Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.1.1. Solution Combustion Synthesis
2.1.2. Sol-Gel Synthesis
2.2. Catalysts Characterization
2.3. Catalytic Activity Tests
2.3.1. Total Oxidation of VOC
2.3.2. Oxidation of Soot
3. Results and Discussion
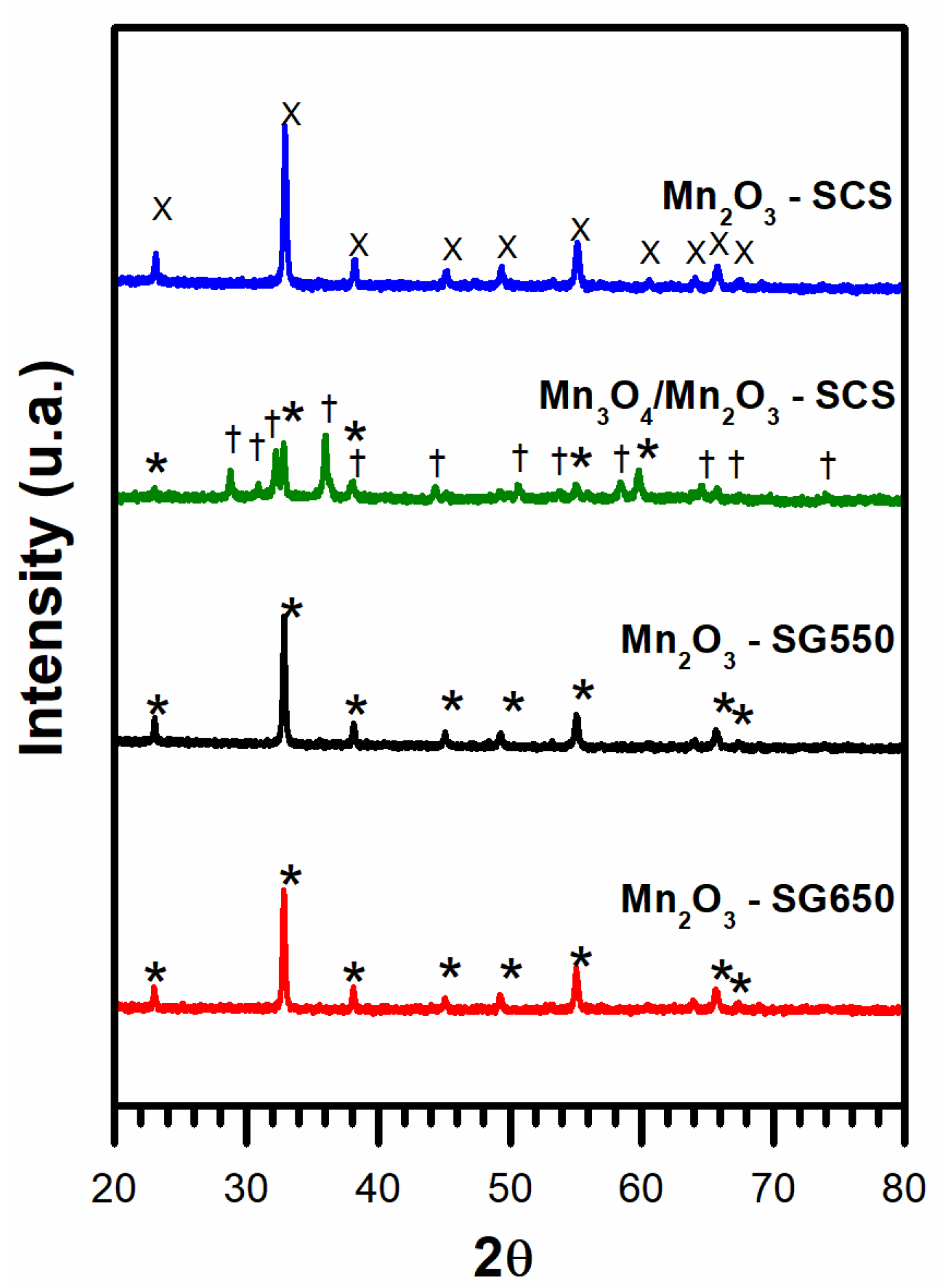
3.1. Material Textural Properties
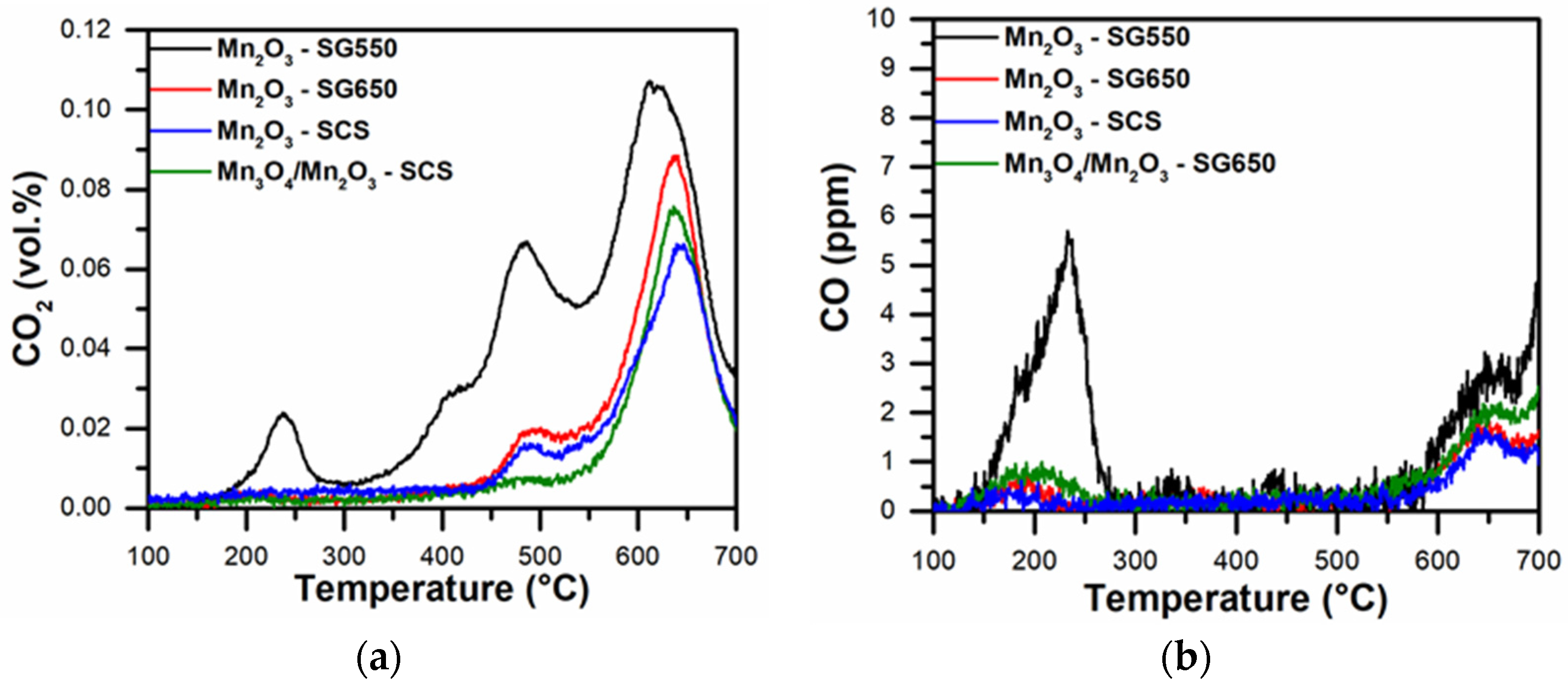
3.2. Temperature-Programmed Analyses
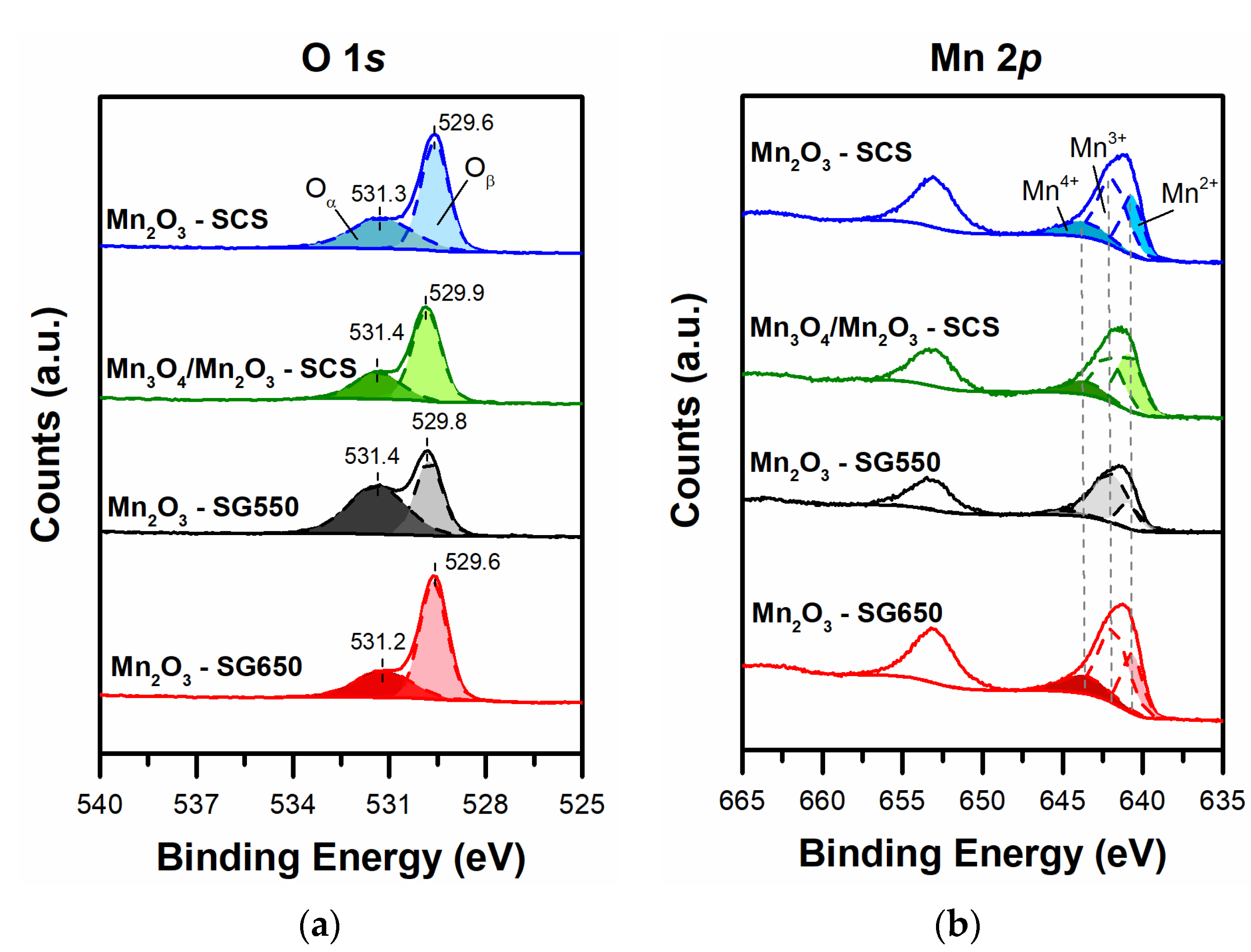
3.3. X-ray Photoelectron Spectroscopy
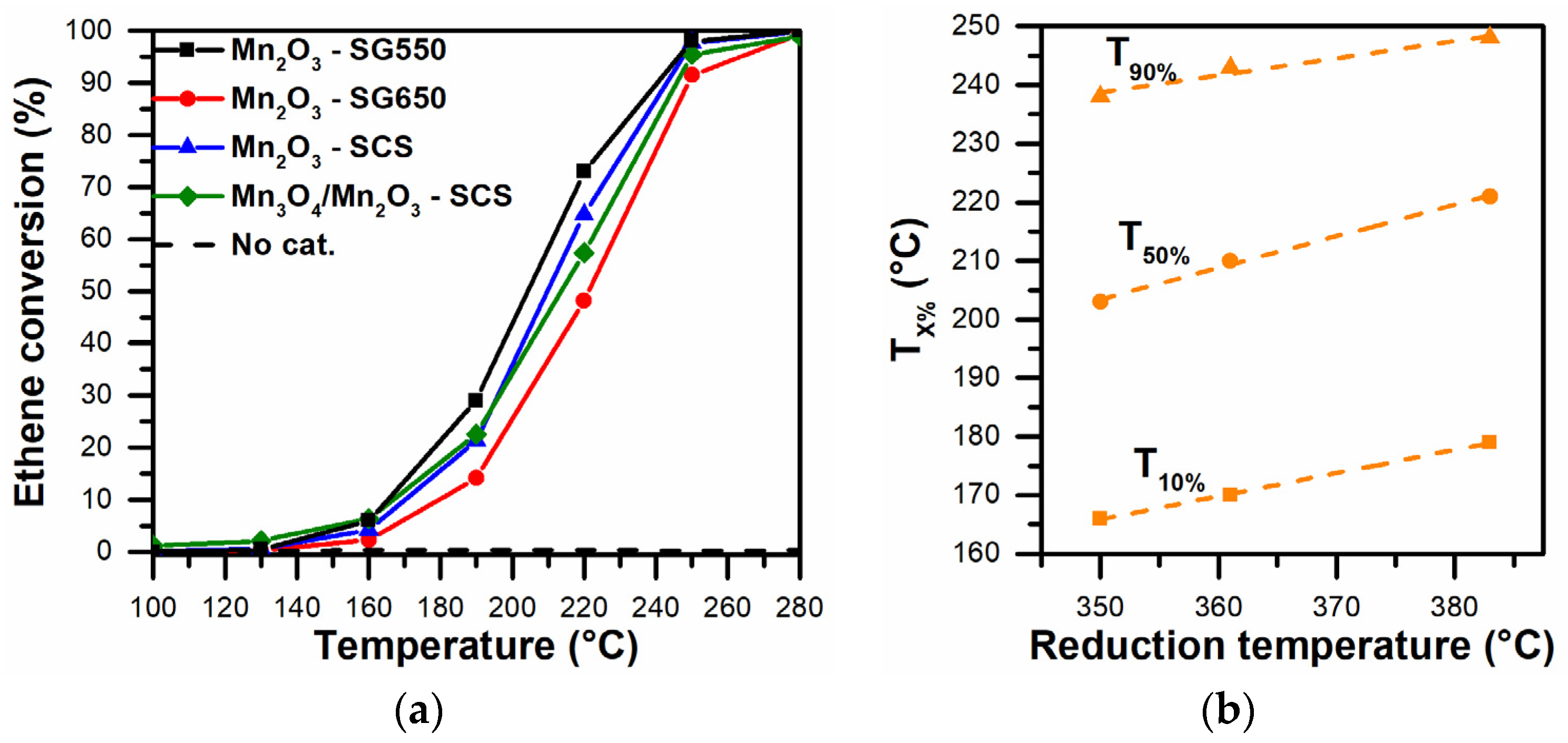
3.4. Catalytic Activity
3.4.1. Oxidation of VOCs
3.4.2. Oxidation of Carbonaceous Matter (Soot)
4. Conclusions
- The elevated relative amounts of active surface Oα species, which can significantly improve the total catalytic oxidation of hydrocarbons at low temperatures.
- The improved low-temperature reducibility of the catalysts due to the enhanced mobility of the oxygen taking part in the oxidation reactions.
- The appearance of small crystallites, which may contain an elevated amount of surface defects that enhance the catalytic performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicard, P.; Khaniabadi, Y.O.; Perez, S.; Gualtieri, M.; De Marco, A. Effect of O3, PM10 and PM2.5 on cardiovascular and respiratory diseases in cities of France, Iran and Italy. Environ. Sci. Pollut. Res. 2019, 26, 32645–32665. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, J.; Von Brockdorff, P. Transboundary air pollution and respiratory disease mortality: Evidence from European countries. J. Econ. Stud. 2020. [Google Scholar] [CrossRef]
- Viegi, G.; Baldacci, S.; Maio, S.; Fasola, S.; Annesi-Maesano, I.; Pistelli, F.; Carrozzi, L.; La Grutta, S.; Forastiere, F. Health effects of air pollution: A Southern European perspective. Chin. Med. J. 2020, 133, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. EUR-Lex. 2008. Available online: https://eur-lex.europa.eu/eli/dir/2008/50/oj.
- Particulate Matter (PM) Implementation Regulatory Actions. United States Environmental Protection Agency. 2016. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-implementation-regulatory-actions.
- Koppmann, R. Volatile Organic Compounds in the Atmosphere; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Gupta, T.; Kumar Singh, D. Organic species emitted as a part of combustion residue: Fate and transformation in the ambient air. J. Energy Environ. Sustain. 2016, 1, 10–18. [Google Scholar] [CrossRef]
- Gelles, T.; Krishnamurthy, A.; Adebayo, B.; Rownaghi, A.; Rezaei, F. Abatement of gaseous volatile organic compounds: A material perspective. Catal. Today 2020, 350, 3–18. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Schiavon, M.; Torretta, V.; Casazza, A.; Ragazzi, M. Non-thermal Plasma as an Innovative Option for the Abatement of Volatile Organic Compounds: A Review. Water Air Soil Pollut. 2017, 228, 388. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; De Geyter, N.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, A.; Adebayo, B.; Gelles, T.; Rownaghi, A.; Rezaei, F. Abatement of gaseous volatile organic compounds: A process perspective. Catal. Today 2020, 350, 100–119. [Google Scholar] [CrossRef]
- I Khan, F.; Ghoshal, A.K. Removal of Volatile Organic Compounds from polluted air. J. Loss Prev. Process. Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in Diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Santos, V.; Pereira, M.; Órfão, J.; Figueiredo, J. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 85th ed.; CRC Press LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Aguilera, D.A.; Pérez-Flórez, A.; Molina, R.; Moreno, S. Cu–Mn and Co–Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Russo, N.; Pirone, R.; Fino, D. Cerium-copper oxides prepared by solution combustion synthesis for total oxidation reactions: From powder catalysts to structured reactors. Appl. Catal. B Environ. 2017, 205, 455–468. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured ceria-based materials: Effect of the hydrothermal synthesis conditions on the structural properties and catalytic activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Gandhe, A.R.; Rebello, J.S.; Figueiredo, J.; Fernandes, J. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate. Appl. Catal. B Environ. 2007, 72, 129–135. [Google Scholar] [CrossRef]
- Spinicci, R.; Tofanari, A. Characterization of catalysts for methane-coupling by means of temperature programmed desorption. Catal. Today 1990, 6, 473–479. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Zha, Y.; Dai, F.; Hu, T.; Xie, Y.; Zhang, J. Highly efficient multifunctional dually-substituted perovskite catalysts La1−xKxCo1−yCuyO3−δ used for soot combustion, NOx storage and simultaneous NOx-soot removal. Appl. Catal. B Environ. 2012, 121–122, 65–74. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Nanostructured ceria-praseodymia catalysts for diesel soot combustion. Appl. Catal. B Environ. 2016, 197, 125–137. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Zawadzki, M.; Trawczyński, J. Synthesis, characterization and catalytic performance of LSCF perovskite for VOC combustion. Catalysts 2011, 176, 449–452. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Piumetti, M.; Russo, N. Notes on Catalysis for Environment and Energy; CLUT-Politecnico di Torino: Turin, Italy, 2017. [Google Scholar]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Andana, T.; Novara, C.; Giorgis, F.; Fino, D.; Russo, N. Novel Mn–Cu-Containing CeO2 Nanopolyhedra for the Oxidation of CO and Diesel Soot: Effect of Dopants on the Nanostructure and Catalytic Activity. Catal. Lett. 2017, 148, 298–311. [Google Scholar] [CrossRef]
- Figueredo, M.J.M.; Andana, T.; Bensaid, S.; Dosa, M.; Fino, D.; Russo, N.; Piumetti, M. Cerium–Copper–Manganese Oxides Synthesized via Solution Combustion Synthesis (SCS) for Total Oxidation of VOCs. Catal. Lett. 2020, 150, 1821–1840. [Google Scholar] [CrossRef]
- Piumetti, M.; van der Linden, B.; Makkee, M.; Miceli, P.; Fino, D.; Russo, N.; Bensaid, S. Contact dynamics for a solid–solid reaction mediated by gas-phase oxygen: Study on the soot oxidation over ceria-based catalysts. Appl. Catal. B Environ. 2016, 199, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Deorsola, F.; Andreoli, S.; Armandi, M.; Bonelli, B.; Pirone, R. Unsupported nanostructured Mn oxides obtained by Solution Combustion Synthesis: Textural and surface properties, and catalytic performance in NO x SCR at low temperature. Appl. Catal. A Gen. 2016, 522, 120–129. [Google Scholar] [CrossRef]
- Wagloehner, S.; Nitzer-Noski, M.; Kureti, S. Oxidation of soot on manganese oxide catalysts. Chem. Eng. J. 2015, 259, 492–504. [Google Scholar] [CrossRef]
- Wagloehner, S.; Baer, J.N.; Kureti, S. Structure–activity relation of iron oxide catalysts in soot oxidation. Appl. Catal. B Environ. 2014, 147, 1000–1008. [Google Scholar] [CrossRef]




| Catalyst. | SBET a | VP a | DP a | Crystallites Size b |
|---|---|---|---|---|
| Mn2O3-SG550 | 15 | 0.12 | 32 | 67 |
| Mn2O3-SG650 | 11 | 0.10 | 37 | 61 |
| Mn2O3-SCS | 22 | 0.15 | 26 | 52 |
| Mn3O4/Mn2O3-SG550 | 21 | 0.13 | 23 | 37/53 |
| Catalyst | Oα, OH− BE (eV) | Oα (at.%) | Oβ BE (eV) | Oβ (at.%) | Oα/Oβ |
|---|---|---|---|---|---|
| Mn2O3-SG550 | 531.4 | 56.7 | 529.8 | 43.3 | 1.31 |
| Mn2O3-SG650 | 531.2 | 31.8 | 529.6 | 68.2 | 0.47 |
| Mn2O3-SCS | 531.3 | 38.5 | 529.6 | 61.5 | 0.63 |
| Mn3O4/Mn2O3-SG550 | 531.4 | 31.8 | 529.9 | 68.2 | 0.47 |
| Catalyst | rpropene a (μmol h−1 m−2) | rethene b (μmol h−1 m−2) |
|---|---|---|
| Mn2O3-SG550 | 0.94 | 1.67 |
| Mn2O3-SG650 | 0.49 | 1.13 |
| Mn2O3-SCS | 0.35 | 1.04 |
| Mn3O4/Mn2O3-SG550 | 1.48 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueredo, M.J.M.; Cocuzza, C.; Bensaid, S.; Fino, D.; Piumetti, M.; Russo, N. Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts. Materials 2021, 14, 4534. https://doi.org/10.3390/ma14164534
Figueredo MJM, Cocuzza C, Bensaid S, Fino D, Piumetti M, Russo N. Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts. Materials. 2021; 14(16):4534. https://doi.org/10.3390/ma14164534
Chicago/Turabian StyleFigueredo, Miguel Jose Marin, Clarissa Cocuzza, Samir Bensaid, Debora Fino, Marco Piumetti, and Nunzio Russo. 2021. "Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts" Materials 14, no. 16: 4534. https://doi.org/10.3390/ma14164534
APA StyleFigueredo, M. J. M., Cocuzza, C., Bensaid, S., Fino, D., Piumetti, M., & Russo, N. (2021). Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts. Materials, 14(16), 4534. https://doi.org/10.3390/ma14164534








