.H.; Rahbari, A. Investigation on microstructural and mechanical properties of B4C–aluminum matrix composites prepared by microwave sintering. J. Mater. Res. Technol. 2015, 4, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, M.; Naveen Sait, A.; Anandakrishnan, V. Workability studies on Al + 2.5%TiO2 + Gr powder metallurgy composites during cold upsetting. Mater. Res. 2014, 29, 1490–1496. [Google Scholar] [CrossRef]
- Kumar, C.A.V.; Rajadurai, J.S. Influence of rutile (TiO2) content on wear and microhardness characteristics of aluminium-based hybrid composites synthesized by powder metallurgy. Trans. Nonferrous Met. Soc. China 2016, 26, 63–73. [Google Scholar] [CrossRef]
- Baradeswaran, A.E.P.A.; Perumal, A.E. Influence of B4C on the tribological and mechanical properties of Al 7075–B4C composites. Compos. Part B 2013, 54, 146–152. [Google Scholar] [CrossRef]
- Prakash, S.K.; Gopal, P.M.; Anburose, D.; Kavimani, V. Mechanical, corrosion and wear characteristics of powder metallurgy. Ain Shams Eng. J. 2016, 7, 2–8. [Google Scholar]
- Harichandran, R.; Selvakumar, N. Effect of nano/micro B4C particles on the mechanical properties of aluminium metal matrix composites fabricated by ultrasonic cavitation-assisted solidification process. Arch. Civ. Mech. Eng. 2015, 16, 147–158. [Google Scholar] [CrossRef]
- Karabulut, S.; Karakoc, H.; Citak, R. Influence of B4C particle reinforcement on mechanical and machining properties of Al6061/B4C composites. Compos. Part B 2016, 101, 87–98. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, C.D.; Luo, G.; Fang, P.; Li, C.; Wang, Y.; Zhang, L. Microstructure and mechanical properties of Al-7075/B4C composites fabricated by plasma activated sintering. J. Alloys Compd. 2014, 588, 265–270. [Google Scholar] [CrossRef]
- Balamurugan, P.; Uthayakumar, M. Influence of process parameters on Cu-Fly ash composite by powder metallurgy technique. Mater. Manuf. Process. 2015, 30, 313–319. [Google Scholar] [CrossRef]
- Dayanand, S.; Boppana, S.B.; Hemanth, J.; Telagu, A. Microstructure and corrosion characteristics of in situ aluminum diboride metal matrix composites. J. Bio Tribo-Corros. 2019, 60, 1–10. [Google Scholar] [CrossRef]
- Ravikumar, M.; Reddappa, H.N.; Suresh, R.; Sreenivasa, R.M.; Babu, E.R.; Reddy, N.C.; Ravikumar, C.R.; Ananda Murth, H.C. Evaluation of corrosion properties of Al2O3 and SiC reinforced aluminium metal matrix composites using taguchi’s techniques. J. Sci. Res. 2021, 65, 253–259. [Google Scholar]
- Li, C.; Qiu, R.; Luan, B.; Li, Z. Effect of carbon nanotubes and high temperature extrusion on the microstructure evolution of Al– Cu alloy. Mater. Sci. Eng. 2017, 704, 38–44. [Google Scholar] [CrossRef]
- El-Kady, O.; Fathy, A. Effect of SiC particle size on the physical and mechanical properties of extruded Al matrix nanocomposites. Mater. Des. 2014, 54, 348–353. [Google Scholar] [CrossRef]
- Sharath, B.N.; Venkatesh, C.V.; Afzal, A. Multi Ceramic Particles Inclusion in the Aluminium Matrix and Wear Characteri-zation through Experimental and Response Surface-Artificial Neural Networks. Materials 2021, 14, 2895. [Google Scholar] [CrossRef]
- Sathish, T.; Kaladgi, A.R.R.; Mohanavel, V.; Arul, K.; Afzal, A.; Aabid, A. Experimental Investigation of the Friction Stir Weldability of AA8006 with Zirconia Particle Reinforcement and Optimized Process Parameters. Materials 2021, 14, 2782. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Khan, M.; Khan, S.A.; Afzal, A.; Subbiah, R.; Bakar, E.A. Determination of Non-Recrystallization Temperature for Niobium Micro alloyed Steel. Materials 2021, 14, 2639. [Google Scholar] [CrossRef]
- Nagaraja, S.; Nagegowda, K.U.; Kumar, V.A.; Alamri, S.; Afzal, A.; Thakur, D.; Kaladgi, A.R.; Panchal, S.; Saleel, C.A. In-fluence of the Fly Ash Material Inoculants on the Tensile and Impact Characteristics of the Aluminum AA 5083/7.5SiC Composites. Materials 2021, 14, 2452. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Mohanavel, V.; Ansari, K.; Saravanan, R.; Karthick, A.; Afzal, A.; Alamri, S.; Saleel, C.A. Synthesis and Characterization of Mechanical Properties and Wire Cut EDM Process Parameters Analysis in AZ61 Magnesium Alloy + B4C + SiC. Materials 2021, 14, 3689. [Google Scholar] [CrossRef] [PubMed]
- Mysore, T.H.M.; Patil, A.Y.; Raju, G.U.; Banapurmath, N.R.; Bhovi, P.M.; Afzal, A.; Alamri, S.; Saleel, C.A. Investigation of Mechanical and Physical Properties of Big Sheep Horn as an Alternative Biomaterial for Structural Applications. Materials 2021, 14, 4039. [Google Scholar] [CrossRef] [PubMed]


| 1 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Time 1 h | Sintering Time 2 h | Sintering Time 3 h | |
| Sintering Temperature 375 °C and 5 wt%.B4C | 2.66 | 2.69 | 2.73 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 2.71 | 2.75 | 2.77 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 2.78 | 2.84 | 2.89 |
| 2 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 5 wt%.B4C | 2.68 | 2.72 | 2.78 |
| Sintering Temperature 475 °C and 5 wt%.B4C | 2.74 | 2.83 | 2.85 |
| Sintering Temperature 475 °C and 5 wt%.B4C | 2.81 | 2.90 | 2.94 |
| 3 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 5 wt%.B4C | 2.73 | 2.75 | 2.84 |
| Sintering Temperature 575 °C and 5 wt%.B4C | 2.77 | 2.82 | 2.89 |
| Sintering Temperature 575 °C and 5 wt%.B4C | 2.84 | 2.93 | 2.96 |
| 4 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 2.76 | 2.79 | 2.86 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 2.81 | 2.85 | 2.94 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 2.87 | 2.95 | 3.01 |
| 5 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 10 wt%.B4C | 2.82 | 2.83 | 2.90 |
| Sintering Temperature 475 °C and 10 wt%.B4C | 2.88 | 2.92 | 3.10 |
| Sintering Temperature 475 °C and 10 wt%.B4C | 2.97 | 3.04 | 3.17 |
| 6 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 10 wt%.B4C | 2.85 | 2.87 | 2.99 |
| Sintering Temperature 575 °C and 10 wt%.B4C | 2.91 | 2.99 | 3.16 |
| Sintering Temperature 575 °C and 10 wt%.B4C | 3.03 | 3.09 | 3.25 |
| 7 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 15 wt%.B4C | 2.89 | 2.89 | 3.07 |
| Sintering Temperature 375 °C and 15 wt%.B4C | 2.96 | 3.08 | 3.20 |
| Sintering Temperature 375 °C and 15 wt%.B4C | 3.11 | 3.19 | 3.32 |
| 8 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 15 wt%.B4C | 2.92 | 2.94 | 3.15 |
| Sintering Temperature 475 °C and 15 wt%.B4C | 3.05 | 3.15 | 3.27 |
| Sintering Temperature 475 °C and 15 wt%.B4C | 3.19 | 3.28 | 3.39 |
| 9 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 15 wt%.B4C | 2.97 | 2.96 | 3.20 |
| Sintering Temperature 575 °C and 15 wt%.B4C | 3.09 | 3.24 | 3.33 |
| Sintering Temperature 575 °C and 15 wt%.B4C | 3.23 | 3.31 | 3.45 |
| 1 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Time 1 h | Sintering Time 2 h | Sintering Time 3 h | |
| Sintering Temperature 375 °C and 5 wt%.B4C | 141.59 | 144.72 | 147.09 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 145.70 | 147.92 | 149.88 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 147.66 | 148.66 | 151.25 |
| 2 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 5 wt%.B4C | 137.9 | 145.27 | 147.82 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 140.85 | 146.75 | 149.73 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 147 | 154.35 | 159.18 |
| 3 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 5 wt%.B4C | 133.27 | 115.92 | 120.09 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 167.30 | 135.24 | 145.75 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 148.21 | 112.62 | 133.41 |
| 4 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 138.97 | 122.75 | 116.32 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 143.89 | 120.45 | 119.66 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 146.71 | 118.85 | 122.02 |
| 5 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 141.71 | 164.85 | 157 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 148.32 | 165.11 | 162.53 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 152.38 | 167 | 164 |
| 6 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 148.52 | 129.25 | 136.06 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 154.70 | 130.11 | 142.70 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 158.71 | 132.41 | 149.82 |
| 7 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 161.56 | 133.77 | 139.65 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 163.87 | 145.69 | 150 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 166.01 | 149.02 | 151.77 |
| 8 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 154.84 | 161.32 | 167.02 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 163.55 | 170.92 | 184.41 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 174.20 | 178.61 | 182.66 |
| 9 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 150.44 | 146 | 165.98 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 159.14 | 153.76 | 169.08 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 163 | 158.03 | 173 |
| 1 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Time 1 h | Sintering Time 2 h | Sintering Time 3 h | |
| Sintering Temperature 375 °C and 5 wt%.B4C | 125.49 | 127.04 | 117.32 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 132.53 | 131.48 | 109.77 |
| Sintering Temperature 375 °C and 5 wt%.B4C | 137.66 | 140 | 105.33 |
| 2 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 5 wt%.B4C | 130.66 | 137.74 | 142.55 |
| Sintering Temperature 475 °C and 5 wt%.B4C | 141 | 144.66 | 149 |
| Sintering Temperature 475 °C and 5 wt%.B4C | 156.03 | 160.93 | 165.53 |
| 3 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 5 wt%.B4C | 132.67 | 135.53 | 140.05 |
| Sintering Temperature 575 °C and 5 wt%.B4C | 142 | 147.75 | 152.90 |
| Sintering Temperature 575 °C and 5 wt%.B4C | 151.55 | 157.11 | 163 |
| 4 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 10 wt%.B4C | 108.09 | 107 | 106.03 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 117.07 | 112.79 | 110.64 |
| Sintering Temperature 375 °C and 10 wt%.B4C | 121.88 | 120.41 | 116.22 |
| 5 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 10 wt%.B4C | 130.66 | 110.81 | 138.07 |
| Sintering Temperature 475 °C and 10 wt%.B4C | 135.04 | 122.66 | 145.98 |
| Sintering Temperature 475 °C and 10 wt%.B4C | 141.06 | 129.32 | 155.11 |
| 6 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 10 wt%.B4C | 144.23 | 139.44 | 136.06 |
| Sintering Temperature 575 °C and 10 wt%.B4C | 154.77 | 146.62 | 142.12 |
| Sintering Temperature 575 °C and 10 wt%.B4C | 159.03 | 148 | 145.88 |
| 7 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 375 °C and 15 wt%.B4C | 139.09 | 141 | 133.16 |
| Sintering Temperature 375 °C and 15 wt%.B4C | 143.22 | 147.19 | 136.42 |
| Sintering Temperature 375 °C and 15 wt%.B4C | 149.18 | 152.88 | 145.02 |
| 8 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 475 °C and 15 wt%.B4C | 151.12 | 126.42 | 147.33 |
| Sintering Temperature 475 °C and 15 wt%.B4C | 156.29 | 139.02 | 150.06 |
| Sintering Temperature 475 °C and 15 wt%.B4C | 164.33 | 148.04 | 157.83 |
| 9 | Compaction Pressure 300 MPa | Compaction Pressure 400 MPa | Compaction Pressure 500 MPa |
| Sintering Temperature 575 °C and 15 wt%.B4C | 149.33 | 159.36 | 122.12 |
| Sintering Temperature 575 °C and 15 wt%.B4C | 156.88 | 167.41 | 135.81 |
| Sintering Temperature 575 °C and 15 wt%.B4C | 161.10 | 171 | 143.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meignanamoorthy, M.; Ravichandran, M.; Mohanavel, V.; Afzal, A.; Sathish, T.; Alamri, S.; Khan, S.A.; Saleel, C.A. Microstructure, Mechanical Properties, and Corrosion Behavior of Boron Carbide Reinforced Aluminum Alloy (Al-Fe-Si-Zn-Cu) Matrix Composites Produced via Powder Metallurgy Route. Materials 2021, 14, 4315. https://doi.org/10.3390/ma14154315
Meignanamoorthy M, Ravichandran M, Mohanavel V, Afzal A, Sathish T, Alamri S, Khan SA, Saleel CA. Microstructure, Mechanical Properties, and Corrosion Behavior of Boron Carbide Reinforced Aluminum Alloy (Al-Fe-Si-Zn-Cu) Matrix Composites Produced via Powder Metallurgy Route. Materials. 2021; 14(15):4315. https://doi.org/10.3390/ma14154315
Chicago/Turabian StyleMeignanamoorthy, M., Manickam Ravichandran, Vinayagam Mohanavel, Asif Afzal, T. Sathish, Sagr Alamri, Sher Afghan Khan, and C. Ahamed Saleel. 2021. "Microstructure, Mechanical Properties, and Corrosion Behavior of Boron Carbide Reinforced Aluminum Alloy (Al-Fe-Si-Zn-Cu) Matrix Composites Produced via Powder Metallurgy Route" Materials 14, no. 15: 4315. https://doi.org/10.3390/ma14154315
APA StyleMeignanamoorthy, M., Ravichandran, M., Mohanavel, V., Afzal, A., Sathish, T., Alamri, S., Khan, S. A., & Saleel, C. A. (2021). Microstructure, Mechanical Properties, and Corrosion Behavior of Boron Carbide Reinforced Aluminum Alloy (Al-Fe-Si-Zn-Cu) Matrix Composites Produced via Powder Metallurgy Route. Materials, 14(15), 4315. https://doi.org/10.3390/ma14154315











