Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Preparation of Chitosan/Eudragit E 100/AgNPs Coatings
2.3. Testing of the Microstructure and Morphology of the Coatings
2.4. Wettability Studies
2.5. Adhesion Studies
2.6. Corrosion Studies
2.7. Silver Release Study
3. Results and Discussion
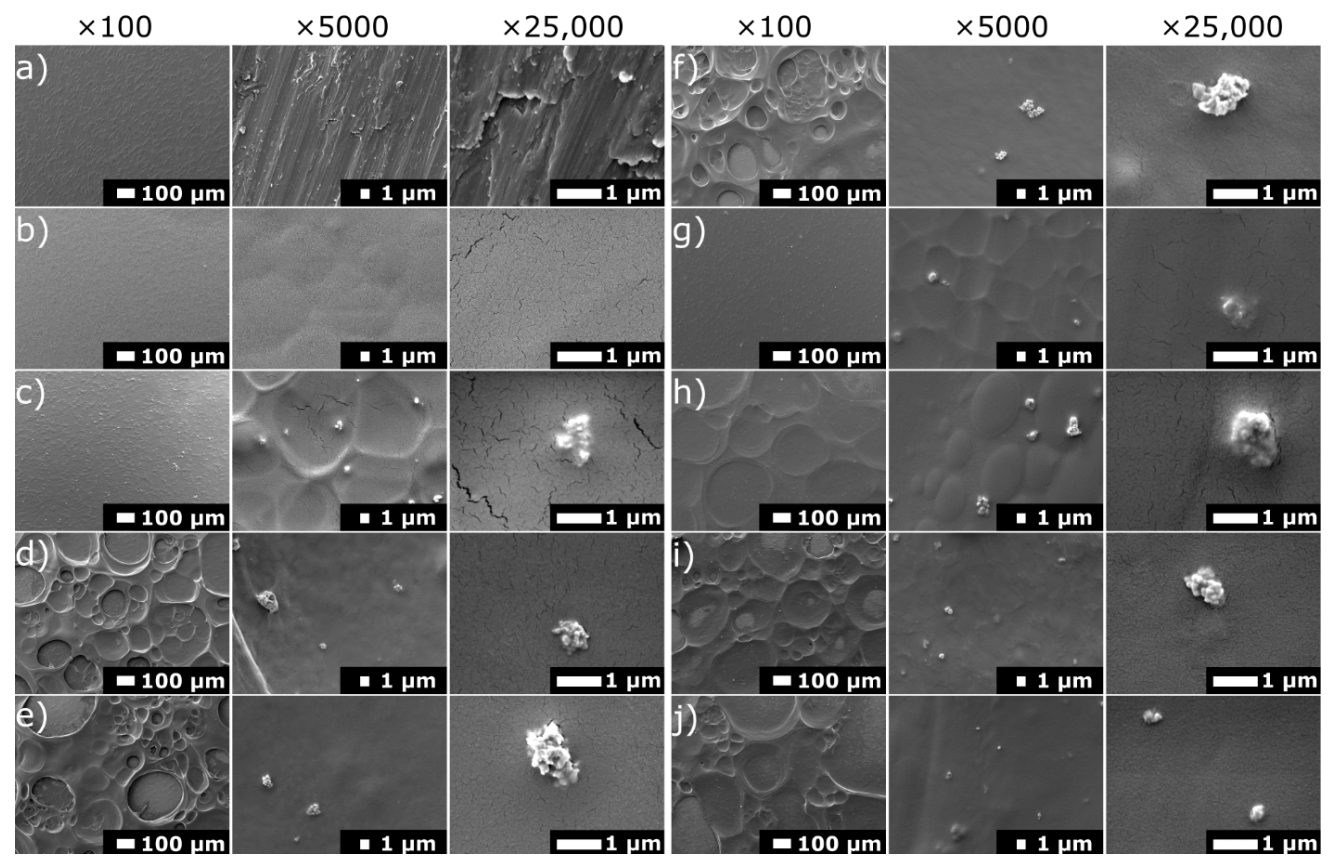
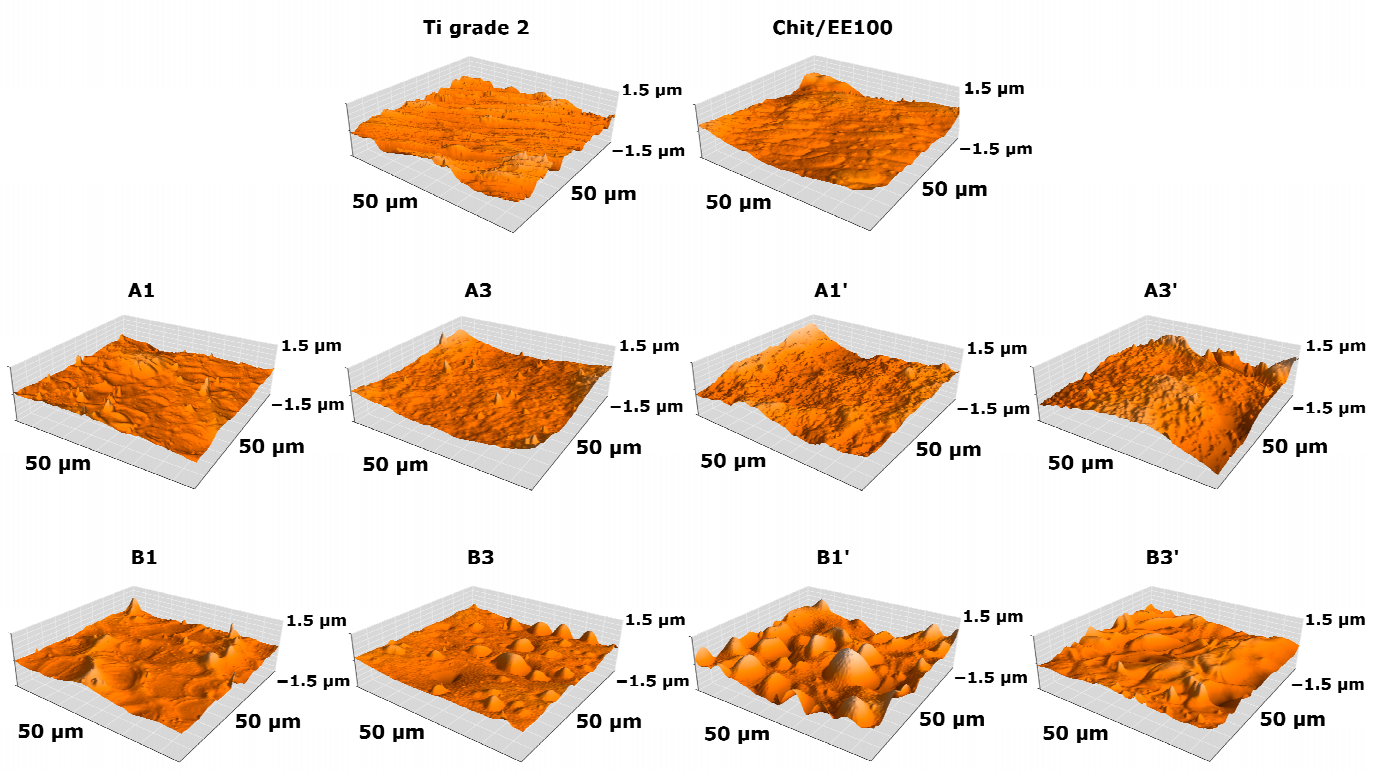
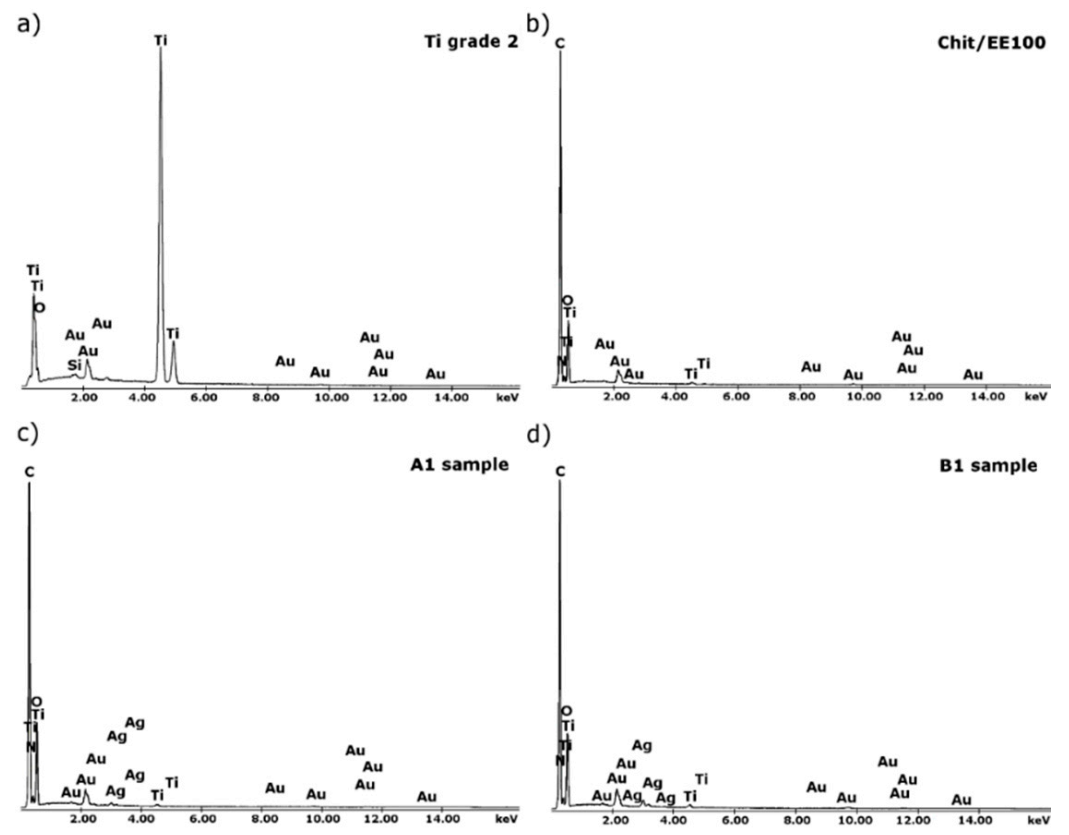
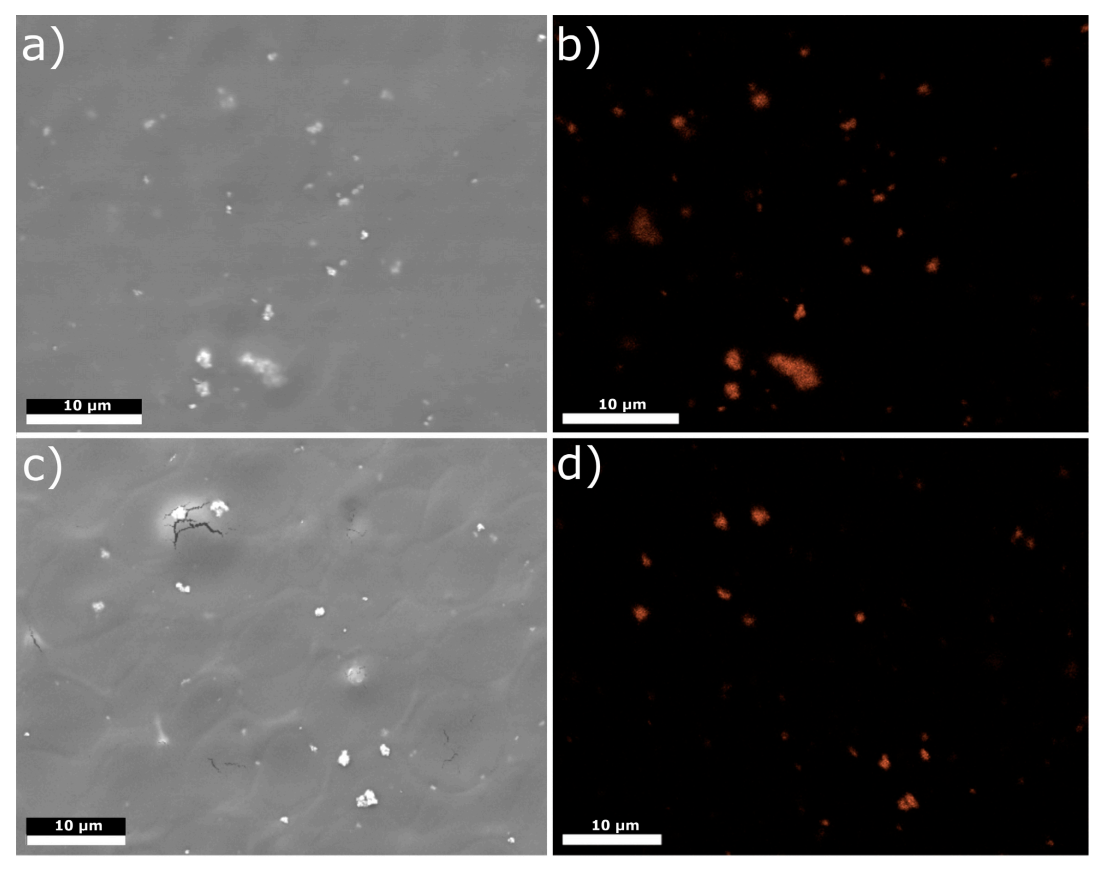
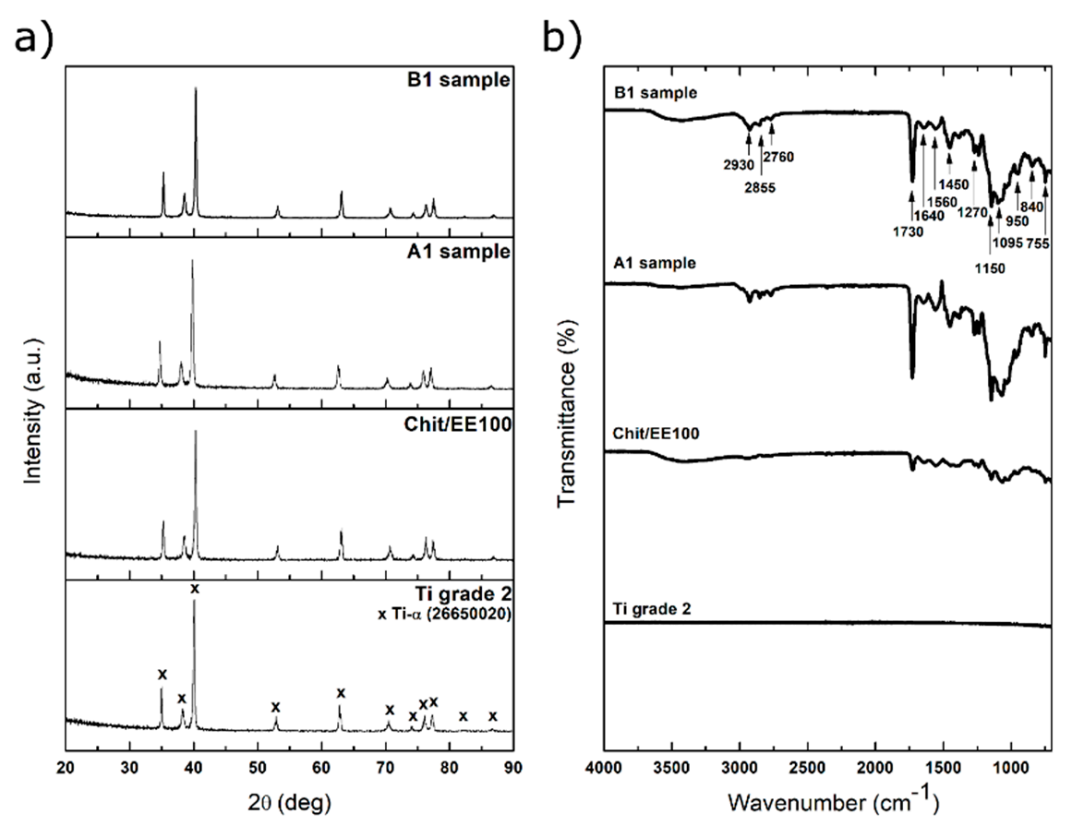
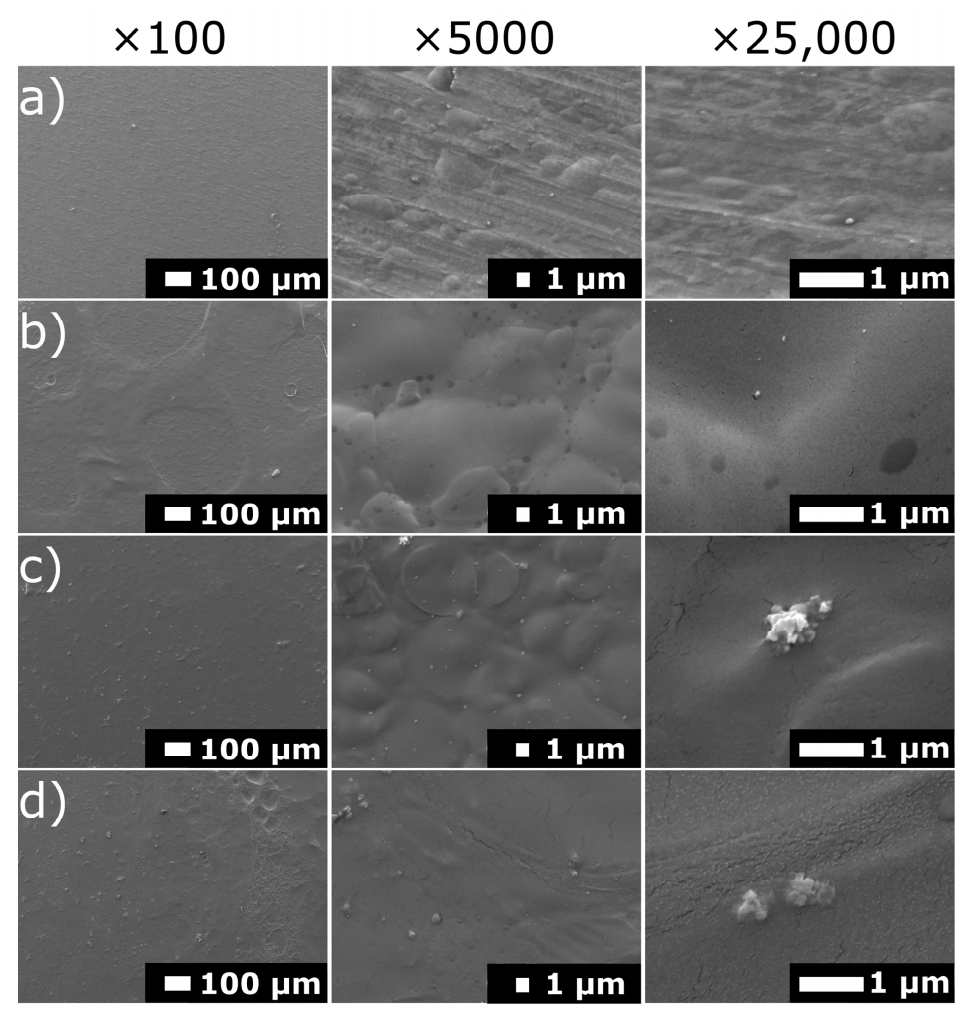
3.1. Structure and Morphology of Chitosan/Eudragit E 100/AgNPs Coatings
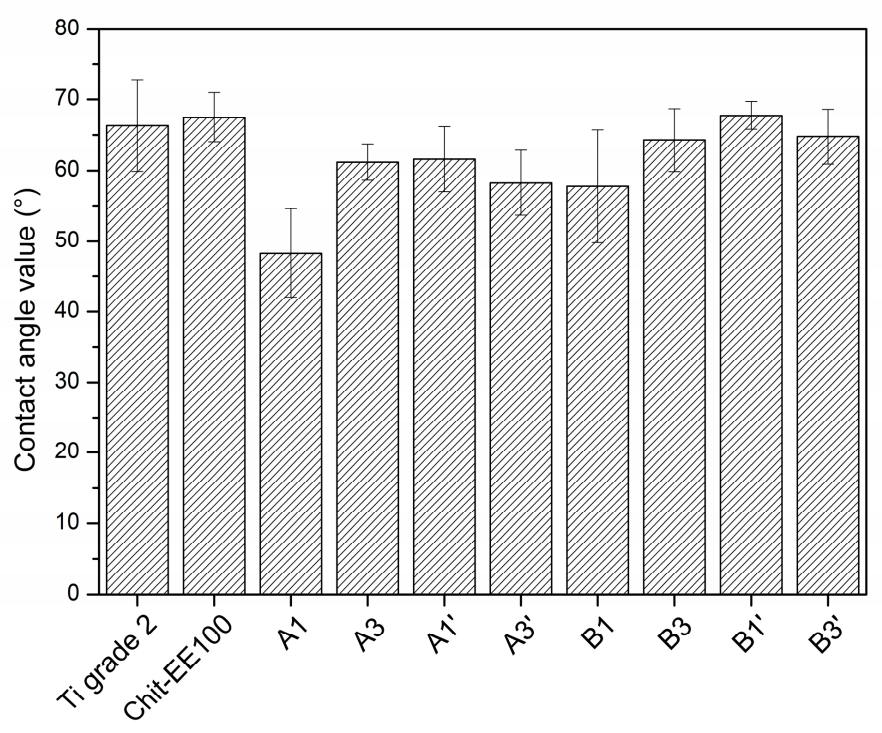
3.2. Wettability Studies
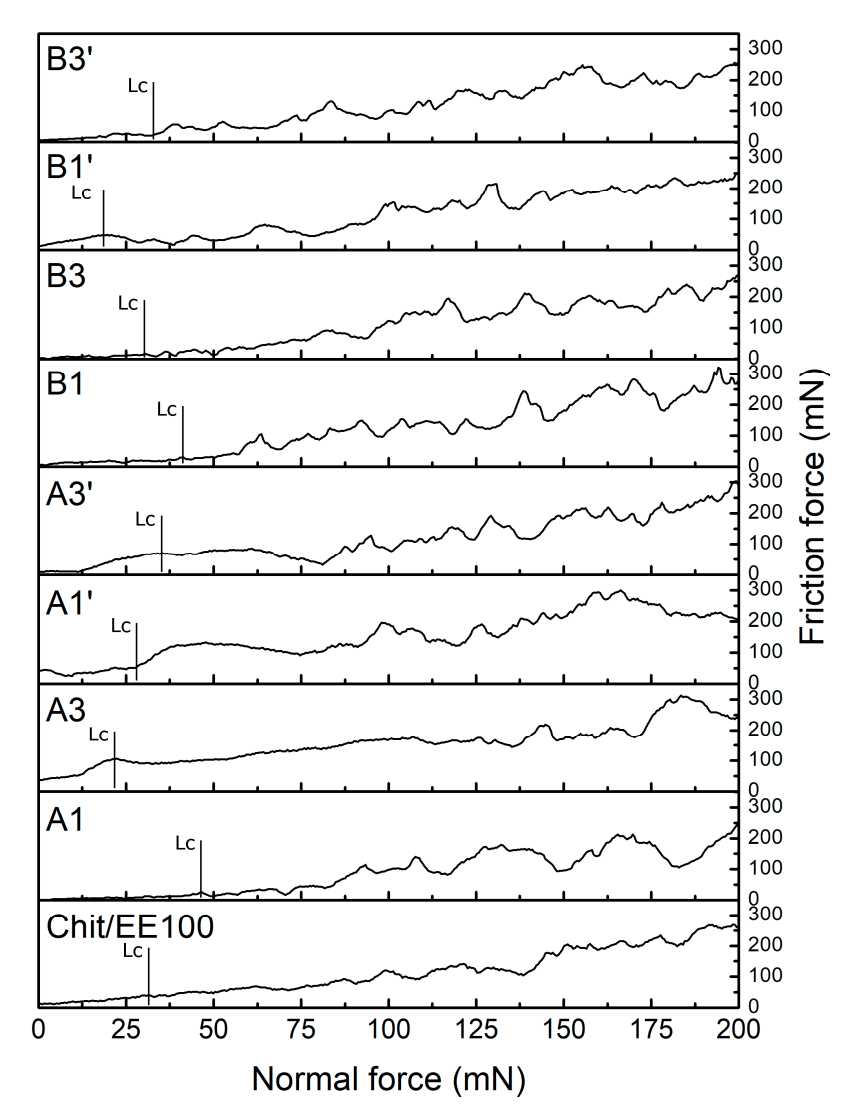
3.3. Mechanical Studies
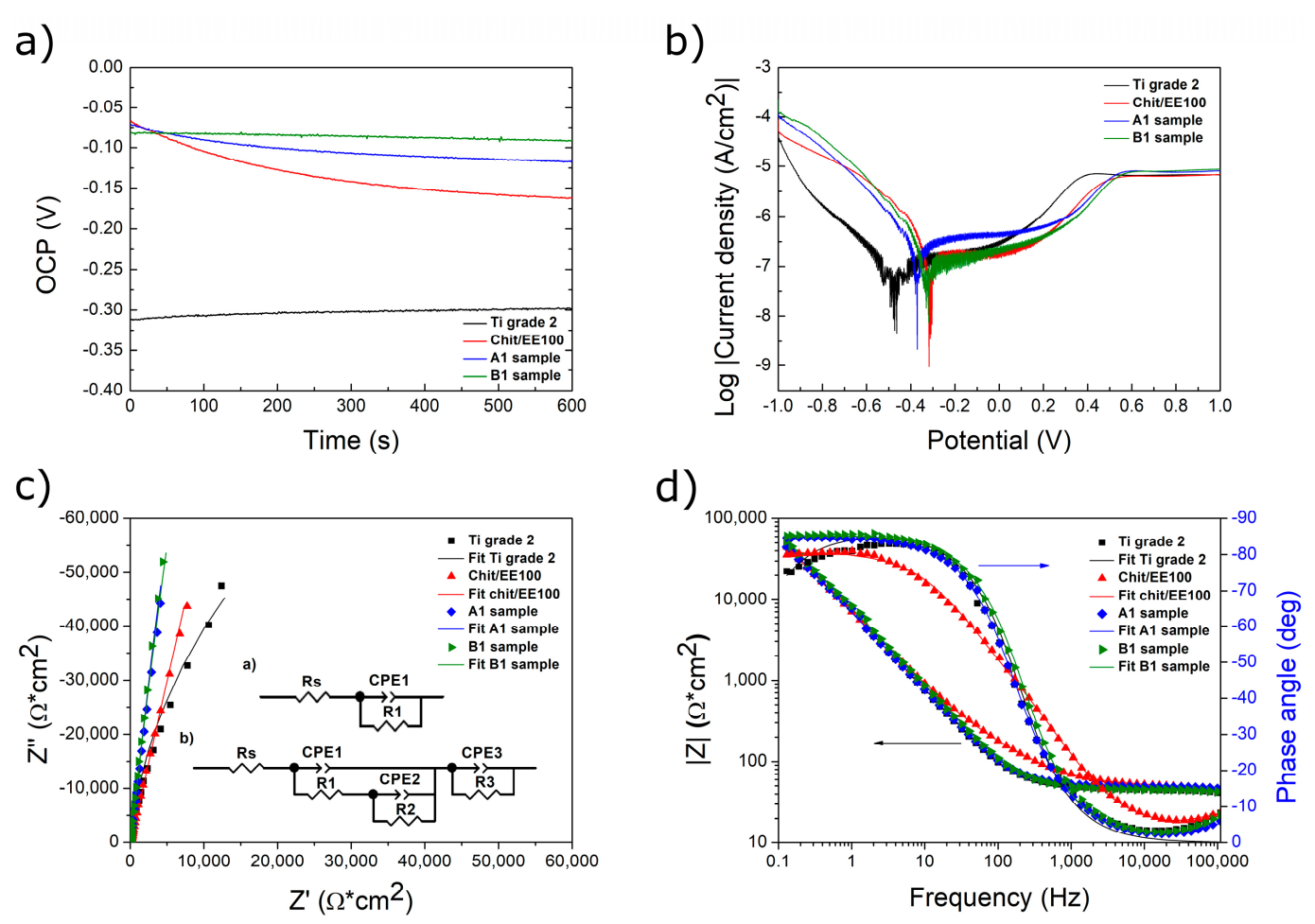
3.4. Corrosion Studies
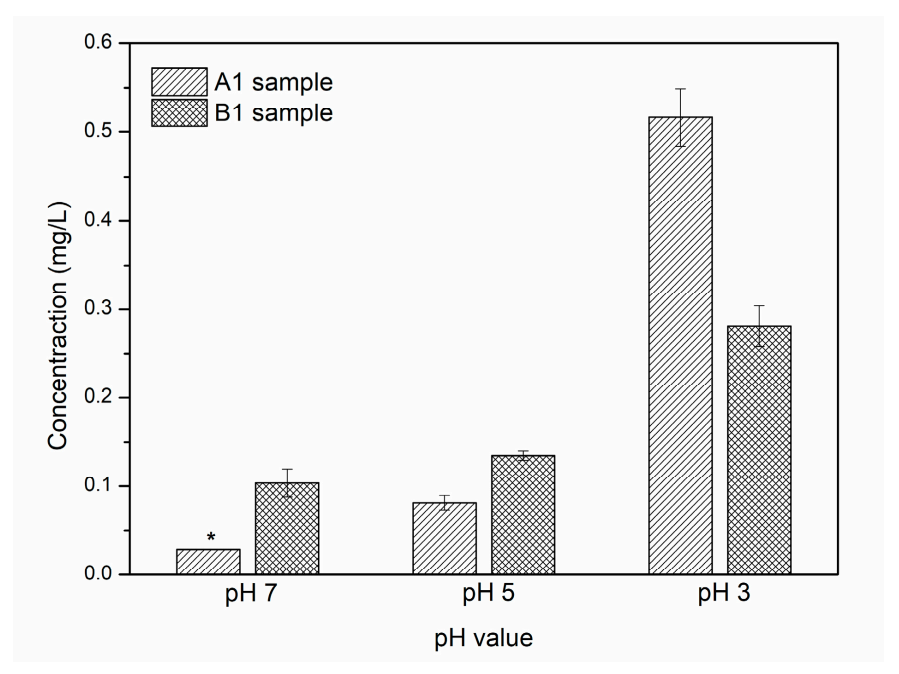
3.5. Silver Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Garcia de Carvalho, G.; Sanchez-Puetate, J.C.; Casalle, N.; Marcantonio Junior, E.; Leal Zandim-Barcelos, D. Antimicrobial photodynamic therapy associated with bone regeneration for peri-implantitis treatment: A case report. Photodiagn. Photodyn. Ther. 2020, 30, 101705. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implants Res. 2012, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.V.V.S.N.; Srihari, S.V.V.S. A Review on Surface Modifications and Coatings on Implants to Prevent Biofilm. Regen. Eng. Transl. Med. 2020, 6, 330–346. [Google Scholar]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl methacrylate modified chitosan: Synthesis, characterization and application in drug and gene delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, W.; Goudouri, O.M.; Ding, Y.; Cabanas-Polo, S.; Boccaccini, A.R. Electrophoretic deposition of antibiotic loaded PHBV microsphere-alginate composite coating with controlled delivery potential. Colloids Surf. B Biointerfaces 2015, 130, 199–206. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan-vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Pawłowski, Ł. pH-dependent composite coatings for controlled drug delivery system—Review. Inz. Mater. 2019, 1, 4–9. [Google Scholar] [CrossRef]
- Świeczko-Żurek, B.; Bartmański, M. Investigations of Titanium Implants Covered with Hydroxyapatite Layer. Adv. Mater. Sci. 2016, 16, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. PH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Doerdelmann, G.; Kozlova, D.; Epple, M. A pH-sensitive poly(methyl methacrylate) copolymer for efficient drug and gene delivery across the cell membrane. J. Mater. Chem. B 2014, 2, 7123–7131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart Biopolymers and their Biomedical Applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Chen, Q.; Hao, T.; Qiao, M.; Zhao, H.; Zhang, J.; Hu, H.; Zhao, X.; Chen, D.; et al. pH-sensitive nanoparticles of poly(L-histidine)-poly(lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef]
- Kofuji, K.; Qian, C.J.; Nishimura, M.; Sugiyama, I.; Murata, Y.; Kawashima, S. Relationship between physicochemical characteristics and functional properties of chitosan. Eur. Polym. J. 2005, 41, 2784–2791. [Google Scholar] [CrossRef]
- Wang, J.; Law, W.C.; Chen, L.; Chen, D.; Tang, C.Y. Fabrication of monodisperse drug-loaded poly(lactic-co-glycolic acid)–chitosan core-shell nanocomposites via pickering emulsion. Compos. Part B Eng. 2017, 121, 99–107. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Taurino, R.; Sciancalepore, C.; Collini, L.; Bondi, M.; Bondioli, F. Functionalization of PVC by chitosan addition: Compound stability and tensile properties. Compos. Part B Eng. 2018, 149, 240–247. [Google Scholar] [CrossRef]
- Jugowiec, D.; Kot, M.; Moskalewicz, T. Electrophoretic deposition and characterisation of chitosan coatings on near-β titanium alloy. Arch. Metall. Mater. 2016, 61, 657–664. [Google Scholar] [CrossRef]
- Ahmed, R.A.; Fadl-Allah, S.A.; El-Bagoury, N.; El-Rab, S.M.F.G. Improvement of corrosion resistance and antibacterial effect of NiTi orthopedic materials by chitosan and gold nanoparticles. Appl. Surf. Sci. 2014, 292, 390–399. [Google Scholar] [CrossRef]
- Ordikhani, F.; Simchi, A. Long-term antibiotic delivery by chitosan-based composite coatings with bone regenerative potential. Appl. Surf. Sci. 2014, 317, 56–66. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Bartmański, M.; Strugała, G.; Mielewczyk-Gryń, A.; Jażdżewska, M.; Zieliński, A. Electrophoretic Deposition and Characterization of Chitosan/Eudragit E 100 Coatings on Titanium Substrate. Coatings 2020, 10, 607. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Designing, preparation and evaluation of the antimicrobial activity of biomaterials based on chitosan modified with silver nanoparticles. Int. J. Biol. Macromol. 2020, 151, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Salem, R.B.S.; Hamdi, M.; Jellouli, K.; Ayadi, W.; Nasri, M.; Boufi, S. Nanocomposite films based on chitosan–poly(vinyl alcohol) and silver nanoparticles with high antibacterial and antioxidant activities. Process. Saf. Environ. Prot. 2017, 111, 112–121. [Google Scholar] [CrossRef]
- Rubina, M.S.; Elmanovich, I.V.; Shulenina, A.V.; Peters, G.S.; Svetogorov, R.D.; Egorov, A.A.; Naumkin, A.V.; Vasil’kov, A.Y. Chitosan aerogel containing silver nanoparticles: From metal-chitosan powder to porous material. Polym. Test. 2020, 86, 106481. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieslik, B. Electrophoretic deposition and characteristics of chitosan/nanosilver composite coatings on the nanotubular TiO 2layer. Coatings 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Biological Evaluation of Medical Devices—Part 15: Identification and Quantification of Degradation Products from Metals and Alloys; PN EN ISO 10993-15: 2009; International Organization for Standardization ISO Central Secretariat: Geneva, Switzerland, 2009.
- Dias, C.I.; Mano, J.F.; Alves, N.M. PH-Responsive biomineralization onto chitosan grafted biodegradable substrates. J. Mater. Chem. 2008, 18, 2493–2499. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.S.; Hwang, M.J.; Jeong, H.N.; Lee, W.Y.; Song, H.J.; Park, Y.J. Evaluation of surface mechanical properties and grindability of binary Ti alloys containing 5 wt % Al, Cr, Sn, and V. Metals 2017, 7, 487. [Google Scholar] [CrossRef] [Green Version]
- Anderson Machado Oliveira, A.; Costa de Santana, R.A.; de Oliveira Wanderley Neto, A. Electrophoretic deposition and characterization of chitosan-molybdenum composite coatings. Carbohydr. Polym. 2020, 255, 117382. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Z.; Zhou, Y.; Liu, Y.; Wang, Z.; Tong, H.; Shen, X.; Wang, Y. Surface functionalization of titanium with chitosan/gelatin via electrophoretic deposition: Characterization and cell behavior. Biomacromolecules 2010, 11, 1254–1260. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; Salzano de Luna, M.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-based coatings for corrosion protection of copper-based alloys: A promising more sustainable approach for cultural heritage applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Gebhardt, F.; Seuss, S.; Turhan, M.C.; Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Characterization of electrophoretic chitosan coatings on stainless steel. Mater. Lett. 2012, 66, 302–304. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of chitosan in different alcohols. J. Coat. Technol. Res. 2014, 11, 739–746. [Google Scholar] [CrossRef]
- Pawlik, A.; Rehman, M.A.U.; Nawaz, Q.; Bastan, F.E.; Sulka, G.D.; Boccaccini, A.R. Fabrication and characterization of electrophoretically deposited chitosan-hydroxyapatite composite coatings on anodic titanium dioxide layers. Electrochim. Acta 2019, 307, 465–473. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, X.; Yao, Y.; Luo, J.; Tang, Q.; Wu, H.; Lin, S.; Han, C.; Wei, Q.; Chen, L. Electrophoretic deposition of chitosan/gelatin coatings with controlled porous surface topography to enhance initial osteoblast adhesive responses. J. Mater. Chem. B 2016, 4, 7584–7595. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Liang, J.; Cheng, J.; Ma, W.; Zhao, Y. Staphylococcus aureus adhesion to different implant surface coatings: An in vitro study. Surf. Coat. Technol. 2009, 203, 3454–3458. [Google Scholar] [CrossRef]
- Ferreira Soares, P.B.; Moura, C.C.G.; Claudino, M.; Carvalho, V.F.; Rocha, F.S.; Zanetta-Barbosa, D. Influence of implant surfaces on osseointegration: A histomorphometric and implant stability study in rabbits. Braz. Dent. J. 2015, 26, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Dudek, K.; Dulski, M.; Goryczka, T.; Gerle, A. Structural changes of hydroxyapatite coating electrophoretically deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 11292–11300. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Jang, Y.S.; Kim, J.H.; Lee, M.H. Improvement of osteogenesis by a uniform PCL coating on a magnesium screw for biodegradable applications. Sci. Rep. 2018, 8, 13264. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ. Sci. Technol. 2013, 47, 10293–10301. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B. Synthesis of silver nanoparticles: A safer alternative to conventional antimicrobial and antibacterial agents Amrut. J. Chem. Pharm. Res. 2011, 3, 287–294. [Google Scholar]
- Balagani, P.G.A. Formulation and evaluation of nizatidine solid dispersions. World J. Pharm. Pharm. Sci. 2015, 4, 810–817. [Google Scholar]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz. Oral Res. 2012, 26, 505–510. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Gao, H.; Gilabert, J.; Sanchez, E.; Roether, J.A.; Schubert, D.W.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of nanostructured-TiO2/chitosan composite coatings on stainless steel. RSC Adv. 2013, 3, 11247–11254. [Google Scholar] [CrossRef] [Green Version]
- Brohede, U.; Zhao, S.; Lindberg, F.; Mihranyan, A.; Forsgren, J.; Strømme, M.; Engqvist, H. A novel graded bioactive high adhesion implant coating. Appl. Surf. Sci. 2009, 255, 7723–7728. [Google Scholar] [CrossRef]
- Stevanović, M.; Došić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Crevar Sakač, M.; Rhee, K.Y.; Misković-Stanković, V. Gentamicin-Loaded Bioactive Hydroxyapatite/Chitosan Composite Coating Electrodeposited on Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef]
- Barnes, D.; Johnson, S.; Snell, R.; Best, S. Using scratch testing to measure the adhesion strength of calcium phosphate coatings applied to poly(carbonate urethane) substrates. J. Mech. Behav. Biomed. Mater. 2012, 6, 128–138. [Google Scholar] [CrossRef]
- Kutilek, P.; Miksovsky, J. The procedure of evaluating the practical adhesion strength of new biocompatible nano-and micro-thin films in accordance with international standards. Acta Bioeng. Biomech. Orig. Pap. 2011, 13, 87–94. [Google Scholar]
- Lei, W.S.; Mittal, K.; Yu, Z. Adhesion measurement of coatings on biodevices/implants: A critical review. Rev. Adhes. Adhes. 2016, 4, 367–396. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Maszybrocka, J.; Kubisztal, J.; Skrabalak, G.; Stwora, A. Corrosion resistance of the cpti g2 cellular lattice with tpms architecture for gas diffusion electrodes. Materials 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Savencu, C.E.; Costea, L.V.; Dan, M.L.; Porojan, L. Corrosion behaviour of Co-Cr dental alloys processed by alternative CAD/CAM technologies in artificial saliva solutions. Int. J. Electrochem. Sci. 2018, 13, 3588–3600. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the silver nanoparticles (AgNPs) formation conditions onto titanium dioxide (TiO2) nanotubes based electrodes on their impedimetric response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef] [Green Version]
- Tabesh, E.; Salimijazi, H.R.; Kharaziha, M.; Mahmoudi, M.; Hejazi, M. Development of an in-situ chitosan-copper nanoparticle coating by electrophoretic deposition. Surf. Coat. Technol. 2019, 364, 239–247. [Google Scholar] [CrossRef]
- Ng, K.H.; Liu, H.; Penner, R.M. Subnanometer Silver Clusters Exhibiting Unexpected Electrochemical Metastability on Graphite. Langmuir 2000, 16, 4016–4023. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Kazempour, A. Chitosan, its derivatives and composites with superior potentials for the corrosion protection of steel alloys: A comprehensive review. Carbohydr. Polym. 2020, 237, 116110. [Google Scholar] [CrossRef]
- Boeris, V.; Romanini, D.; Farruggia, B.; Picó, G. Interaction and complex formation between catalase and cationic polyelectrolytes: Chitosan and Eudragit E100. Int. J. Biol. Macromol. 2009, 45, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef] [Green Version]
- Bagherifard, S. Mediating bone regeneration by means of drug eluting implants: From passive to smart strategies. Mater. Sci. Eng. C 2017, 71, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, S.; Loordhuswamy, A.M.; Venkateshwapuram Rengaswami, G.D. Electrophoretic deposition of chitosan/nano silver embedded micro sphere on centrifugal spun fibrous matrices—A facile biofilm resistant biocompatible material. Int. J. Biol. Macromol. 2020, 148, 68–78. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Judith Vijaya, J.; John Kennedy, L.; Priadharsini, K.; Palani, P. Antibacterial activity of silver nanoparticles synthesized from serine. Mater. Sci. Eng. C 2015, 49, 316–322. [Google Scholar] [CrossRef] [PubMed]
| Element | H | N | C | Fe | O | Ti |
|---|---|---|---|---|---|---|
| wt.% | <0.001 | <0.009 | <0.013 | 0.168–0.179 | 0.170–0.190 | rest |
| Sample | Suspension | Voltage (V) | Time (min) | |
|---|---|---|---|---|
| A1 | A (0.005 g AgNPs) | 100 mL of 1 vol.% acetic acid, 0.1 mL of Polysorbate 20, 0.25 g of Eudragit E 100, and 0.1 g of chitosan | 10 | 1 |
| A3 | 3 | |||
| A1′ | 30 | 1 | ||
| A3′ | 3 | |||
| B1 | B (0.01 g AgNPs) | 10 | 1 | |
| B3 | 3 | |||
| B1′ | 30 | 1 | ||
| B3′ | 3 | |||
| Sample | Sa (nm) | Sp (nm) | Sv (nm) | Coating Thickness (µm) |
|---|---|---|---|---|
| Ti grade 2 | 142 ± 19 | 921 ± 310 | −738 ± 158 | - |
| Chit/EE100 | 81 ± 14 | 575 ± 167 | −446 ± 141 | 3.61 ± 0.75 |
| A1 | 82 ± 7 | 931 ± 416 | −373 ± 90 | 2.69 ± 0.97 |
| A3 | 99 ± 14 | 1065 ± 478 | −415 ± 77 | 15.65 ± 2.29 |
| A1′ | 148 ± 35 | 957 ± 128 | −465 ± 122 | 13.88 ± 2.32 |
| A3′ | 199 ± 17 | 1582 ± 346 | −859 ± 41 | 31.79 ± 1.96 |
| B1 | 91 ± 30 | 749 ± 230 | −393 ± 128 | 2.65 ± 0.89 |
| B3 | 86 ± 9 | 805 ± 378 | −494 ± 228 | 13.09 ± 1.73 |
| B1′ | 200 ± 34 | 1223 ± 170 | −896 ± 68 | 10.47 ± 1.05 |
| B3′ | 156 ± 9 | 812 ± 64 | −729 ± 47 | 15.65 ± 1.50 |
| Sample | Critical Load, Lc (mN) | Critical Friction, Lf (mN) |
|---|---|---|
| Chit/EE100 | 32.58 ± 14.52 | 35.70 ± 19.03 |
| A1 | 51.76 ± 13.53 | 20.31 ± 11.40 |
| A3 | 24.95 ± 9.12 | 49.96 ± 31.01 |
| A1′ | 27.58 ± 8.22 | 46.89 ± 18.07 |
| A3′ | 34.53 ± 12.04 | 54.84 ± 29.41 |
| B1 | 40.22 ± 9.17 | 27.61 ± 9.68 |
| B3 | 27.96 ± 8.74 | 30.24 ± 26.11 |
| B1′ | 18.69 ± 10.03 | 33.39 ± 10.57 |
| B3′ | 32.10 ± 10.29 | 31.41 ± 17.40 |
| Sample | OCP (V) | Ecorr (V) | icorr (nA/cm2) |
|---|---|---|---|
| Ti grade 2 | −0.298 | −0.464 | 190.55 |
| Chit/EE100 | −0.162 | −0.348 | 177.83 |
| A1 | −0.108 | −0.371 | 169.82 |
| B1 | −0.092 | −0.338 | 158.49 |
| Sample | Rs (Ωcm2) | CPE1-T (µFcm−2) | CPE1-P | R1 (Ωcm2) | CPE2-T (µFcm−2) | CPE2-P | R2 (MΩcm2) | CPE3-T (µFcm−2) | CPE3-P | R3 (Ωcm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti grade 2 | 35.07 | 1.261 | 0.84 | 12.87 | - | - | - | - | - | - | 0.008665 |
| Chit/EE100 | 34.18 | 0.799 | 0.81 | 16.38 | 26.35 | 0.90 | 6.91 | 141.38 | 0.67 | 100.20 | 0.000398 |
| A1 | 34.20 | 0.299 | 0.86 | 14.89 | 25.06 | 0.95 | 7.27 | 85.57 | 0.92 | 3.98 | 0.004804 |
| B1 | 29.20 | 0.950 | 0.78 | 17.95 | 21.60 | 0.96 | 7.89 | 7030.00 | 0.99 | 50.10 | 0.005371 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowski, Ł.; Bartmański, M.; Mielewczyk-Gryń, A.; Cieślik, B.M.; Gajowiec, G.; Zieliński, A. Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System. Materials 2021, 14, 4533. https://doi.org/10.3390/ma14164533
Pawłowski Ł, Bartmański M, Mielewczyk-Gryń A, Cieślik BM, Gajowiec G, Zieliński A. Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System. Materials. 2021; 14(16):4533. https://doi.org/10.3390/ma14164533
Chicago/Turabian StylePawłowski, Łukasz, Michał Bartmański, Aleksandra Mielewczyk-Gryń, Bartłomiej Michał Cieślik, Grzegorz Gajowiec, and Andrzej Zieliński. 2021. "Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System" Materials 14, no. 16: 4533. https://doi.org/10.3390/ma14164533
APA StylePawłowski, Ł., Bartmański, M., Mielewczyk-Gryń, A., Cieślik, B. M., Gajowiec, G., & Zieliński, A. (2021). Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System. Materials, 14(16), 4533. https://doi.org/10.3390/ma14164533











