Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method
Abstract
:1. Introduction
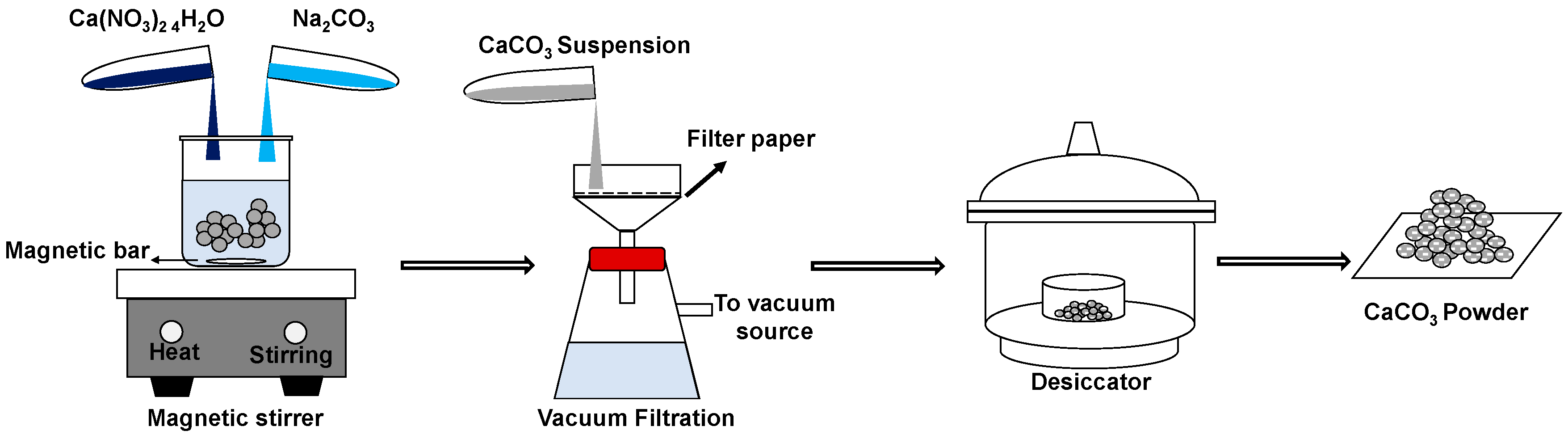
2. Materials and Methods
- = weight of gas adsorbed
- = relative pressure
- = weight of adsorbate as monolayer
- = BET constant
- = weight of adsorbate as monolayer
- = Avogadro’s number (6.023 1023)
- = Molecular weight of adsorbate
- = Adsorbate cross sectional area (16.2 for Å2 Nitrogen)
3. Results and Discussion
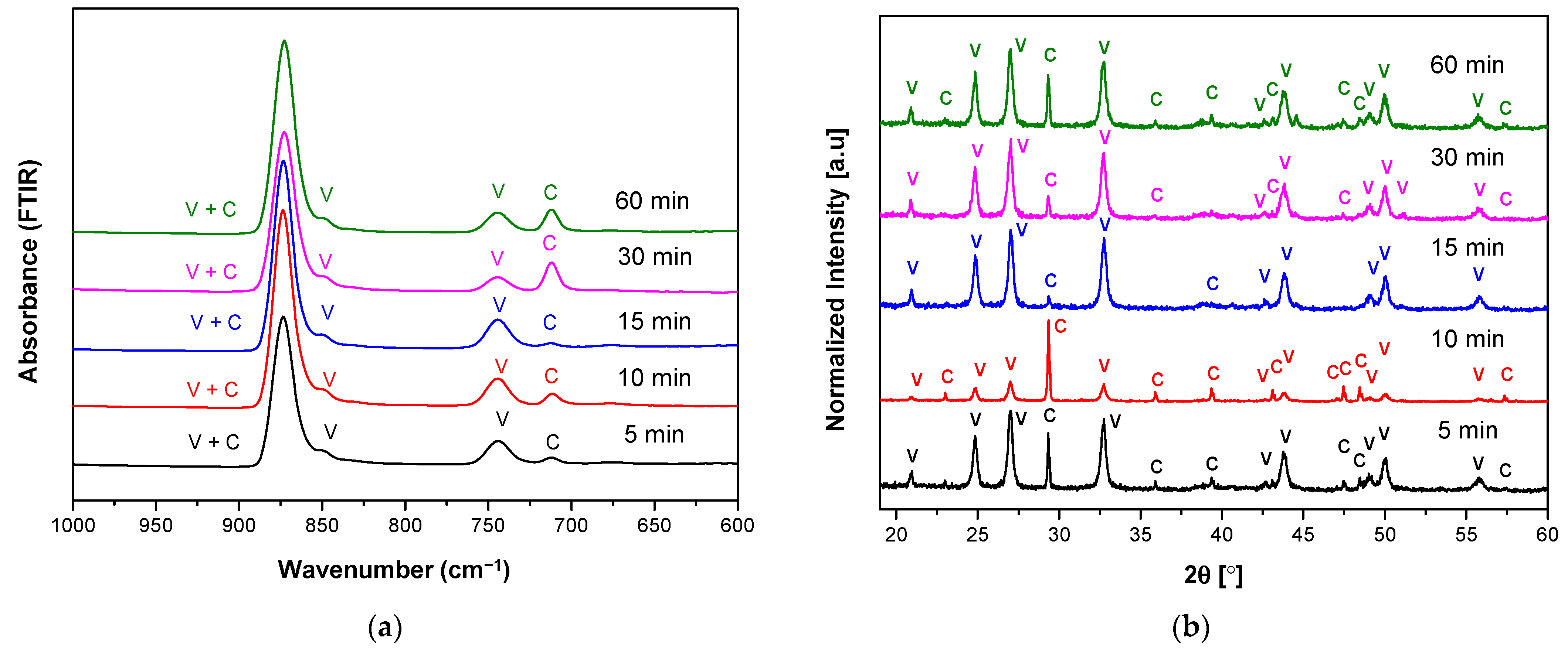
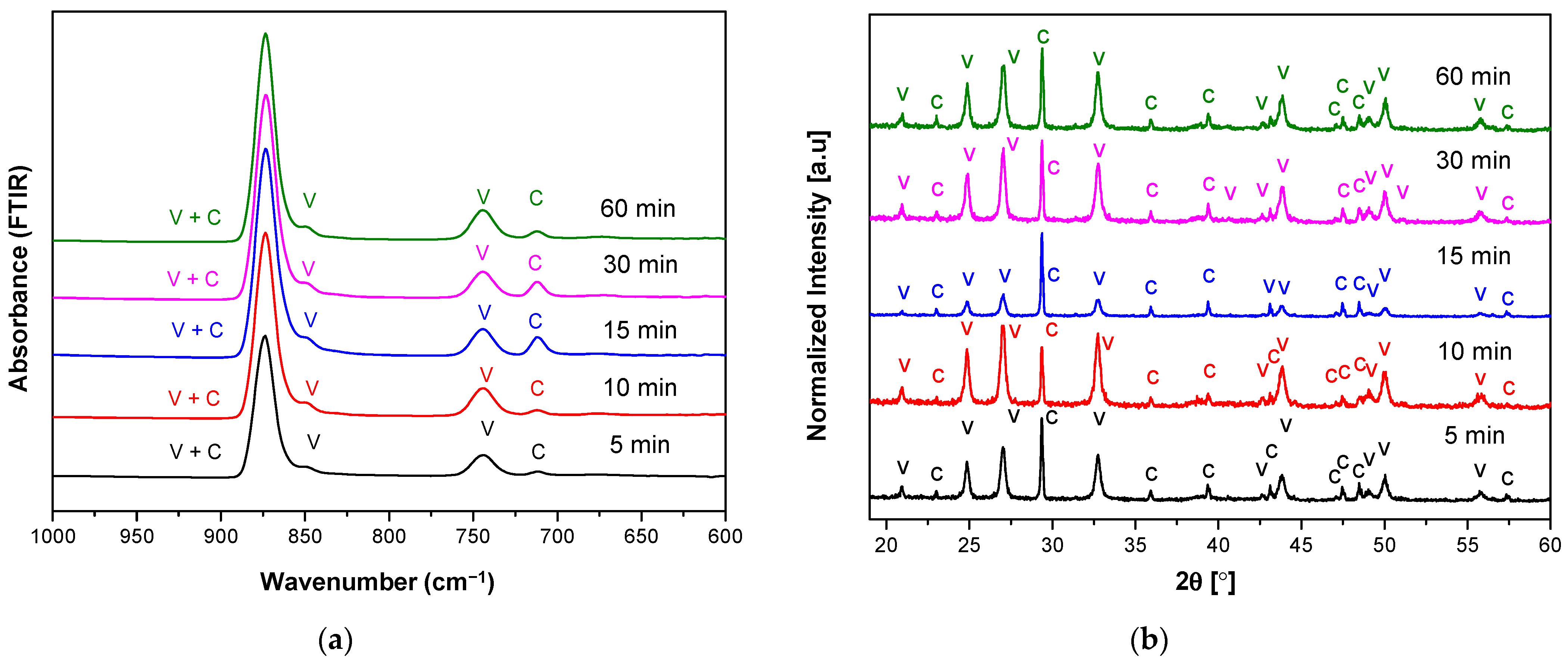
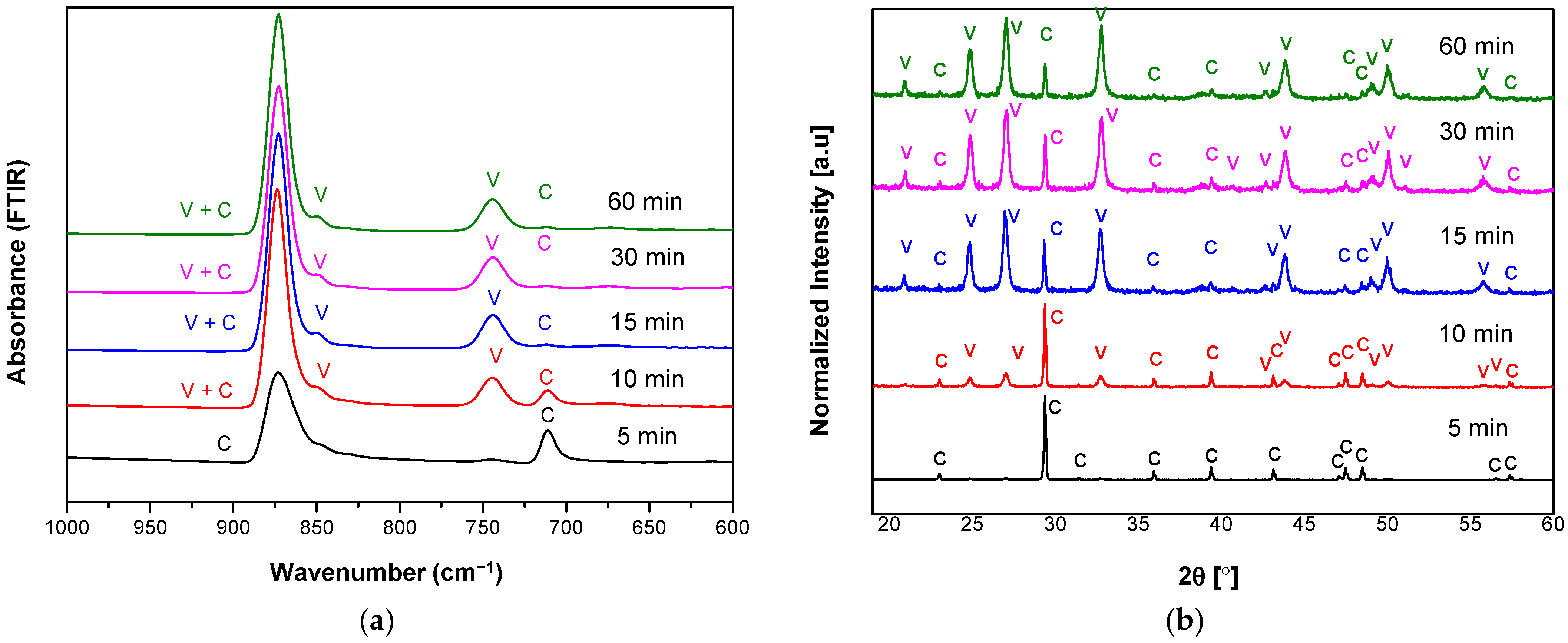
3.1. The Characteristics of Porous CaCO3 Microsphere
3.2. The Proposed Mechanism of Spherulitic Growth of CaCO3 Mesoporous
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maleki Dizaj, S.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.K.; Zakaria, M.Z.A.B.; Razak, I.S.B.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Oral, Ç.M.; Çalışkan, A.; Göçtü, Y.; Kapusuz, D.; Ercan, B. Synthesis of calcium carbonate microspheres via inert gas bubbling for orthopedic applications. Ceram. Int. 2020, 46, 3513–3522. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Hossein Zarrintan, M.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles; Potential in bone and tooth disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Effect of flow rate and CO2 content on the phase and morphology of CaCO3 prepared by bubbling method. J. Cryst. Growth 2005, 276, 541–548. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2015, 45, 644–658. [Google Scholar] [CrossRef]
- Ševčík, R.; Pérez-Estébanez, M.; Viani, A.; Šašek, P.; Mácová, P. Characterization of vaterite synthesized at various temperatures and stirring velocities without use of additives. Powder Technol. 2015, 284, 265–271. [Google Scholar] [CrossRef]
- Miyazaki, T.; Arii, T.; Shirosaki, Y. Control of crystalline phase and morphology of calcium carbonate by electrolysis: Effects of current and temperature. Ceram. Int. 2019, 45, 14039–14044. [Google Scholar] [CrossRef]
- Kogo, M.; Umegaki, T.; Kojima, Y. Effect of pH on formation of single-phase vaterite. J. Cryst. Growth 2019, 517, 35–38. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Takabait, F.; Mahtout, L.; Carrasco-Hurtado, B.; Eliche-Quesada, D.; Sánchez-Soto, P.J. Synthesis of vaterite CaCO3 as submicron and nanosized particles using inorganic precursors and sucrose in aqueous medium. Ceram. Int. 2018, 44, 5291–5296. [Google Scholar] [CrossRef]
- Yang, B.; Nan, Z. Abnormal polymorph conversion of calcium carbonate from calcite to vaterite. Mater. Res. Bull. 2012, 47, 521–526. [Google Scholar] [CrossRef]
- Cahyanto, A.; Maruta, M.; Tsuru, K.; Matsuya, S.; Ishikawa, K. Basic properties of carbonate apatite cement consisting of vaterite and dicalcium phosphate anhydrous. Key Eng. Mater. 2013, 529–530, 192–196. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, X.; Cao, W.; Zhou, G. Controlled synthesis and microstructure of metastable flower-like vaterite. Materials 2018, 11, 2300. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.C.; Yuan, X.; Xiao, Q.; Xiao, W.X.; Luo, Y.K.; Wang, X.L.; Song, F.; Wang, Y.Z. Facile batch synthesis of porous vaterite microspheres for high efficient and fast removal of toxic heavy metal ions. J. Environ. Chem. Eng. 2017, 5, 4505–4515. [Google Scholar] [CrossRef]
- Nehrke, G.; Van Cappellen, P. Framboidal vaterite aggregates and their transformation into calcite: A morphological study. J. Cryst. Growth 2006, 287, 528–530. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, L. Controllable synthesis of calcium carbonate polymorphs at different temperatures. Powder Technol. 2009, 189, 64–69. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Tao, K.; Zhao, K.; Wang, J.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Influence of ovalbumin on CaCO3 precipitation during in vitro biomineralization. J. Phys. Chem. B 2010, 114, 5301–5308. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.-Y.; Chia, C.-H.; Zakaria, S. Polymorphs calcium carbonate on temperature reaction. In Proceedings of the AIP Conference Proceedings, Surakarta, Indonesia, 12 May 2014; Volume 1614, pp. 52–56. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Y.; Chen, C.; Wang, X.; Zhao, H.; Xu, S.; Yang, C.C.; Xiao, B. A novel route to prepare the metastable vaterite phase of CaCO3 from CaCl2 ethanol solution and Na2CO3 aqueous solution. Adv. Powder Technol. 2018, 29, 2416–2422. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. Biochem. Pharmacol. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Kirboga, S.; Oner, M. Effect of the experimental parameters on calcium carbonate precipitation. J. Environ. Chem. Eng. Trans. 2013, 32, 2119–2124. [Google Scholar] [CrossRef]
- Shivkumara, C.; Singh, P.; Gupta, A.; Hegde, M.S. Synthesis of vaterite CaCO3 by direct precipitation using glycine and l-alanine as directing agents. Mater. Res. Bull. 2006, 41, 1455–1460. [Google Scholar] [CrossRef]
- Pan, L.; Li, Q.; Zhou, Y.; Song, N.; Yu, L.; Wang, X.; Xiong, K.; Yap, L.; Huo, J. Effects of different calcium sources on the mineralization and sand curing of CaCO3 by carbonic anhydrase-producing bacteria. RSC Adv. 2019, 9, 40827–40834. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Türk, S.; Altınsoy, İ.; ÇelebiEfe, G.; İpek, M.; Özacar, M.; Bindal, C. Microwave–assisted biomimetic synthesis of hydroxyapatite using different sources of calcium. Mater. Sci. Eng. C 2017, 76, 528–535. [Google Scholar] [CrossRef]
- Vagenas, N. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Impact Crystal; MATCH! Version 3.7.0.124 [Computer Software]. 2018. Available online: https://www.crystalimpact.com/match/download_previous_version.htm (accessed on 6 August 2021).
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Stávek, J.; Šípek, M.; Hirasawa, I.; Toyokura, K. Controlled double-jet precipitation of sparingly soluble salts. A method for the preparation of high added value materials. Chem. Mater. 1992, 4, 545–555. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, H.; Zhou, Y.; Huang, Y.; Yang, H.; Wang, D.; Xu, D. Properties of amorphous calcium carbonate and the template action of vaterite spheres. J. Phys. Chem. B 2006, 110, 2994–3000. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Pouget, E.M.; Bomans, P.H.H.; Dey, A.; Frederik, P.M.; De With, G.; Sommerdijk, N.A.J.M. The development of morphology and structure in hexagonal vaterite. J. Am. Chem. Soc. 2010, 132, 11560–11565. [Google Scholar] [CrossRef]
- Andreassen, J.P. Formation mechanism and morphology in precipitation of vaterite-Nano-aggregation or crystal growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.D.; Roncal-Herrero, T.; Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the vaterite CaCO3 crystal pores. Design of tailor-made polymer based microcapsules by hard templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.; Andreassen, J.P. Spherulitic growth of calcium carbonate. Cryst. Growth Des. 2010, 10, 2934–2947. [Google Scholar] [CrossRef]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. Growth and form of spherulites. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2005, 72, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bałdyga, J. Mixing and fluid dynamics effects in particle precipitation processes. KONA Powder Part. J. 2016, 2016, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Vacassy, R.; Lemaître, J.; Hofmann, H.; Gerlings, J.H. Calcium carbonate precipitation using new segmented flow tubular reactor. AIChE J. 2000, 46, 1241–1252. [Google Scholar] [CrossRef]
- Song, X.; Liu, H.; Wang, J.; Cao, Y.; Luo, X. A study of the effects of NH4+ on the fast precipitation of vaterite CaCO3 formed from steamed ammonia liquid waste and K2CO3/Na2CO3. CrystEngComm 2021, 23, 4284–4300. [Google Scholar] [CrossRef]

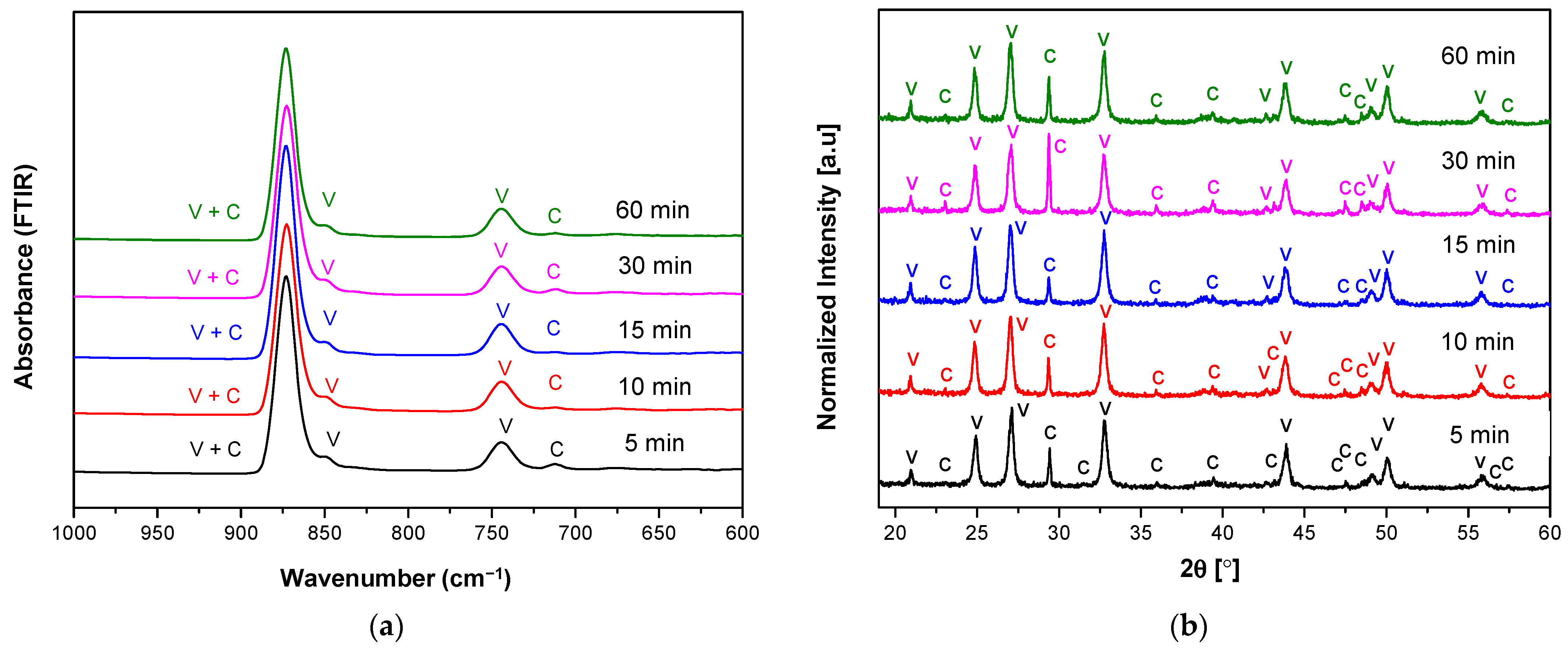
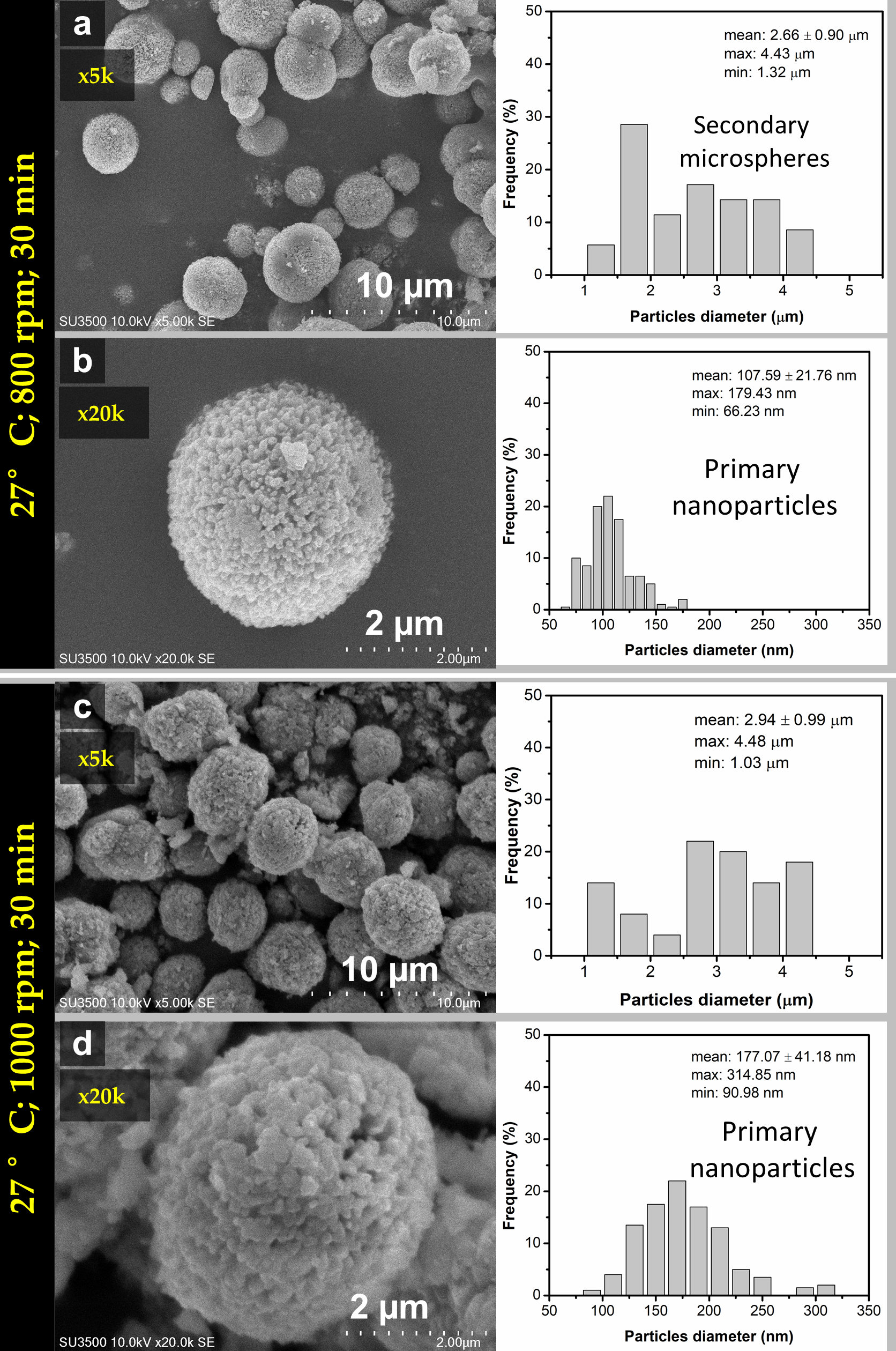
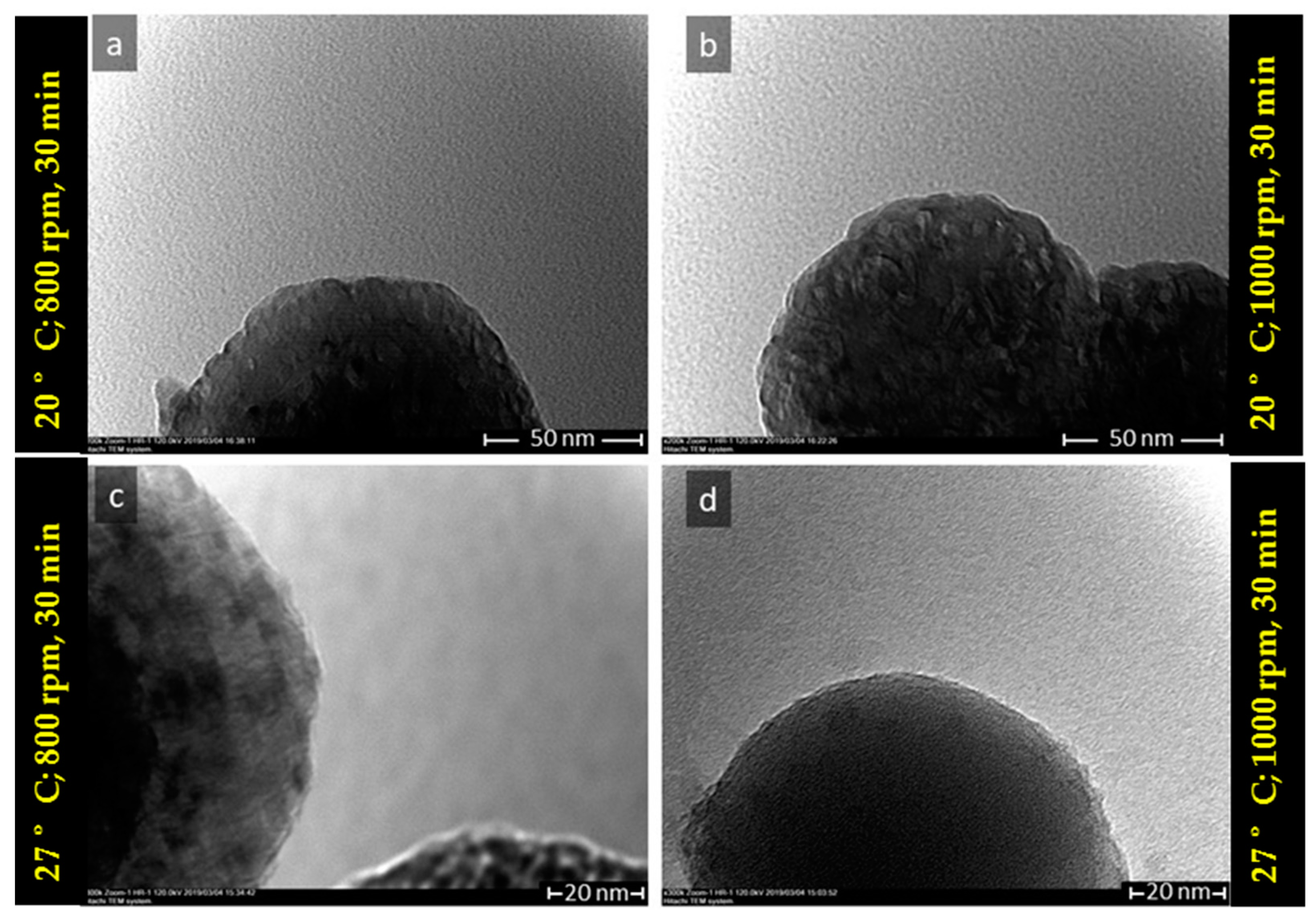

| No. | Vaterite | Calcite | ||
|---|---|---|---|---|
| hkl | 2Theta [deg] | hkl | 2Theta [deg] | |
| 1 | 002 | 20.89 | 104 | 29.32 |
| 2 | 100 | 24.82 | 110 | 35.90 |
| 3 | 101 | 26.99 | 113 | 39.36 |
| 4 | 102 | 32.72 | 018 | 47.47 |
| 5 | 110 | 43.71 | 116 | 48.43 |
| 6 | 112 | 49.07 | 122 | 57.37 |
| 7 | 104 | 50.06 | - | - |
| 8 | 202 | 55.74 | - | - |
| Sample Name | Reaction Time (min) | Calcite (%) | Vaterite (%) | Crystallite Size (nm) |
|---|---|---|---|---|
| R05 T20 S800 | 5 | 8.2 | 91.8 | 26.02 |
| R10 T20 S800 | 10 | 37.8 | 62.2 | 27.18 |
| R15 T20 S800 | 15 | 2.9 | 97.1 | 23.90 |
| R30 T20 S800 | 30 | 4.7 | 95.3 | 23.91 |
| R60 T20 S800 | 60 | 12.7 | 87.3 | 24.83 |
| Mean = 25.17 ± 1.42 |
| Sample Name | Reaction Time (min) | Calcite (%) | Vaterite (%) | Crystallite Size (nm) |
|---|---|---|---|---|
| R05 T20 S1000 | 5 | 19.0 | 81.0 | 24.16 |
| R10 T20 S1000 | 10 | 13.5 | 86.5 | 27.82 |
| R15 T20 S1000 | 15 | 36.3 | 63.7 | 25.07 |
| R30 T20 S1000 | 30 | 17.8 | 82.2 | 26.34 |
| R60 T20 S1000 | 60 | 14.4 | 85.6 | 27.57 |
| Mean = 26.19 ± 1.58 |
| Sample Name | Reaction Time (min) | Calcite (%) | Vaterite (%) | Crystallite Size (nm) |
|---|---|---|---|---|
| R05 T27 S800 | 5 | 100 | 0 | 29.94 |
| R10 T27 S800 | 10 | 46.3 | 53.7 | 26.25 |
| R15 T27 S800 | 15 | 8.1 | 91.9 | 26.00 |
| R30 T27 S800 | 30 | 9.6 | 90.4 | 23.92 |
| R60 T27 S800 | 60 | 5.9 | 94.1 | 25.08 |
| Mean = 26.24 ± 2.26 |
| Sample Name | Reaction Time (min) | Calcite (%) | Vaterite (%) | Crystallite Size (nm) |
|---|---|---|---|---|
| R05 T27 S1000 | 5 | 7.5 | 92.5 | 25.11 |
| R10 T27 S1000 | 10 | 5.6 | 94.4 | 22.99 |
| R15 T27 S1000 | 15 | 4.3 | 95.7 | 24.33 |
| R30 T27 S1000 | 30 | 6.5 | 93.5 | 22.58 |
| R60 T27 S1000 | 60 | 6.8 | 93.2 | 23.90 |
| Mean = 23.78 ± 1.02 |
| Sample | Specific Surface Area (m2/g) | Pore Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter Dv [d] (nm) |
|---|---|---|---|---|
| T20 SS800 | 10.853 | 20.640 | 0.036 | 3.5578 |
| T20 SS1000 | 4.767 | 13.093 | 0.030 | 3.5731 |
| T27 SS800 | 10.331 | 20.715 | 0.042 | 3.5757 |
| T27 SS1000 | 7.721 | 13.724 | 0.026 | 3.5801 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Febrida, R.; Cahyanto, A.; Herda, E.; Muthukanan, V.; Djustiana, N.; Faizal, F.; Panatarani, C.; Joni, I.M. Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method. Materials 2021, 14, 4425. https://doi.org/10.3390/ma14164425
Febrida R, Cahyanto A, Herda E, Muthukanan V, Djustiana N, Faizal F, Panatarani C, Joni IM. Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method. Materials. 2021; 14(16):4425. https://doi.org/10.3390/ma14164425
Chicago/Turabian StyleFebrida, Renny, Arief Cahyanto, Ellyza Herda, Vanitha Muthukanan, Nina Djustiana, Ferry Faizal, Camellia Panatarani, and I Made Joni. 2021. "Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method" Materials 14, no. 16: 4425. https://doi.org/10.3390/ma14164425
APA StyleFebrida, R., Cahyanto, A., Herda, E., Muthukanan, V., Djustiana, N., Faizal, F., Panatarani, C., & Joni, I. M. (2021). Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method. Materials, 14(16), 4425. https://doi.org/10.3390/ma14164425







