Microstructure and Mechanical Properties of Wire Arc Additively Manufactured MoNbTaWTi High Entropy Alloys
Abstract
:1. Introduction
2. Experimental
Procedures
3. Results
3.1. Microstructure and Morphology
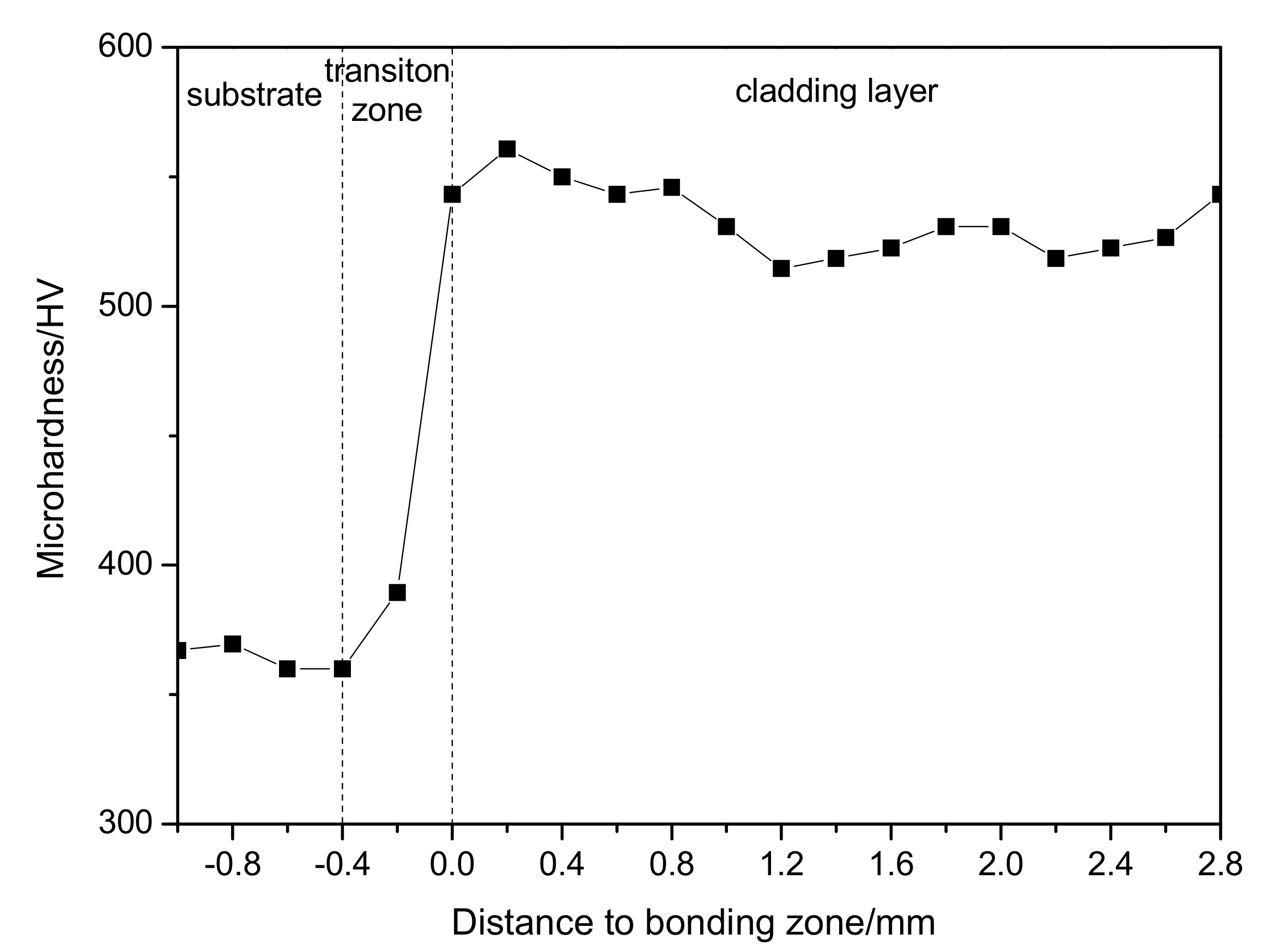
3.2. Hardness of WNbMoTaTi Surfacing Layer
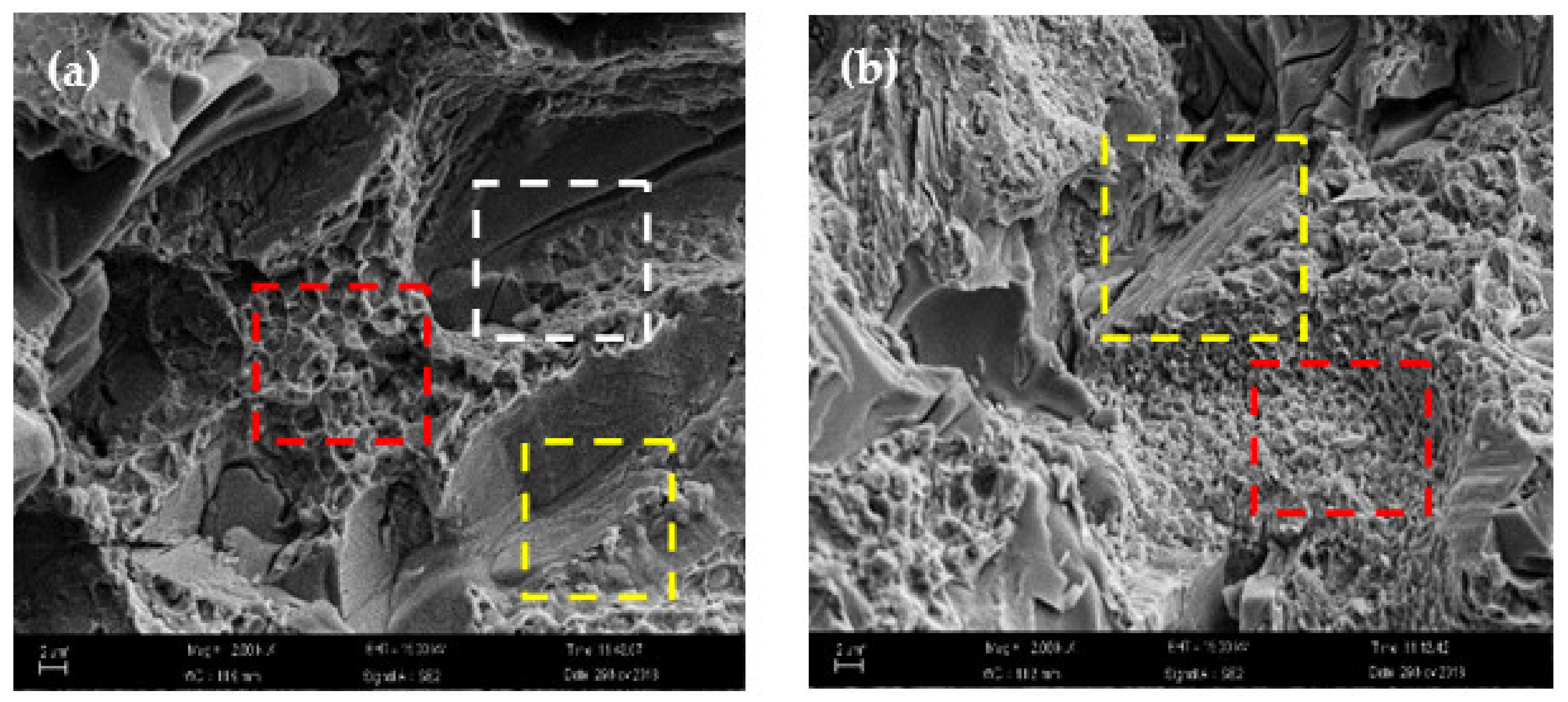
3.3. Compression Performance of WNbMoTaTi Surfacing Layer
3.4. Discussion
4. Conclusions
- (1)
- Cable-type welding wire (CTWW) is an effective way to solve the bottleneck that engineering preparation of HEAs wire is impossible to realize using traditional methods, and wire is helpful to achieve high efficiency, high quality and low-cost preparation of HEAs.
- (2)
- The overlaying formed layer of WNbMoTaTi showed a single disordered BCC solid solution phase structure with no obvious component segregation, but certain dendrite segregation occurred. The bottom structure of the overlaying formed layer was identified as a columnar cellular structure, and the upper part was a “cauliflower-like” fine dendrite and equiaxed crystal structure.
- (3)
- The serious lattice distortion and slow diffusion effect led to the high-temperature structural stability of the overlaying formed layer of WNbMoTaTi, and the single-phase BCC structure was preserved from 0 to 1400 °C.
- (4)
- The average hardness of the overlaying formed layer of WNbMoTaTi at room temperature was estimated to be about 533 HV0.2. At 1000 °C, the hardness fluctuated around 110 HV1, close to that of Inconel 718 alloy 125 HV1. The compressive strength was 602 MPa, much higher than that of Inconel 718 alloy (200 MPa) under the same conditions.
- (5)
- As the temperature increased from 500 to 1000 °C, the compressive strength of the overlaying formed layer of WNbMoTaTi changed from 629 MPa to 602 MPa, equivalent to only 27 MPa decrease. However, the fracture strain increased from 2.1 to 4.9%, respectively. High energy promoted the dynamic softening behavior and starting of dislocation slip system during compression deformation. The latter was the main reason why the overlaying formed layer of WNbMoTaTi maintained high strength at 1000 °C with greatly increased fracture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375-377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Lee, P.H. Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 2007; Volume 560, pp. 1–9. [Google Scholar]
- Lin, L. The Structure and Properties of Quinary High Entropy Alloys with High Melting Temperature; Harbin Institute of Technology: Shenzhen, China, 2007; pp. 1–63. [Google Scholar]
- Ji, Y.Z.; Zheng, Y.; Bao, C.G. Progress in equipment and melting techniques of vacuum arc furnace. Foundry Technol. 2008, 29, 827–829. [Google Scholar]
- Wu, H. Research on Microstructure and Properties of Tungsten High-Entropy Alloy; Shenyang University of Technology: Shenyang, China, 2017; pp. 1–54. [Google Scholar]
- Stepanov, N.D.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Aging behavior of the HfNbTaTiZr high entropy alloy. Mater. Lett. 2018, 211, 87–90. [Google Scholar] [CrossRef]
- Qu, H.Q. Process and Mechanical Properties of Refractory High-Entropy Alloy Coatings Prepared by Cable Welding Wires; HoHai University: Nanjing, China, 2019; pp. 1–16. [Google Scholar]
- Jayaraj, J.; Thirathipviwat, P.; Han, J.; Gebert, A. Microstructure, mechanical and thermal oxidation behavior of AlNbTiZr high entropy alloy. Intermetallics 2018, 10, 9–19. [Google Scholar] [CrossRef]
- Guo, W.M.; Liu, B.; Liu, Y.; Li, T.C. Microstructures and mechanical properties of ductile NbTaTiV refractory high entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2018, 5, 73–78. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Chen, J.; Fang, Q.; Liu, Y. Ultra-High Strength TiC/Refractory High-Entropy-Alloy Composite Prepared by Powder Metallurgy. JOM 2017, 69, 651–656. [Google Scholar] [CrossRef]
- Feng, X.B.; Zhang, J.Y.; Xia, Z.R. Stable nanocrystalline NbMoTaW high entropy alloythin films with excellent mechanical and electrical properties. Mater. Lett. 2018, 2, 84–87. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)Nx coatings by reactive magnetron sputtering. Thin Solid Film. 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Guo, W. Microsturcture and Mechanical Properties of Ni MoTaW(V) High-Entropy Alloy Prepared by Mechanical Alloying; South China University of Technology: Guangzhou, China, 2016; pp. 1–50. [Google Scholar]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Microstructure, mechanical property and Hall-Petch relationship of a light-weight refractory Al0.1CrNbVMo high entropy alloy fabricated by powder metallurgical process. J. Alloys Compd. 2018, 8, 32–36. [Google Scholar] [CrossRef]
- Waseem, O.A.; Lee, J.; Lee, H.M.; Ryu, H.J. The effect of Ti on the sintering and mechanical properties of refractory high-entropy alloy TixWTaVCr fabricated via spark plasmasintering for fusion plasma-facing materials. Mater. Chem. Phys. 2017, 8, 1–8. [Google Scholar]
- Qi, P.B.; Liang, X.B.; Tong, Y.G.; Chen, Y.X.; Zhang, Z.B. Preparation and characterization of NbMoTaW High-entropy alloy coating. Appl. Laser 2018, 38, 66–70. [Google Scholar]
- Zhang, M.; Zhou, X.; Yu, X.; Li, J. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Q.B.; Zheng, B. Effect of silicon and aluminum on microsturcture and properties of MoFeCrTiW high-entropy alloy coating. China Laser 2016, 43, 52–59. [Google Scholar]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Me-chanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinié, J.P.; Perrière, L.; Bourgon, J.; Emery, N.; Guillot, I. New structure in refractory high-entropy alloys. Mater. Lett. 2014, 132, 123–125. [Google Scholar] [CrossRef]
- Juan, C.C.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Wang, W.R.; Yang, C.C.; Chen, S.K.; Lin, S.J.; Yeh, J.W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 6, 76–83. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-Transformation Ductilization of Brittle High-Entropy Alloys via Metastability Engineering. Adv. Mater. 2017, 35, 32–38. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef]
- Yao, J.Q.; Liu, X.W.; Gao, N.; Jiang, Q.H.; Li, N.; Liu, G.; Zhang, W.B.; Fan, Z.T. Phase stability of a ductile single-phase BCC Hf0.5Nb0.5Ta 0.5Ti1.5Zr refractory high-entropy alloy. Intermetallics 2018, 9, 79–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.B.; Yang, X. Advanced Technology in High-Entropy Alloys; Chemical Industry Press: Beijing, China, 2019. [Google Scholar]
- Tong, Y.G.; Bai, L.H.; Liang, X.B.; Chen, Y.X.; Zhang, Z.B.; Liu, J.; Li, Y.J.; Hu, Y.L. Influence of alloying elements on mechanical and electronic properties of NbMoTaWX (X = Cr, Zr, V, Hf and Re) refractory high entropy alloys. Intermetallics 2020, 126, 106928. [Google Scholar] [CrossRef]
- Guo, N. Microsturcture and Mechanical Properties of Mo-Ni-Hf-Zr-Ti Refractory High-Entropy Alloy; Harbin Institute of Technology: Shenzhen, China, 2016; pp. 1–27. [Google Scholar]



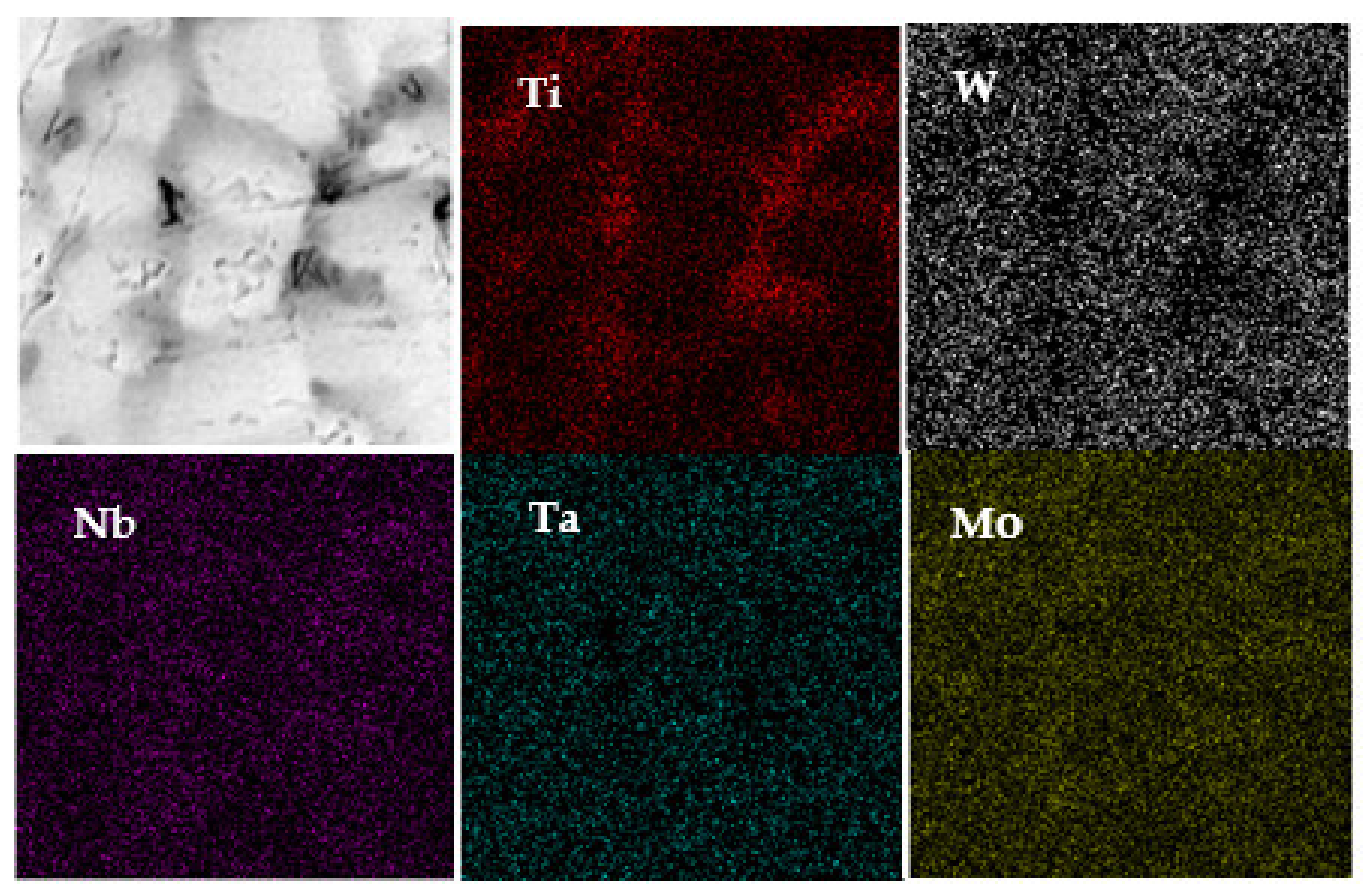



| W | Nb | Mo | Ta | Ti | ||
|---|---|---|---|---|---|---|
| Atomic percentage | Dendrites (white color) | 20.88 | 12.87 | 33.87 | 14.95 | 17.63 |
| Intergranular (dark color) | 8.45 | 15.31 | 30.64 | 9.08 | 36.52 | |
| Segregation rate SR | 0.40 | 1.19 | 0.90 | 0.61 | 2.07 |
| Element | W | Nb | Mo | Ta | Ti |
|---|---|---|---|---|---|
| W | 0 | −8 | 0 | −7 | 6 |
| Nb | - | 0 | −6 | 0 | 2 |
| Mo | - | - | 0 | −5 | −4 |
| Ta | - | - | - | 0 | 1 |
| Ti | - | - | - | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, J.; Du, X.; Tong, Y.; Wang, R.; He, D.; Cai, Z.; Wang, H. Microstructure and Mechanical Properties of Wire Arc Additively Manufactured MoNbTaWTi High Entropy Alloys. Materials 2021, 14, 4512. https://doi.org/10.3390/ma14164512
Liu J, Li J, Du X, Tong Y, Wang R, He D, Cai Z, Wang H. Microstructure and Mechanical Properties of Wire Arc Additively Manufactured MoNbTaWTi High Entropy Alloys. Materials. 2021; 14(16):4512. https://doi.org/10.3390/ma14164512
Chicago/Turabian StyleLiu, Jian, Jing Li, Xian Du, Yonggang Tong, Rui Wang, Dongyu He, Zhihai Cai, and Haidou Wang. 2021. "Microstructure and Mechanical Properties of Wire Arc Additively Manufactured MoNbTaWTi High Entropy Alloys" Materials 14, no. 16: 4512. https://doi.org/10.3390/ma14164512
APA StyleLiu, J., Li, J., Du, X., Tong, Y., Wang, R., He, D., Cai, Z., & Wang, H. (2021). Microstructure and Mechanical Properties of Wire Arc Additively Manufactured MoNbTaWTi High Entropy Alloys. Materials, 14(16), 4512. https://doi.org/10.3390/ma14164512








