Functional and Antioxidant Properties of Plastic Bottle Caps Incorporated with BHA or BHT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
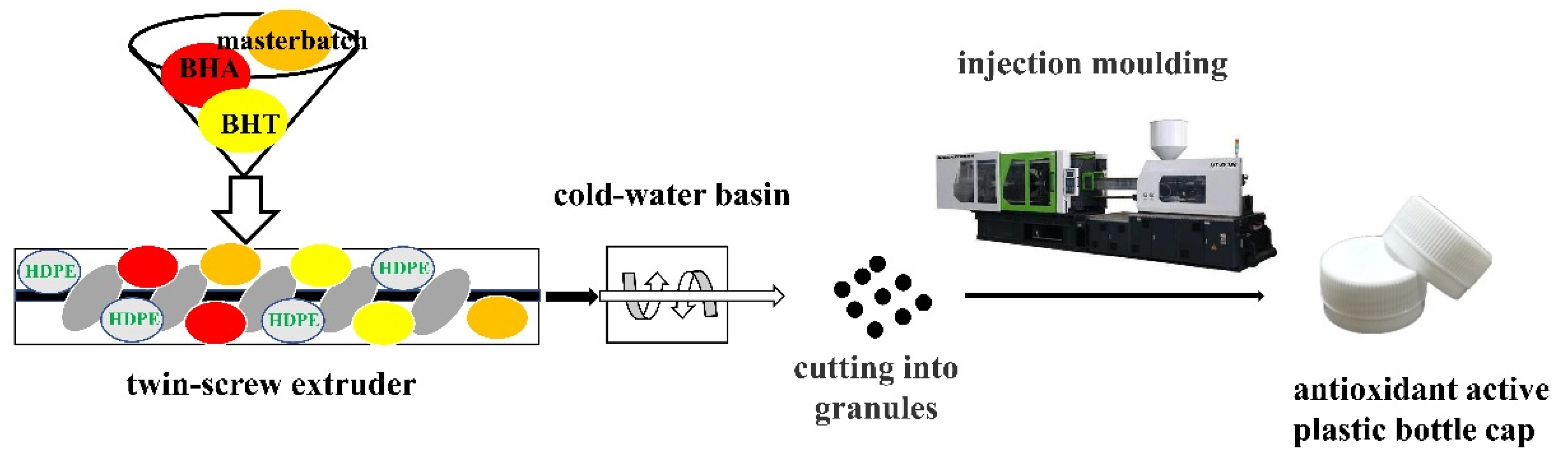
2.2. Sample Preparation
2.3. Color Attributes
2.4. Thermal Analysis
2.5. Fourier-Transform Infrared (FT-IR) Spectrometry
2.6. Torque Tests
2.7. Sensory Evaluation
2.8. Antioxidant Assessment
2.9. Specific Migration Experiment
2.9.1. Migration Conditions
2.9.2. Migration Experiment
2.9.3. Determination and Quantification of Initial Antioxidant Concentrations in Plastic Bottle Caps
2.9.4. Chromatographic Analysis of the Antioxidants
2.9.5. Method Validation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Color Attributes
3.2. Thermal Analysis
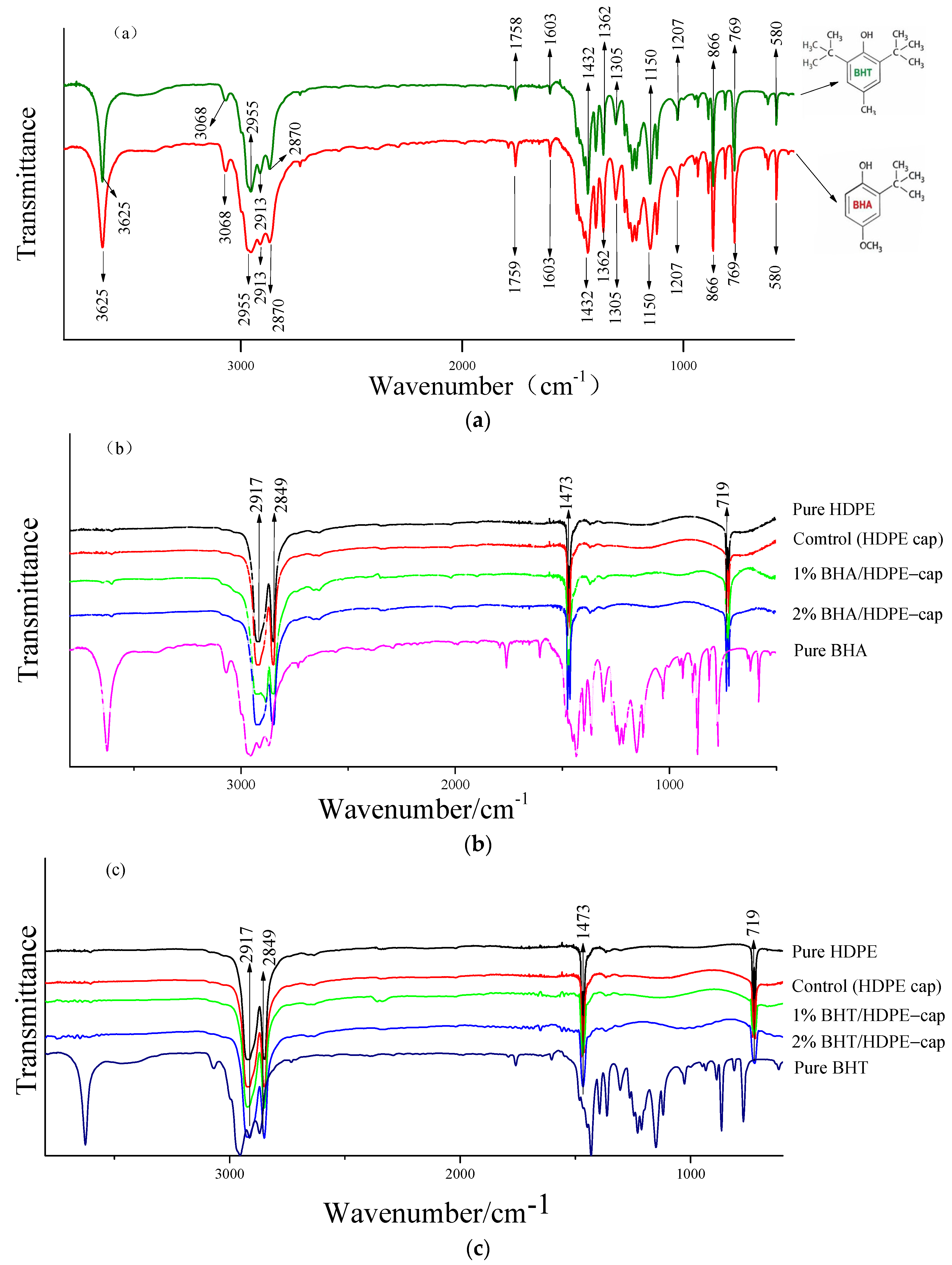
3.3. Fourier-Transform Infrared (FT-IR) Spectrometry
3.4. Torque Tests
3.5. Sensory Evaluation
3.6. Antioxidant Assessment
3.7. Specific Migration Experiment
3.7.1. Method Validation
3.7.2. Determination and Quantification of Initial Antioxidant Concentrations in Plastic Bottle Caps
3.7.3. Migration Experiment and Safety Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welle, F. Twenty years of PET bottle to bottle recycling-An overview. Resour. Conserv. Recy. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Berlinet, C.; Brat, P.; Ducruet, V. Quality of orange juice in barrier packaging material. Packag. Technol. Sci. 2008, 21, 279–286. [Google Scholar] [CrossRef]
- Ros-Chumillas, M.; Belissario, Y.; Iguaz, A.; Lopez, A. Quality and shelf life of orange juice aseptically packaged in PET bottles. J. Food Eng. 2007, 79, 234–242. [Google Scholar] [CrossRef]
- Silva, M.A.; Julien, M.; Jourdes, M.; Teissedre, P. Impact of closures on wine post-bottling development: A review. Eur. Food Res. Technol. 2011, 233, 905–914. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, X.; Chagnon, M.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, D.; Goldbeck, C.; Humpf, H.; Fuerst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Kenawy, E.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, J. Antibacterial activity of polyvinyl alcohol (PVA)/epsilon-polylysine packaging films and the effect on longan fruit. Food Sci. Tech. 2020, 40, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. 2004, 44, 223–237. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerin, C. Polyamide modified with green tea extract for fresh minced meat active packaging applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef]
- Wrona, M.; Cran, M.J.; Nerin, C.; Bigger, S.W. Development and characterisation of HPMC films containing PLA nanoparticles loaded with green tea extract for food packaging applications. Carbohyd. Polym. 2017, 156, 108–117. [Google Scholar] [CrossRef]
- Bodai, Z.; Kirchkeszner, C.; Novak, M.; Nyiri, Z.; Kovacs, J.; Magyar, N.; Rikker, T.; Ivan, B.; Eke, Z. Migration of Tinuvin P and Irganox 3114 into milk and the corresponding authorised food simulant. Food Addit. Contam. A 2015, 32, 1358–1366. [Google Scholar] [CrossRef]
- Kasza, G.; Mosnackova, K.; Nador, A.; Osvath, Z.; Stumphauser, T.; Szarka, G.; Czaniková, K.; Rychly, J.; Chmela, S.; Ivan, B.; et al. Synthesis of hyperbranched poly (ethyleneimine) based macromolecular antioxidants and investigation of their efficiency in stabilization of polyolefins. Eur. Polym J. 2015, 68, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf 2020, 24, 100–111. [Google Scholar]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf 2019, 22, 100393. [Google Scholar] [CrossRef]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Stabilization of beef meat by a new active packaging containing natural antioxidants. J. Agric. Food Chem 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Dominici, F.; Jiménez, A.; Garrigós, M.D.C.; Kenny, J.M. Influence of thymol and silver nanoparticles on the degradation of poly(lactic acid) based nanocomposites: Thermal and morphological properties. Polym. Degrad. Stabil. 2014, 108, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohyd. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alonso, I.; Jimenez-Escrig, A.; Saura-Calixto, F.; Borderias, A.J. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT Food Sci. Technol. 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Peltzer, M.; Valente, A.J.M.; Garrigos, M.D.C. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Lv, X.; Lin, Q.; Li, Z.; Liao, J.; Xu, C.; Zhong, W. Migration of metal elements from polylactic acid dinner plate into acidic food simulant and its safety evaluation. Food Packag. Shelf. 2019, 22, 1–7. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohyd. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, E.J.; Chang, C.; Lam, R.S.H.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.G.; Wu, N.F.; Fan, M.M.; Shen, X.L.; Chen, M.T.; Jiang, A.M.; Lai, L. Effect of hsian-tsao gum (HG) content upon rheological properties of film-forming solutions (FFS) and physical properties of soy protein/hsian-tsao gum films. Food Hydrocolloid 2015, 50, 211–218. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with alpha-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Ramos, M.; Jimenez, A.; Peltzer, M.; Garrigos, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Ammawath, W.; Man, Y.; Baharin, B.S.; Rahman, R. Analysis of butylated hydroxyanisole (BHA) in RBD palm oil and RBD palm olein using partial least squares based on FTIR spectroscopy. J. Food Lipids 2005, 12, 198–208. [Google Scholar] [CrossRef]
- Kang, K.; Chang, Y.; Choi, J.C.; Park, S.; Han, J. Migration Study of Butylated Hydroxytoluene and Irganox 1010 from Polypropylene Treated with Severe Processing Conditions. J. Food Sci. 2018, 83, 1005–1010. [Google Scholar] [CrossRef]
- Sharif, A.; Aalaie, J.; Shariatpanahi, H.; Hosseinkhanli, H.; Khoshniyat, A. Fabrication of a novel polyethylene/starch blend through mediation of a high-energy ball milling process: Mechanical properties and formation mechanism. J. Appl. Polym. Sci. 2012, 128, 145–152. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Karim, R. Effect of different types of plastic packaging films on the moisture and aflatoxin contents of pistachio nuts during storage. J. Food Sci. Technol. 2013, 50, 409–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, H.; Hong, W.; Yu, F.; Sun, W.; Yin, X. Investigation of the Influence of Tamper-evident Band Bridge Strength on Opening Property of Tamper-evident Plastic Closures. Packag. Eng. 2015, 36, 69–73. [Google Scholar]
- Carse, B.; Thomson, A.; Stansfield, B. A novel device for evaluating forces during the dynamic jar opening action—Do older and younger adults do things differently? Med. Eng. Phys. 2010, 33, 521–525. [Google Scholar] [CrossRef]
- Lajmi, A.; Champliaud, H.; Van Ngan, L. Computation of the Maximum Torque of a Cap Liner Using a Power-Law Friction and Finite Element Analysis. Packag. Technol. Sci. 2011, 24, 103–121. [Google Scholar] [CrossRef]
- Wessling, C.; Nielsen, T.; Leufvén, A. The influence of α-tocopherol concentration on the stability of linoleic acid and the properties of low-density polyethylene. Packag. Technol. Sci. 2015, 13, 19–28. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Sendra, E.; Sayas-Barbera, E.; Perez-Alvarez, J.A. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res. Int. 2011, 44, 1217–1223. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Hernandez Cocoletzi, H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Lopez De Dicastillo, C.; Rodriguez, F.; Guarda, A.; Jose Galotto, M. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohyd. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Mueller, L.; Froehlich, K.; Boehm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (alpha TEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Dopico-Garcia, M.S.; Lopez-Vilarino, J.M.; Gonzalez-Rodriguez, M.V. Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages. J. Agric. Food Chem. 2007, 55, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Kasza, G.; Stumphauser, T.; Nador, A.; Osvath, Z.; Szarka, G.; Domjan, A.; Mosnacek, J.; Ivan, B. Hyperbranched polyglycerol nanoparticles based multifunctional, nonmigrating hindered phenolic macromolecular antioxidants: Synthesis, characterization and its stabilization effect on poly (vinyl chloride). Polymer 2017, 124, 210–218. [Google Scholar] [CrossRef] [Green Version]




| Project | Characteristic | Score |
|---|---|---|
| Taste | Taste is normal | 9 |
| The taste is slightly different to that of mineral water | 7 | |
| The taste is obviously different to that of mineral water | 5 | |
| The mineral water has a heavy taste | 3 | |
| The mineral water has a pungent taste | 1 |
| Application | Method Used | References |
|---|---|---|
| Packaging of salmon slices | DPPH, TBARS | [16] |
| Packaging of fresh meat | MetMb, color, PV, aldehydes | [17] |
| Nano-biocomposite antioxidant films | DPPH | [18] |
| Antioxidant chitosan film | DPPH, ABTS, FRAP | [19] |
| Packaging of frozen fish | TH, CD, TBARS, FRAP, ABTS, color | [20] |
| Antioxidant polyolefin film | DPPH | [21] |
| Film Sample | L* | a* | b* | WI |
|---|---|---|---|---|
| Control (HDPE cap) | 89.57 ± 0.43 a | 1.10 ± 0.18 a | 3.74 ± 0.51 cd | 88.85 ± 0.26 a |
| 1% BHA/ HDPE cap | 84.70 ± 1.42 b | 1.37 ± 0.40 a | 6.01 ± 0.90 b | 83.33 ± 0.92 c |
| 2% BHA/ HDPE cap | 84.83 ± 1.90 b | 1.72 ± 0.02 b | 6.79 ± 0.47 b | 84.47 ± 1.80 bc |
| 1% BHT/ HDPE cap | 83.35 ± 1.09 b | 1.41 ± 0.42 a | 4.89 ± 0.87 bc | 82.59 ± 0.78 c |
| 2% BHT/ HDPE cap | 83.20 ± 0.67 b | 1.93 ± 0.40 b | 7.45 ± 0.23 a | 86.72 ± 0.47 b |
| Cap Sample | Tg (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| Control (HDPE cap) | 123.93 | 136.20 | 149.15 | 51.91 |
| 1% BHA/ HDPE cap | 123.40 | 135.50 | 145.19 | 50.54 |
| 2% BHA/ HDPE cap | 123.40 | 135.87 | 142.77 | 49.69 |
| 1% BHT/ HDPE cap | 123.93 | 135.80 | 146.31 | 50.93 |
| 2% BHT/ HDPE cap | 123.90 | 135.20 | 142.39 | 49.56 |
| Cap Sample | Taste | ||
|---|---|---|---|
| 24 h | 48 h | 144 h | |
| Control HDPE cap | 7.80 ± 1.78 a | 6.60 ± 0.89 a | 5.40 ± 0.89 a |
| 1% BHA/HDPE cap | 7.00 ± 2.00 a | 5.80 ± 1.78 a | 5.00 ± 0.92 a |
| 2% BHA/HDPE cap | 7.40 ± 1.67 a | 6.60 ± 1.67 a | 5.80 ± 1.09 a |
| 1% BHT/HDPE cap | 7.40 ± 1.67 a | 6.60 ± 1.67 a | 5.80 ± 1.09 a |
| 2% BHT/HDPE cap | 7.00 ± 2.00 a | 7.00 ± 1.41 a | 5.40 ± 0.89 a |
| Cap Type | DPPH SA (%) | ABTS SB (%) | FRAP (mg AAE/g DW) |
|---|---|---|---|
| Control HDPE cap | 2.09 ± 0.80 c | 4.58 ± 1.01 c | 21.22 ± 0.79 d |
| 1% BHA/HDPE cap | 10.56 ± 1.17 b | 27.01 ± 3.61 b | 44.13 ± 3.15 b |
| 2% BHA/HDPE cap | 22.02 ± 2.31 a | 42.10 ± 3.34 a | 66.61 ± 1.82 c |
| 1% BHT/HDPE cap | 8.26 ± 1.81 b | 26.39 ± 1.91 b | 43.46 ± 2.78 b |
| 2% BHT/HDPE cap | 18.7 ± 1.48 a | 43.52 ± 2.01 a | 59.33 ± 1.72 a |
| Antioxidant | Food Simulant | Linear Equation | Linear Ranges (mg/kg) | Correlation Coefficient (r2) | LOD (mg/L) | LOQ (mg/L) | Slope Confidence Intervals | Intercept Confidence Intervals |
|---|---|---|---|---|---|---|---|---|
| BHA | 3% acetic acid | y = 1.92x − 0.86 | 0.5–10 | 0.99908 | 0.14 | 0.47 | 1.88–1.95 | −0.89–0.83 |
| 10% ethanol | y = 1.15x + 0.01 | 0.5–10 | 0.99939 | 0.15 | 0.47 | 1.13–1.17 | −0.09–0.11 | |
| 50% ethanol | y = 2.36x + 0.24 | 0.2–50 | 0.99993 | 0.06 | 0.28 | 2.16–1.56 | 0.13–0.35 | |
| 95% ethanol | y = 2.17x + 0.52 | 0.5–100 | 0.99984 | 0.16 | 0.48 | 2.16–2.18 | 0.49–0.55 | |
| BHT | 3% acetic acid | y = 1.37x + 0.12 | 0.5–10 | 0.99948 | 0.15 | 0.51 | 1.34–1.41 | 0.06–0.18 |
| 10% ethanol | y = 1.46x + 0.06 | 0.5–10 | 0.99957 | 0.05 | 0.22 | 1.44–1.48 | 0.04–0.08 | |
| 50% ethanol | y = 2.63x + 0.70 | 0.2–50 | 0.99992 | 0.05 | 0.11 | 2.61–2.65 | 0.69–0.71 | |
| 95% ethanol | y = 2.25x − 0.16 | 0.5–100 | 0.99991 | 0.16 | 0.52 | 2.18–2.32 | −0.52–0.20 |
| Figure | Spiked Concentration (mg/L) | Recoveries (%) | ||
|---|---|---|---|---|
| BHA | BHT | BHA | BHT | |
| 3% acetic acid | 0.8 | 0.8 | 87.24 ± 3.53 | 88.06 ± 4.61 |
| 3 | 6 | 93.86 ± 1.48 | 85.21 ± 6.66 | |
| 8 | 8 | 90.45 ± 1.66 | 84.54 ± 2.47 | |
| 10% ethanol | 2 | 1.5 | 85.13 ± 2.93 | 93.02 ± 2.94 |
| 6 | 4 | 94.65 ± 2.23 | 94.59 ± 1.49 | |
| 8 | 8 | 102.13 ± 1.93 | 99.39 ± 1.25 | |
| 50% ethanol | 0.4 | 0.4 | 89.37 ± 0.61 | 98.76 ± 2.12 |
| 15 | 15 | 94.40 ± 2.82 | 106.55 ± 1.66 | |
| 40 | 40 | 99.01 ± 4.45 | 110.92 ± 0.95 | |
| 95% ethanol | 4 | 4 | 106.60 ± 1.59 | 101.22 ± 0.68 |
| 25 | 25 | 105.42 ± 0.11 | 98.89 ± 0.31 | |
| 80 | 80 | 108.21 ± 0.20 | 101.35 ± 0.28 | |
| Cap Sample | Extraction Solvent | ||
|---|---|---|---|
| Methanol | Methylene Chloride | Ethyl Acetate | |
| Control HDPE cap | ND | ND | ND |
| 1% BHA/HDPE cap | 466.30 ± 34.88 a | 3870.55 ± 74.12 a | 2961.61 ± 218.92 a |
| 2%BHA/HDPE cap | 787.95 ± 50.13 b | 6630.82 ± 291.84 b | 5991.24 ± 277.72 b |
| 1% BHT/HDPE cap | 345.96 ± 11.57 c | 2323.18 ± 93.60 c | 1940.02 ± 70.59 c |
| 2% BHT/HDPE cap | 981.48 ± 45.55 d | 3171.66 ± 141.36 d | 4283.13 ± 84.64 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-W.; Li, Y.-N.; Lin, Q.-B.; Wang, X.; Li, Z.-H.; Wu, K.-X. Functional and Antioxidant Properties of Plastic Bottle Caps Incorporated with BHA or BHT. Materials 2021, 14, 4545. https://doi.org/10.3390/ma14164545
Wang Y-W, Li Y-N, Lin Q-B, Wang X, Li Z-H, Wu K-X. Functional and Antioxidant Properties of Plastic Bottle Caps Incorporated with BHA or BHT. Materials. 2021; 14(16):4545. https://doi.org/10.3390/ma14164545
Chicago/Turabian StyleWang, Yu-Wen, Ya-Na Li, Qin-Bao Lin, Xiao Wang, Zeng-Hui Li, and Kai-Xuan Wu. 2021. "Functional and Antioxidant Properties of Plastic Bottle Caps Incorporated with BHA or BHT" Materials 14, no. 16: 4545. https://doi.org/10.3390/ma14164545
APA StyleWang, Y.-W., Li, Y.-N., Lin, Q.-B., Wang, X., Li, Z.-H., & Wu, K.-X. (2021). Functional and Antioxidant Properties of Plastic Bottle Caps Incorporated with BHA or BHT. Materials, 14(16), 4545. https://doi.org/10.3390/ma14164545





