Insights in the Ionic Conduction inside Nanoporous Metal-Organic Frameworks by Using an Appropriate Equivalent Circuit
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
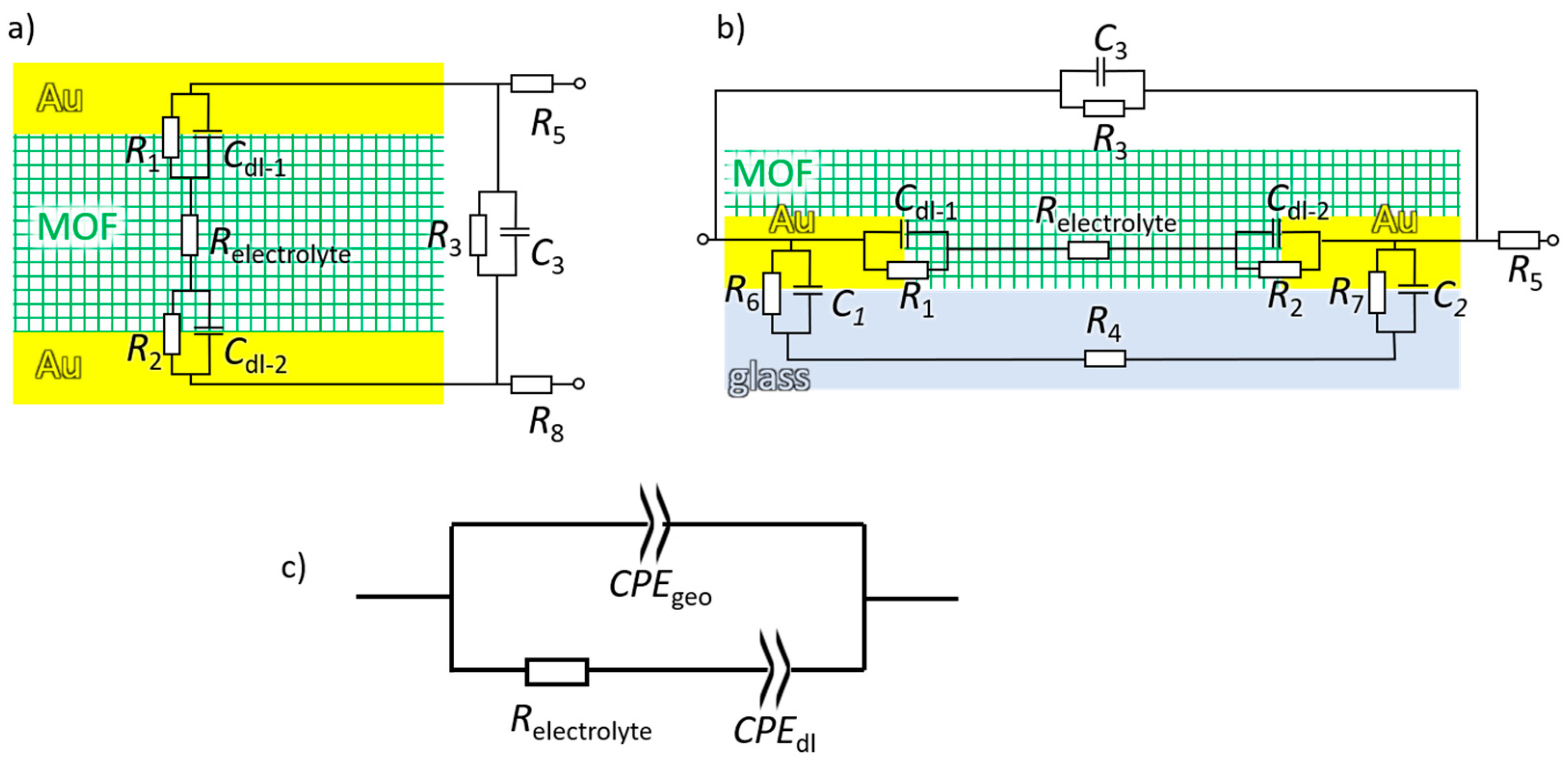
3.1. Introduction of Equivalent Circuit
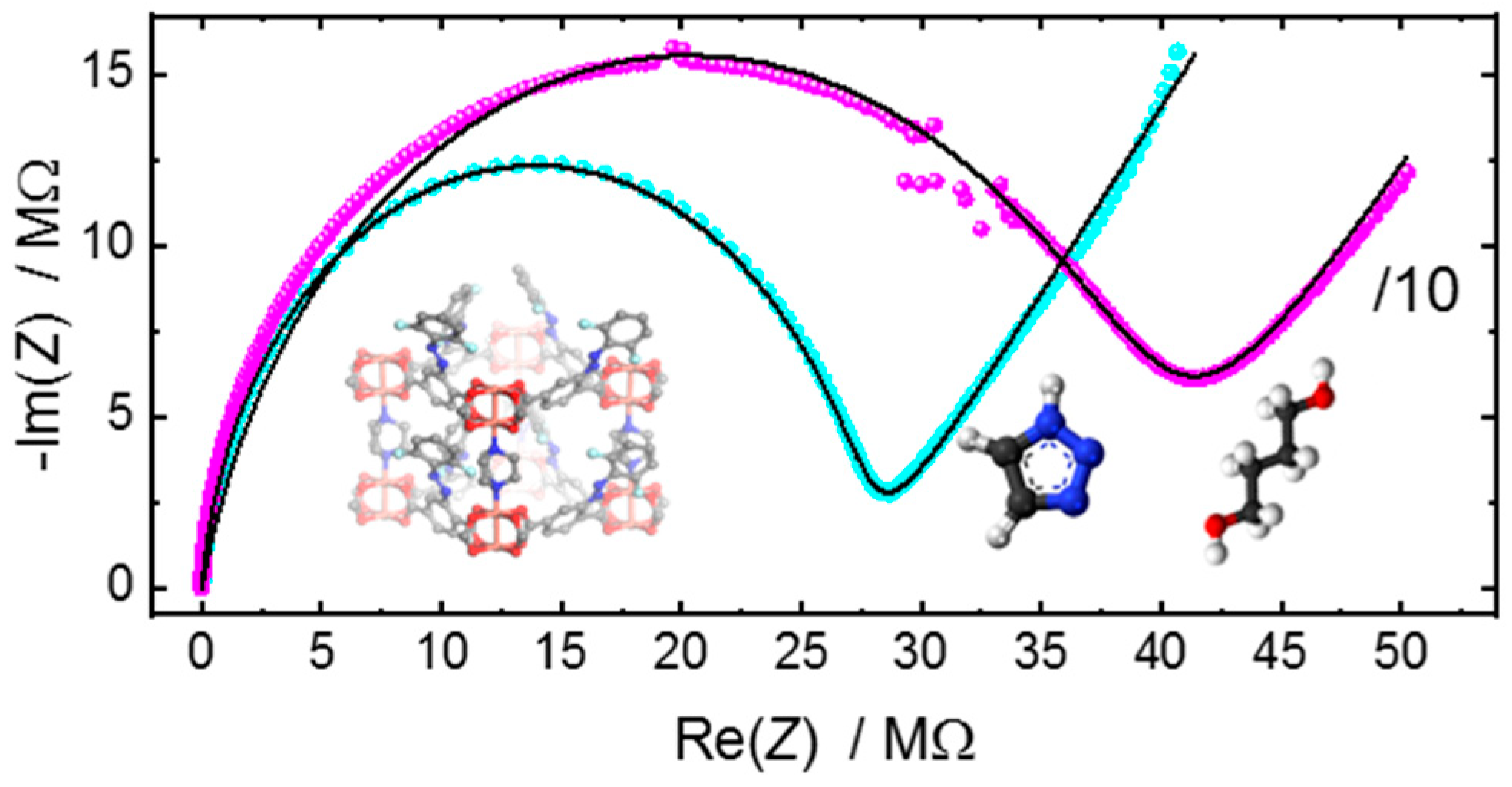
3.2. Application of the Equivalent Circuit to the Ionic Conduction in MOF Films and Pellets
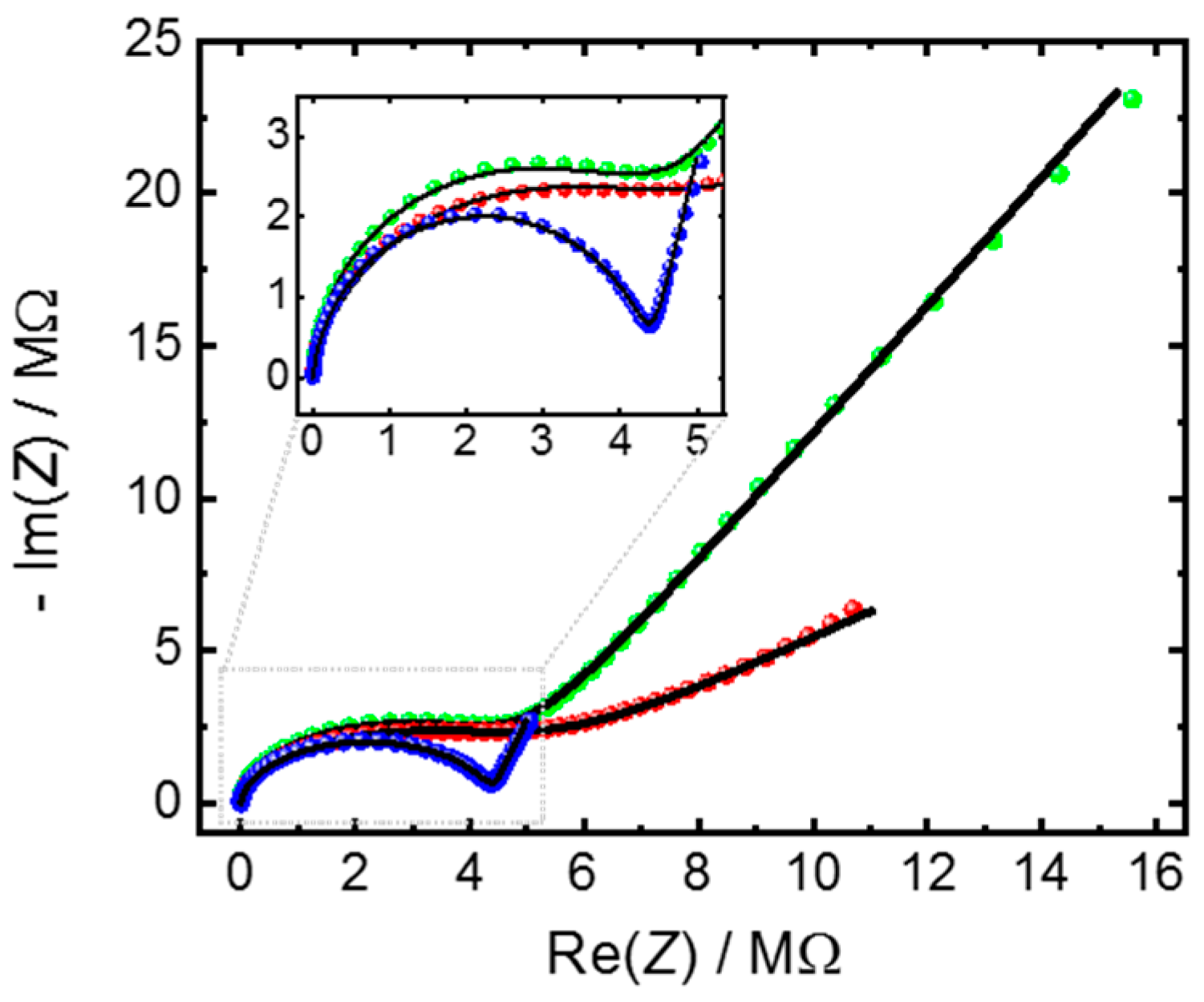
3.3. Insights in the MOF-Electrode Interface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. MOF Powder Synthesis, IL Loading and Pellet Preparation
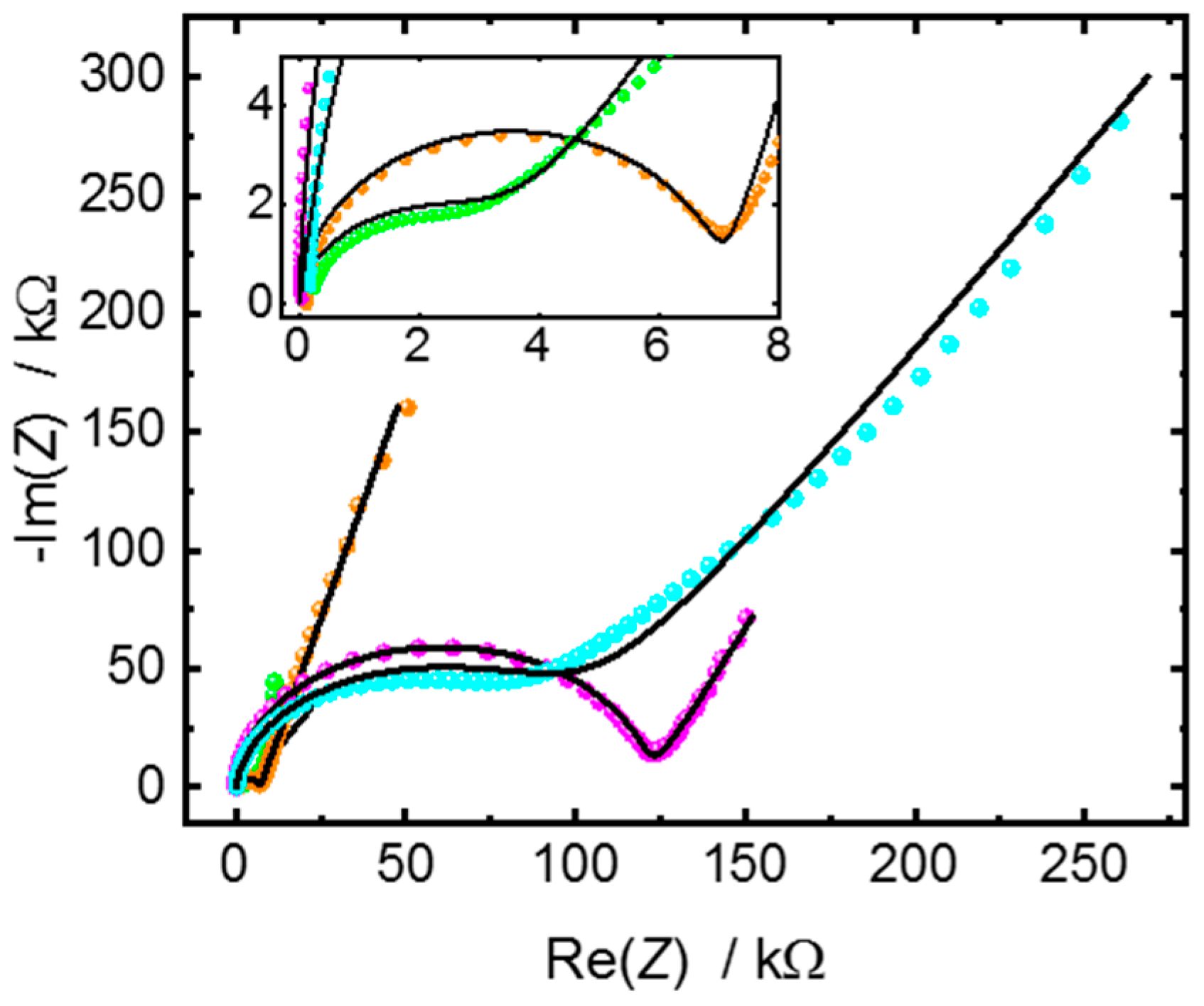
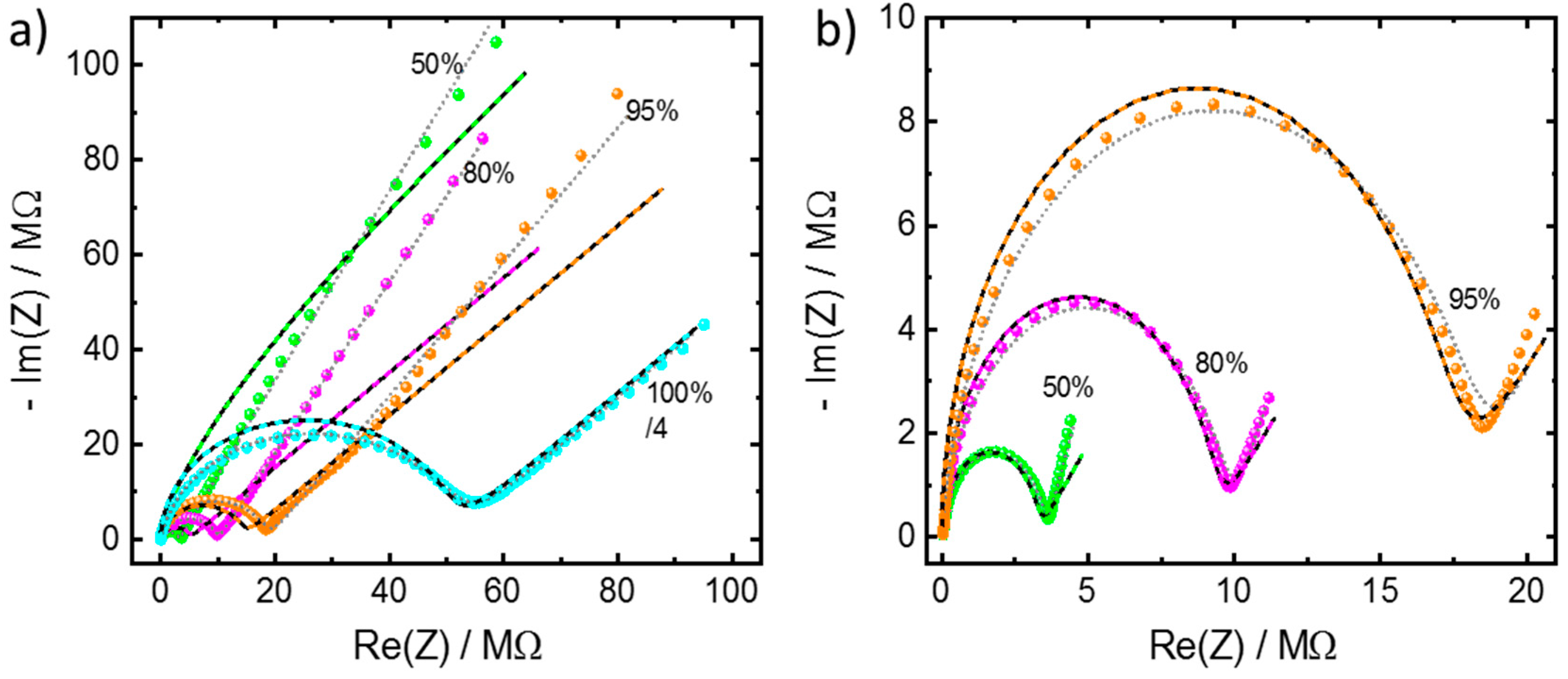
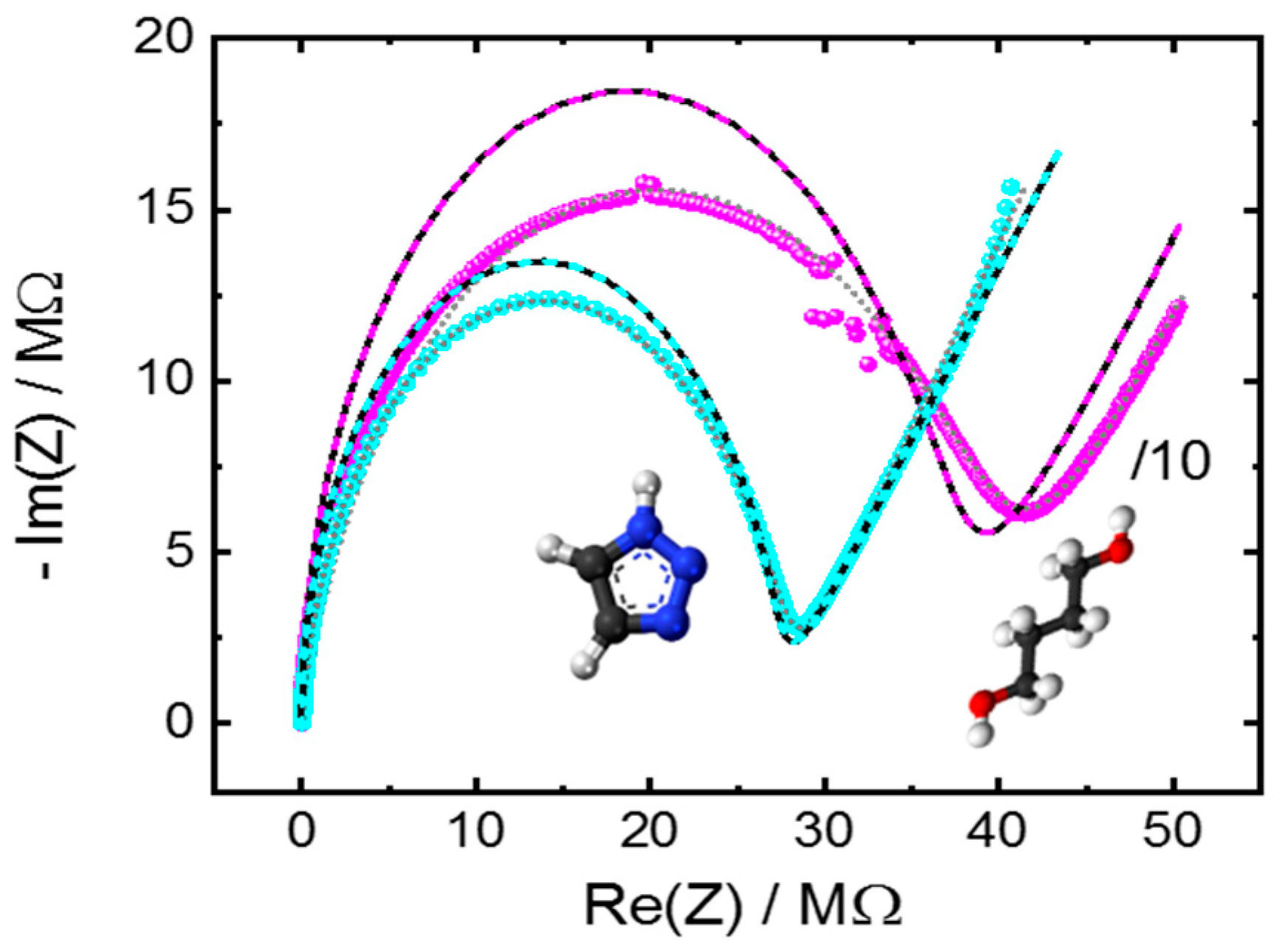
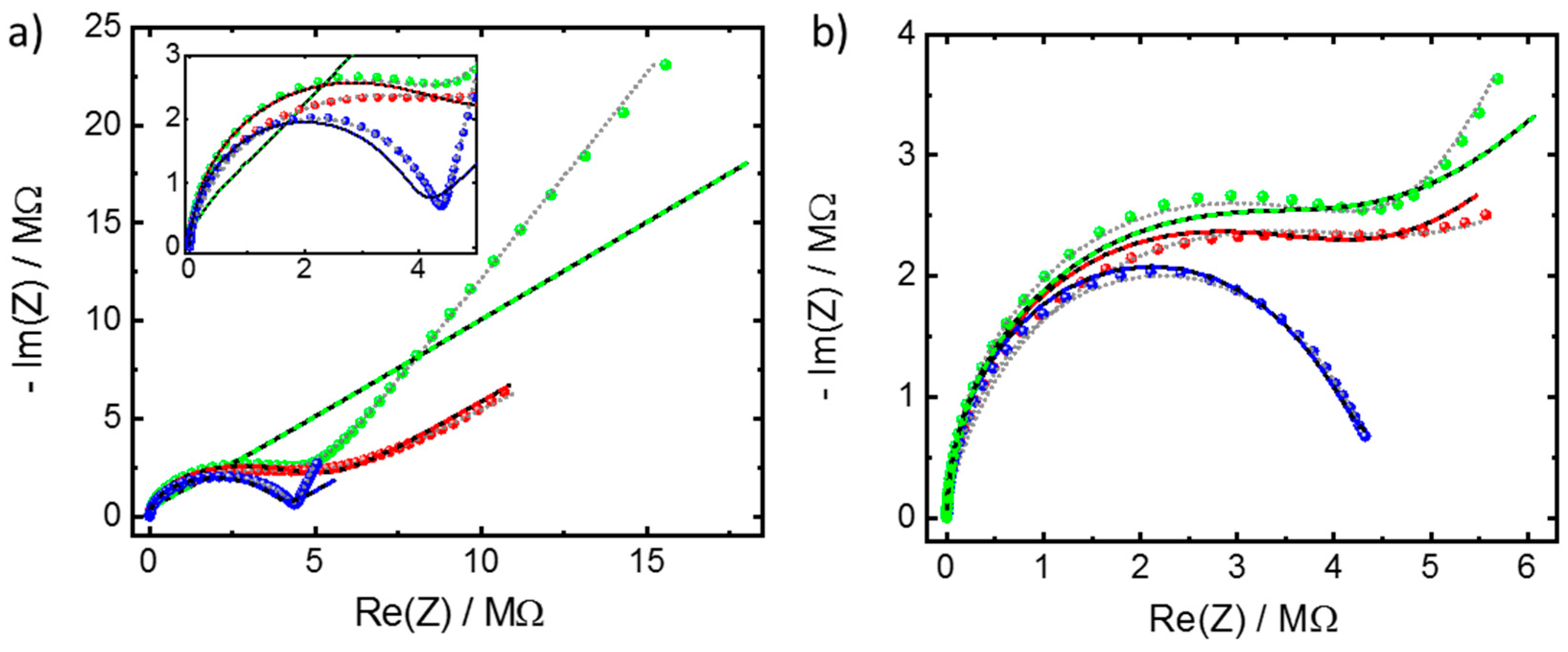
Appendix A.2. Additional Data—Nyquist Plots, Determined Parameters, SEM Images and XRD
| Electrolyte | R/kΩ | CPEdl | ndl | CPEgeo | ngeo | Double Layer Capacitance Cdl/nF | Estimated Double Layer Thickness λdl/nm |
|---|---|---|---|---|---|---|---|
| IL | 3.7 | 68 | 0.67 | 179 | 0.95 | 1.15 | 1 |
| butanediol | 121 | 1242 | 0.73 | 270 | 0.99 | 609 | 7.8 |
| triazole | 101 | 82 | 0.66 | 168 | 0.94 | 6.94 | 0.5 |
| water | 7.1 | 353 | 0.84 | 248 | 1 | 113 | 105 |

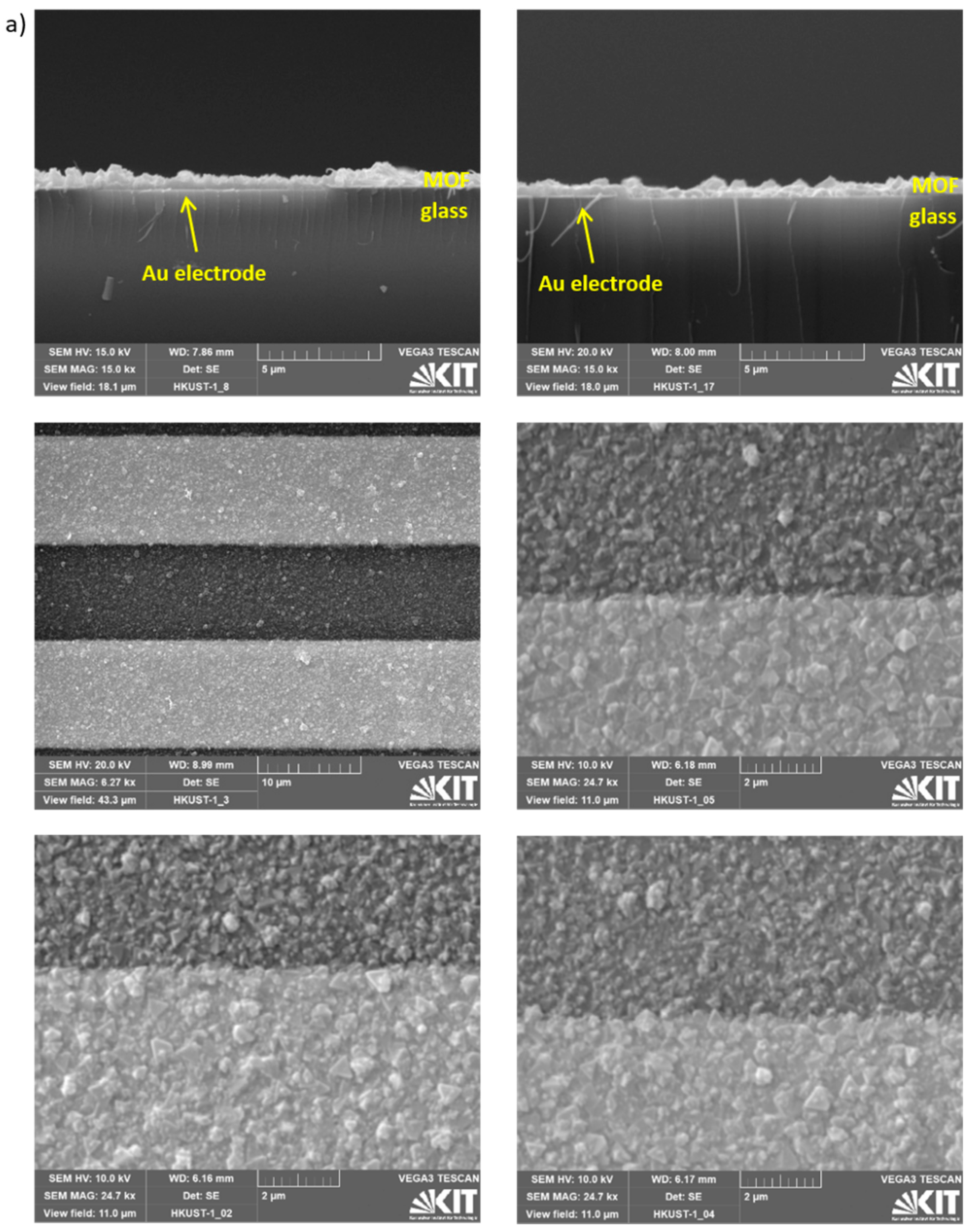
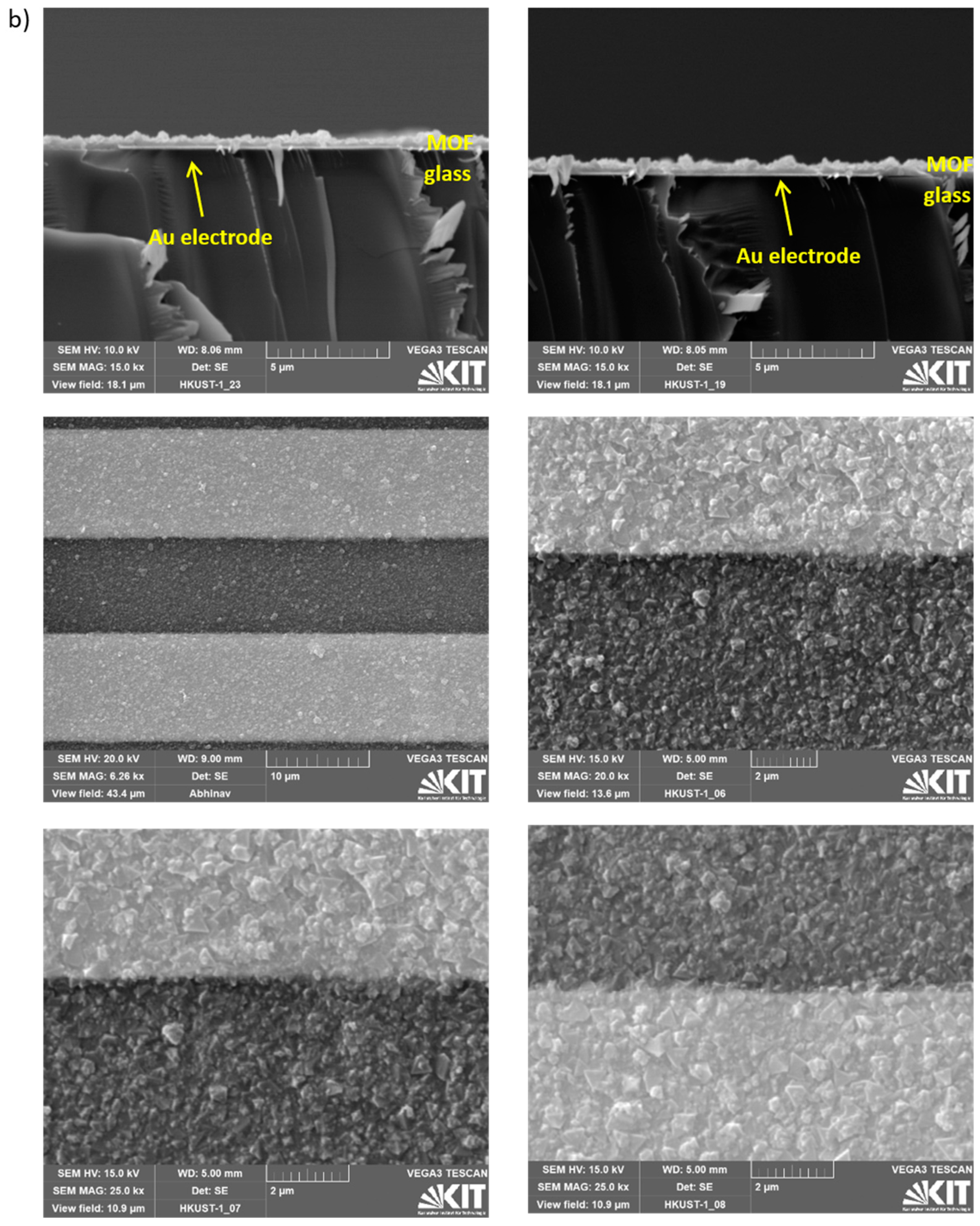
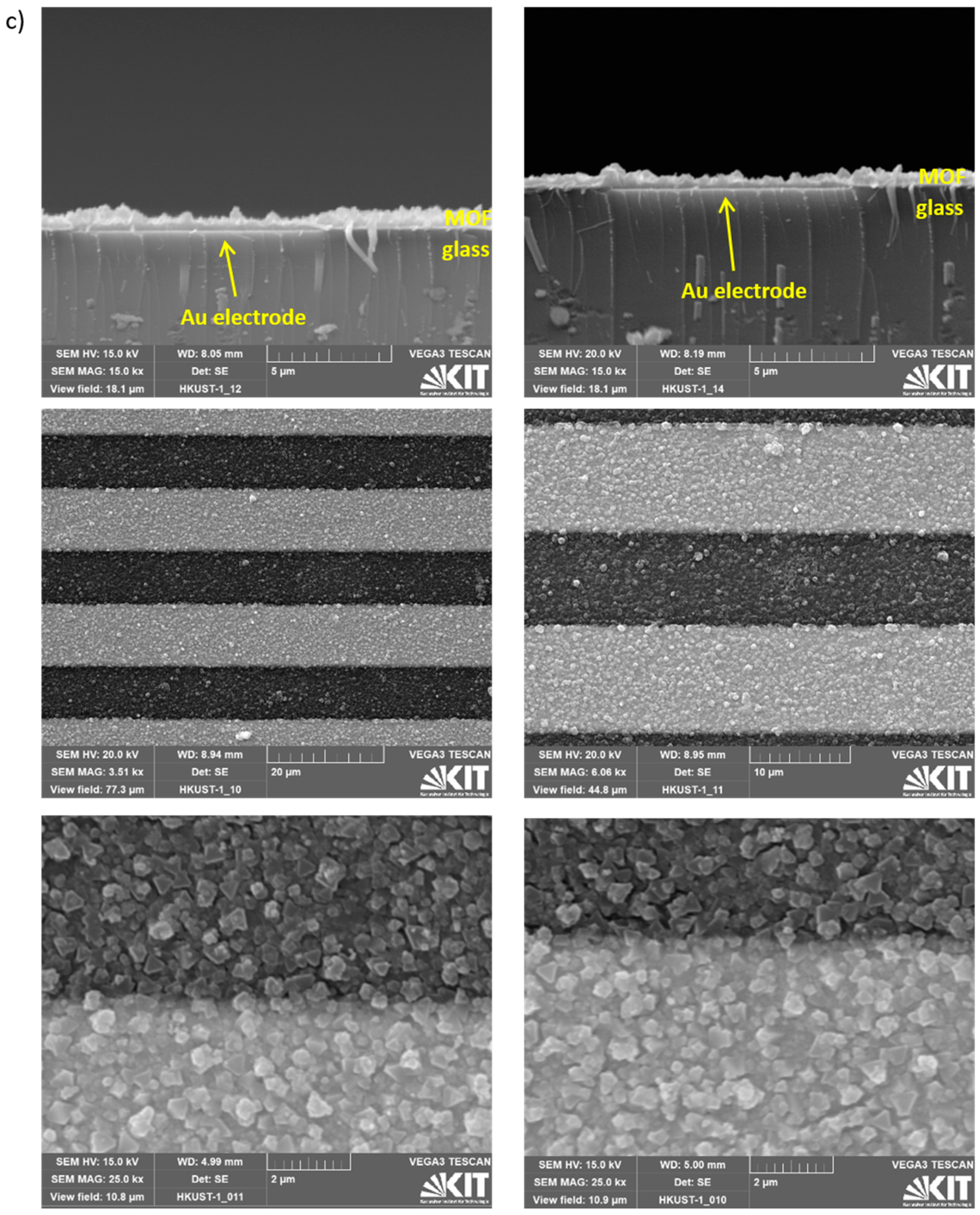
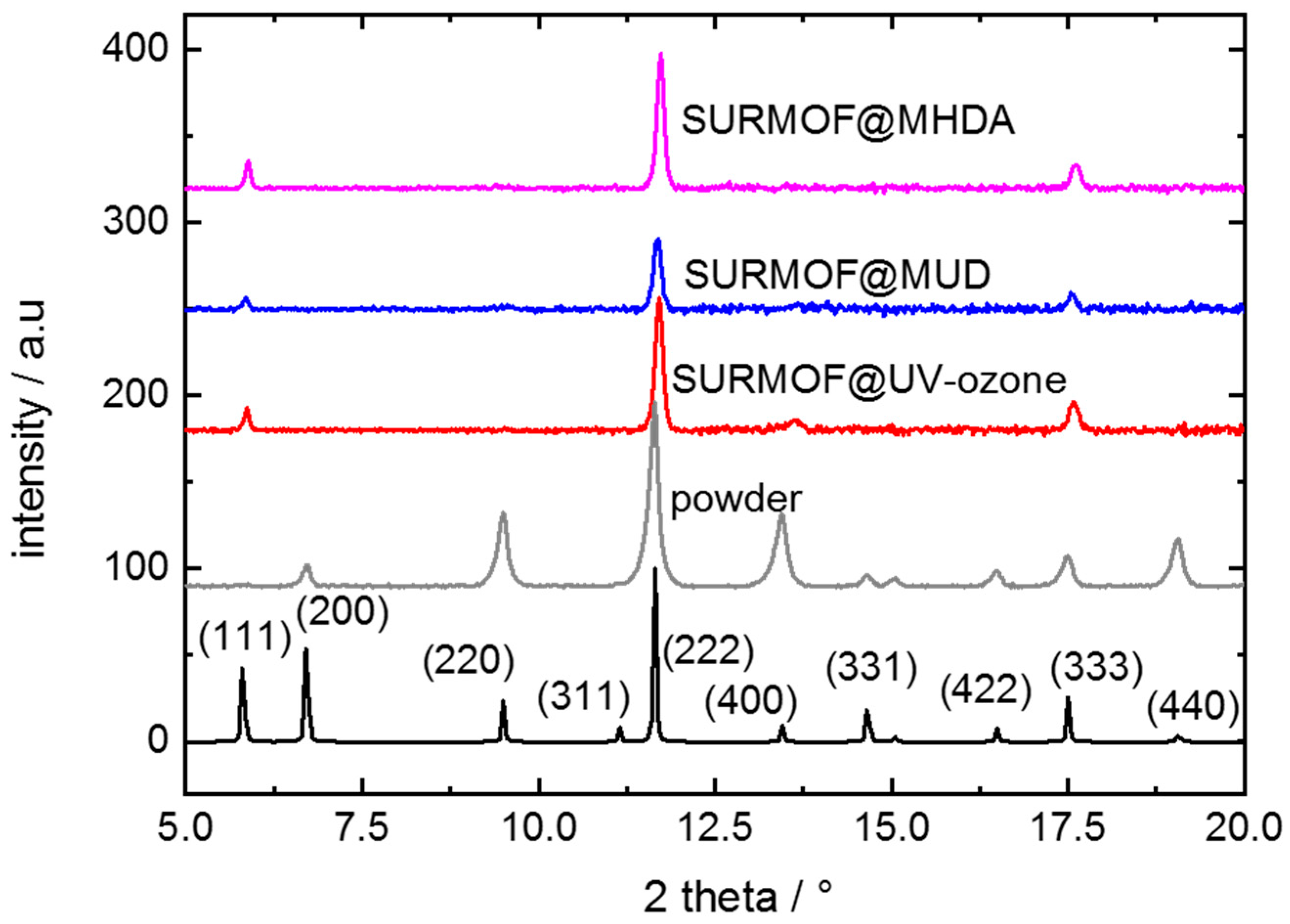
References
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.T.; Lakshmanan, B.; Mathias, M.F. Electrochemistry and the Future of the Automobile. J. Phys. Chem. Let. 2010, 1, 2204–2219. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic liquids in electrochemical devices and processes: Managing interfacial Electrochemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 88–96. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef]
- Morozan, A.; Jaouen, F. Metal organic frameworks for electrochemical applications. Energy Environ. Sci. 2012, 5, 9269–9290. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors—Challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Burtch, N.C.; Talin, A.A.; Falcaro, P.; Allendorf, M.D.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal-Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Liang, W.B.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS App. Mat. Int. 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Masa, J.; Andronescu, C.; Muhler, M.; Fischer, R.A.; Schuhmann, W. MOFs for Electrocatalysis: From Serendipity to Design Strategies. Small Methods 2019, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Nagarkar, S.S.; Unni, S.M.; Sharma, A.; Kurungot, S.; Ghosh, S.K. Two-in-One: Inherent Anhydrous and Water-Assisted High Proton Conduction in a 3D Metal-Organic Framework. Angew. Chem. Int. Ed. 2014, 53, 2638–2642. [Google Scholar] [CrossRef]
- Grancha, T.; Ferrando-Soria, J.; Cano, J.; Amoros, P.; Seoane, B.; Gascon, J.; Bazaga-Garcia, M.; Losilla, E.R.; Cabeza, A.; Armentano, D.; et al. Insights into the Dynamics of Grotthuss Mechanism in a Proton Conducting Chiral bioMOF. Chem. Mat. 2016, 28, 4608–4615. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Horike, S.; Itakura, T.; Le Ouay, B.; Demessence, A.; Tsujimoto, M.; Kitagawa, S. Enhanced and Optically Switchable Proton Conductivity in a Melting Coordination Polymer Crystal. Angew. Chem. Int. Ed. 2017, 56, 4976–4981. [Google Scholar] [CrossRef]
- Umeyama, D.; Horike, S.; Inukai, M.; Hijikata, Y.; Kitagawa, S. Confinement of Mobile Histamine in Coordination Nanochannels for Fast Proton Transfer. Angew. Chem. Int. Ed. 2011, 50, 11706–11709. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Broersen, R.; Hageman, W.; Yan, N.; Mittelmeijer-Hazeleger, M.C.; Rothenberg, G.; Tanase, S. High proton conductivity in cyanide-bridged metal-organic frameworks: Understanding the role of water. J. Mat. Chem. A 2015, 3, 22347–22352. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Tao, S.S.; Jiang, D.L. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 2016, 15, 722–726. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Zhou, Y.; Suriyakumar, S.; Dhanalakshmi, R.B.; Satishrajan, M.; Alwarappan, S.; Alkordi, M.H.; Stephan, A.M. Microporous Metal-Organic Framework (MOF)-Based Composite Polymer Electrolyte (CPE) Mitigating Lithium Dendrite Formation in All-Solid-State-Lithium Batteries. ACS Omega 2020, 5, 7885–7894. [Google Scholar] [CrossRef] [Green Version]
- Zima, V.; Patil, D.S.; Raja, D.S.; Chang, T.-G.; Lin, C.-H.; Shimakawa, K.; Wagner, T. New MOF based on lithium tetrahydrofuran-2,3,4,5-tetracarboxylate: Its structure and conductivity behavior. J. Solid State Chem. 2014, 217, 150–158. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Slawin, A.M.Z.; Carpenter-Warren, C.L.; Chen, W.; Zheng, A. Ultrafast post-synthetic modification of a pillared cobalt(ii)-based metal–organic framework via sulfurization of its pores for high-performance supercapacitors. J. Mat. Chem. A 2019, 7, 11953–11966. [Google Scholar] [CrossRef]
- Shalini, S.; Dhavale, V.M.; Eldho, K.M.; Kurungot, S.; Ajithkumar, T.G.; Vaidhyanathan, R. 1000-fold enhancement in proton conductivity of a MOF using post-synthetically anchored proton transporters. Sci. Rep. 2016, 6, 32489. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Helfferich, J.; Zhao, F.L.; Verma, R.; Kanj, A.B.; Meded, V.; Bléger, D.; Wenzel, W.; Heinke, L. Switching the Proton Conduction in Nanoporous, Crystalline Materials by Light. Adv. Mater. 2018, 30, 1706551. [Google Scholar] [CrossRef]
- Kanj, A.B.; Verma, R.; Liu, M.; Helfferich, J.; Wenzel, W.; Heinke, L. Bunching and Immobilization of Ionic Liquids in Nanoporous Metal–Organic Framework. Nano Lett. 2019, 19, 2114–2120. [Google Scholar] [CrossRef]
- Kanj, A.B.; Chandresh, A.; Gerwien, A.; Grosjean, S.; Bräse, S.; Wang, Y.; Dube, H.; Heinke, L. Proton-conduction photomodulation in spiropyran-functionalized MOFs with large on–off ratio. Chem. Sci. 2020, 11, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Shalini, S.; Vaid, T.P.; Matzger, A.J. Salt nanoconfinement in zirconium-based metal-organic frameworks leads to pore-size and loading-dependent ionic conductivity enhancement. Chem. Comm. 2020, 56, 7245–7248. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Thornton, K.; Ajdari, A. Diffuse-charge dynamics in electrochemical systems. Phys. Rev. E 2004, 70, 021506. [Google Scholar] [CrossRef] [Green Version]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Jamnik, J.; Maier, J. Generalized equivalent circuits for mixed conductors: Silver sulfide as a model system. Mon. Fur. Chem. 2009, 140, 1113–1119. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.; Montes, R.; Baeza, M. Trends in electrochemical impedance spectroscopy involving nanocomposite transducers: Characterization, architecture surface and bio-sensing. Trac Trends Anal. Chem. 2017, 97, 201–215. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Small, L.J.; Nenoff, T.M. Direct Electrical Detection of Iodine Gas by a Novel Metal-Organic-Framework-Based Sensor. ACS App. Mat. Int. 2017, 9, 44649–44655. [Google Scholar] [CrossRef]
- Fischerauer, A.; Fischerauer, G.; Hagen, G.; Moos, R. Integrated impedance based hydro-carbon gas sensors with Na-zeolite/Cr2O3 thin-film interfaces: From physical modeling to devices. Phys. Status Solidi A Appl. Mater. Sci. 2011, 208, 404–415. [Google Scholar] [CrossRef]
- Hagen, G.; Dubbe, A.; Rettig, F.; Jerger, A.; Birkhofer, T.; Mueller, R.; Plog, C.; Moos, R. Selective impedance based gas sensors for hydrocarbons using ZSM-5 zeolite films with chromium(III)oxide interface. Sens. Actuator B-Chem. 2006, 119, 441–448. [Google Scholar] [CrossRef]
- Matysik, S.; Matysik, F.M.; Schulze, K.D.; Einicke, W.D. Impedance spectroscopic investigations of zeolite-polydimethylsiloxane electrodes. Electrochim. Acta 2002, 48, 297–301. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Woo, R.P.; Grayson, S.M.; Majumder, A.; Raman, R.K.S. Influence of Zeolite Coating on the Corrosion Resistance of AZ91D Magnesium Alloy. Materials 2014, 7, 6092–6104. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhang, Y.; Yin, L.; Ding, D.; Jing, W.; Gu, X. Electrochemical impedance spectroscopy for Analyzing microstructure evolution of NaA zeolite membrane in acid water/ethanol solution. Chem. Eng. Sci. 2016, 153, 1–9. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material Cu-3(TMA)(2)(H2O)(3) (n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Heinke, L.; Wöll, C. Surface-Mounted Metal–Organic Frameworks: Crystalline and Porous Molecular Assemblies for Fundamental Insights and Advanced Applications. Adv. Mater. 2019, 31, 1806324. [Google Scholar] [CrossRef] [PubMed]
- Hurrle, S.; Friebe, S.; Wohlgemuth, J.; Wöll, C.; Caro, J.; Heinke, L. Sprayable, Large-Area Metal–Organic Framework Films and Membranes of Varying Thickness. Chem. Eur. J. 2017, 23, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X.L. Mapping the Cu-BTC metal-organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, M.; Rizzo, C.; Millini, R.; Dietzel, P.D.C.; Blom, R.; Zanardi, S. Methane storage on CPO-27-Ni pellets. J. Porous Mat. 2011, 18, 289–296. [Google Scholar] [CrossRef]
- MacKay, S.; Hermansen, P.; Wishart, D.; Chen, J. Simulations of Interdigitated Electrode Interactions with Gold Nanoparticles for Impedance-Based Biosensing Applications. Sensors 2015, 15, 22192–22208. [Google Scholar] [CrossRef] [Green Version]
- De Levie, R. The influence of surface roughness of solid electrodes on electrochemical measurements. Electrochim. Acta 1965, 10, 113–130. [Google Scholar] [CrossRef]
- Kerner, Z.; Pajkossy, T. On the origin of capacitance dispersion of rough electrodes. Electrochim. Acta 2000, 46, 207–211. [Google Scholar] [CrossRef]
- Singh, M.B.; Kant, R. Debye-Falkenhagen dynamics of electric double layer in presence of electrode heterogeneities. J. Electroanal. Chem. 2013, 704, 197–207. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Pfriem, A.; Hamsch, S.; Breitwieser, H.; Wohlgemuth, J.; Heinke, L.; Gliemann, H.; Wöll, C. Transparent films of metal-organic frameworks for optical applications. Micropor. Mesopor. Mat. 2015, 211, 82–87. [Google Scholar] [CrossRef]
- Okur, S.; Zhang, Z.; Sarheed, M.; Nick, P.; Lemmer, U.; Heinke, L. Towards a MOF e-Nose: A SURMOF sensor array for detection and discrimination of plant oil scents and their mixtures. Sens. Actuators B. Chem. 2020, 306, 127502. [Google Scholar] [CrossRef]
- Müller, K.; Malhi, J.S.; Wohlgemuth, J.; Fischer, R.A.; Wöll, C.; Gliemann, H.; Heinke, L. Water as a modulator in the synthesis of surface-mounted metal–organic framework films of type HKUST-1. Dalt. Trans. 2018, 47, 16474–16479. [Google Scholar] [CrossRef]
- Wang, Z.; Knebel, A.; Grosjean, S.; Wagner, D.; Bräse, S.; Wöll, C.; Caro, J.; Heinke, L. Tunable molecular separation by nanoporous membranes. Nat. Comm. 2016, 7, 13872. [Google Scholar] [CrossRef] [Green Version]
- Shekhah, O. Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs). Materials 2010, 3, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shekhah, O.; Stammer, X.; Arslan, H.K.; Liu, B.; Schüpbach, B.; Terfort, A.; Wöll, C. Deposition of Metal-Organic Frameworks by Liquid-Phase Epitaxy: The Influence of Substrate Functional Group Density on Film Orientation. Materials 2012, 5, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Dragässer, A.; Shekhah, O.; Zybaylo, O.; Shen, C.; Buck, M.; Wöll, C.; Schlettwein, D. Redox mediation enabled by immobilised centres in the pores of a metal-organic framework grown by liquid phase epitaxy. Chem. Comm. 2012, 48, 663–665. [Google Scholar] [CrossRef]
- Kakaei, M.N.; Neshati, J.; Rezaierod, A.R. On the Extraction of the Effective Capacitance from Constant Phase Element Parameters. Prot. Met. Phys. Chem. Surf. 2018, 54, 548–556. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Ruzanov, A.; Lembinen, M.; Jakovits, P.; Srirama, S.N.; Voroshylova, I.V.; Cordeiro, M.N.D.S.; Pereira, C.M.; Rossmeisl, J.; Ivaništšev, V.B. On the thickness of the double layer in ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 10275–10285. [Google Scholar] [CrossRef] [Green Version]
- Lockett, V.; Horne, M.; Sedev, R.; Rodopoulos, T.; Ralston, J. Differential capacitance of the double layer at the electrode/ionic liquids interface. Phys. Chem. Chem. Phys. 2010, 12, 12499–12512. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic Liquids at Electrified Interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delen, G.; Ristanovic, Z.; Mandemaker, L.D.B.; Weckhuysen, B.M. Mechanistic Insights into Growth of Surface-Mounted Metal-Organic Framework Films Resolved by Infrared (Nano-) Spectroscopy. Chem. A Eur. J. 2018, 24, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Babal, A.S.; Chaudhari, A.K.; Yeung, H.H.M.; Tan, J.-C. Guest-Tunable Dielectric Sensing Using a Single Crystal of HKUST-1. Adv. Mater. Interfaces 2020, 7, 2000408. [Google Scholar] [CrossRef]
- Huang, M.-M.; Jiang, Y.; Sasisanker, P.; Driver, G.W.; Weingartner, H. Static Relative Dielectric Permittivities of Ionic Liquids at 25 °C. J. Chem. Eng. Data 2011, 56, 1494–1499. [Google Scholar] [CrossRef]
- Pulst, M.; Balko, J.; Golitsyn, Y.; Reichert, D.; Busse, K.; Kressler, J. Proton conductivity and phase transitions in 1,2,3-triazole. Phys. Chem. Chem. Phys. 2016, 18, 6153–6163. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Wilding, W.V.; Giles, N.F.; Rowley, R.L. A Quantitative Structure Property Relation Correlation of the Dielectric Constant for Organic Chemicals. J. Chem. Eng. Data 2010, 55, 41–45. [Google Scholar] [CrossRef]

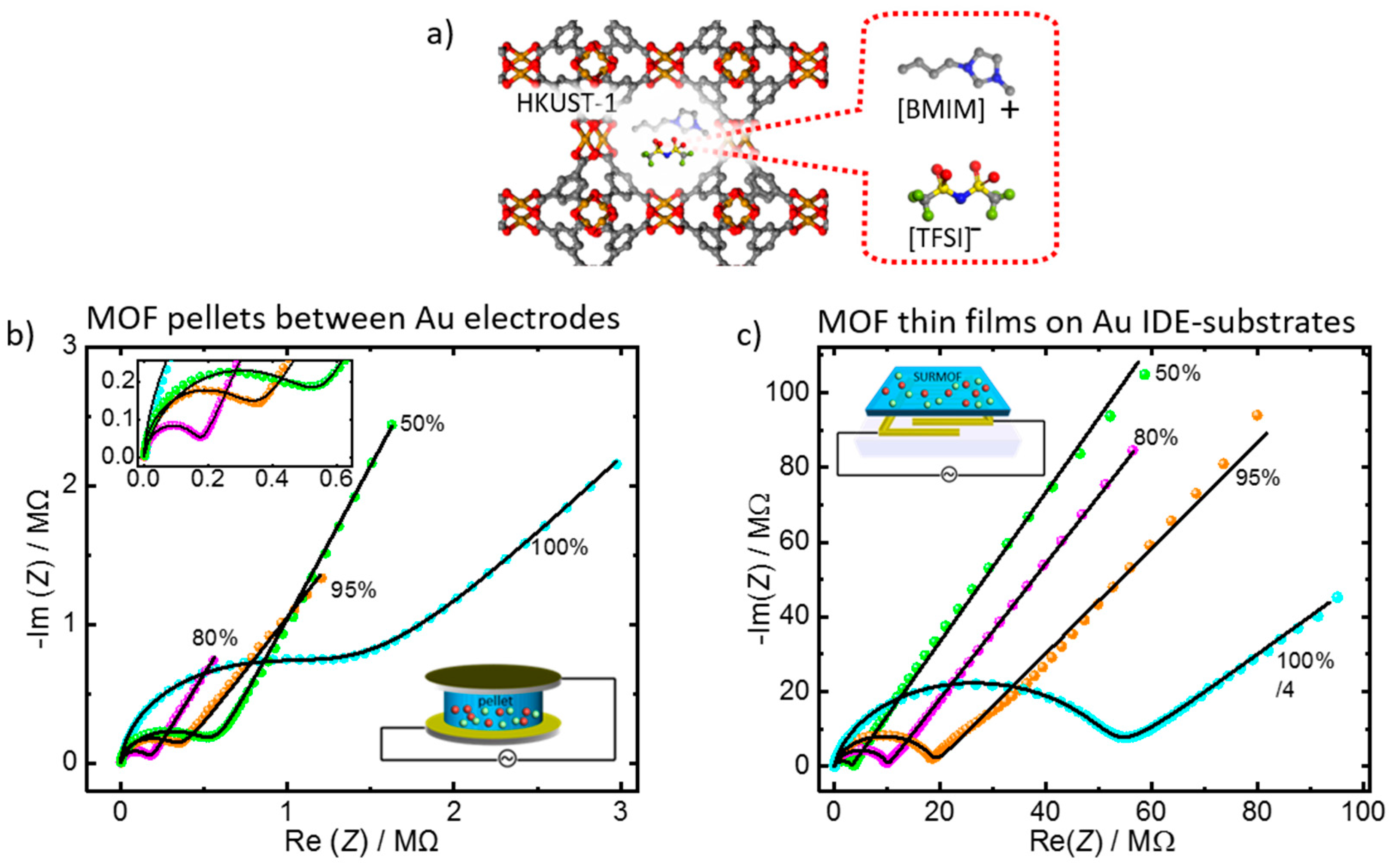
| Form | Electrolyte | MOF Host | R/MΩ | ndl | ngeo | Conductivity σ/µS m−1 | ||
|---|---|---|---|---|---|---|---|---|
| MOF pellets between Au-electrodes | IL 100% | HKUST-1 | 1.29 | 20.4 | 0.55 | 25.1 | 0.95 | 5.8 |
| IL 95% | HKUST-1 | 0.34 | 19.9 | 0.64 | 12.0 | 0.98 | 22.2 | |
| IL 80% | HKUST-1 | 0.18 | 25 | 0.70 | 18.0 | 0.96 | 43.1 | |
| IL 50% | HKUST-1 | 0.57 | 6.36 | 0.73 | 34.7 | 0.84 | 13.2 | |
| MOF thin film on IDE-substrate | IL 100% | HKUST-1 | 205 | 4.58 | 0.51 | 1.91 | 0.90 | 0.12 |
| IL 95% | HKUST-1 | 18.7 | 9.14 | 0.61 | 1.91 | 0.89 | 1.3 | |
| IL 80% | HKUST-1 | 9.98 | 9.56 | 0.68 | 1.83 | 0.90 | 2.4 | |
| IL 50% | HKUST-1 | 3.21 | 7.58 | 0.71 | 1.85 | 0.91 | 7.4 | |
| butanediol | pl-MOF | 393 | 6.64 | 0.52 | 8.878 | 0.84 | 8.5 | |
| triazole | pl-MOF | 27.3 | 52 | 0.53 | 9.9 | 0.93 | 122 |
| Substrate Functionalization | R/MΩ | CPEdl | ndl | CPEgeo | ngeo | Conductivity σ/µS m−1 | Double Layer Capacitance Cdl/nF | Estimated Double Layer Thickness λdl/nm # |
|---|---|---|---|---|---|---|---|---|
| UV-ozone | 4.40 | 51.9 | 0.85 | 99.2 | 0.94 | 2.7 | 40.2 | 10.4 |
| MUD SAM | 4.87 | 8.2 | 0.71 | 53.0 | 0.99 | 2.4 | 2.3 | 179.9 |
| MHDA SAM | 4.57 | 51.0 | 0.46 | 154 | 0.89 | 2.6 | 9.6 | 43.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandresh, A.; Zhang, Z.; Heinke, L. Insights in the Ionic Conduction inside Nanoporous Metal-Organic Frameworks by Using an Appropriate Equivalent Circuit. Materials 2021, 14, 4352. https://doi.org/10.3390/ma14164352
Chandresh A, Zhang Z, Heinke L. Insights in the Ionic Conduction inside Nanoporous Metal-Organic Frameworks by Using an Appropriate Equivalent Circuit. Materials. 2021; 14(16):4352. https://doi.org/10.3390/ma14164352
Chicago/Turabian StyleChandresh, Abhinav, Zejun Zhang, and Lars Heinke. 2021. "Insights in the Ionic Conduction inside Nanoporous Metal-Organic Frameworks by Using an Appropriate Equivalent Circuit" Materials 14, no. 16: 4352. https://doi.org/10.3390/ma14164352






