Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition of Raw Materials and Calorific Value of Bread
2.3. Black Cumin Seed Waste Pressing
2.4. Process of Starch Bread Baking
2.5. Determination of Basic Physical Parameters of Bread
2.6. Determination of Texture and Sensory Parameters of Bread
2.7. Extraction and Derivatization of Phenolic Compounds
2.8. C-MS Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Starch and BCS and BCW
3.2. Physical Properties and Color Values of Starch Bread with BCS and BCW
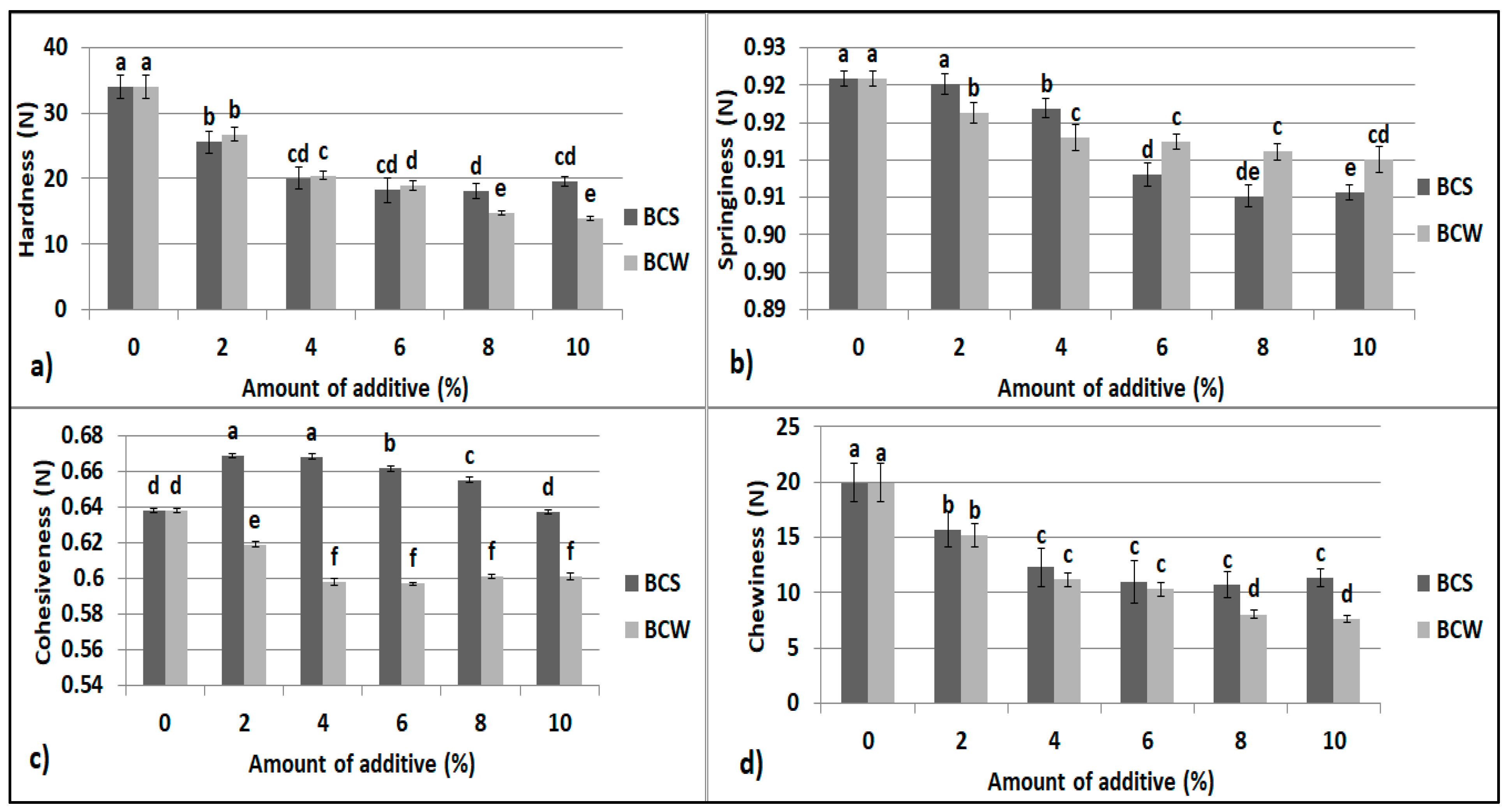
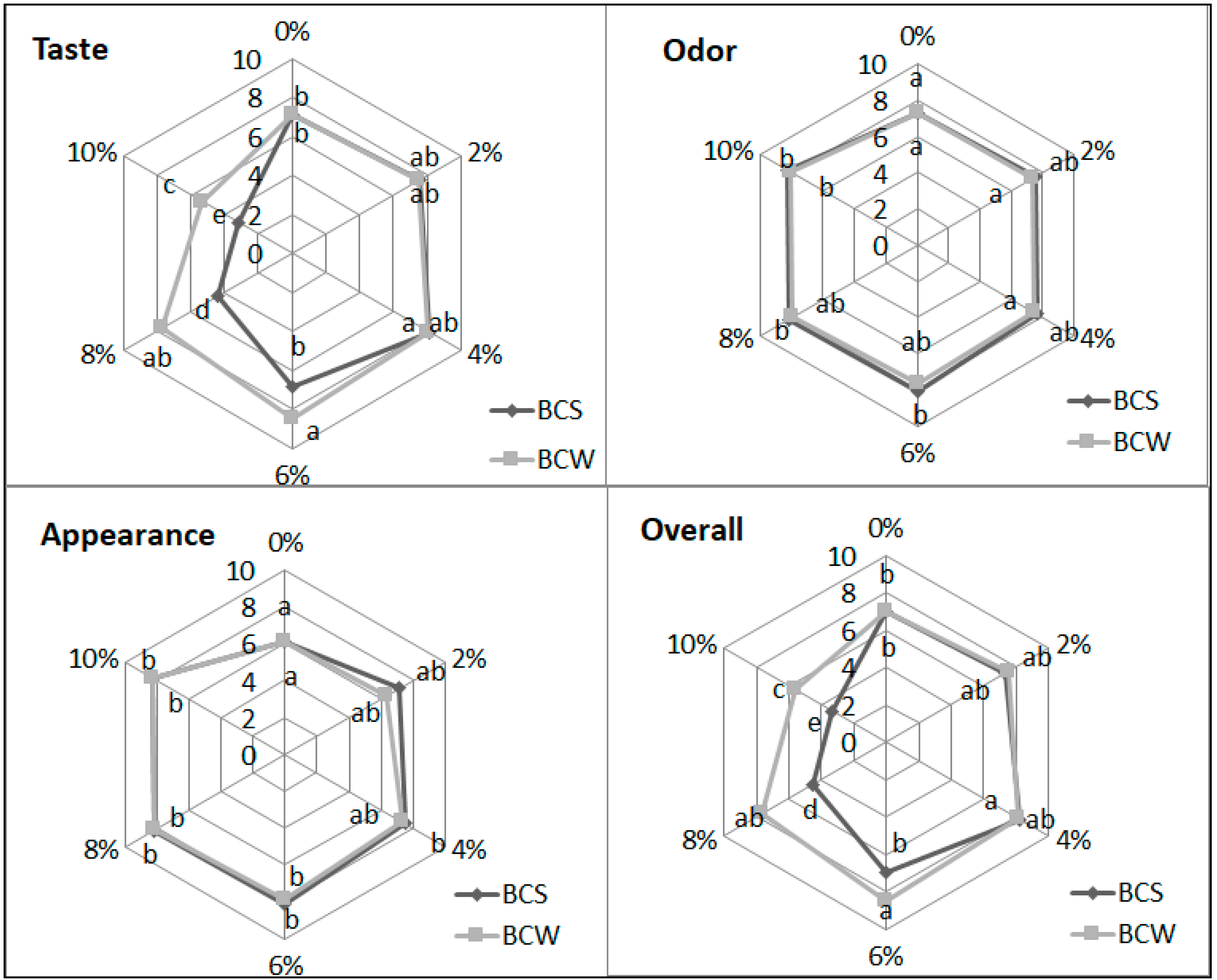
3.3. Texture and Sensory Evaluation of Starch Bread with BCS and BCW
3.4. Calorific Value of Starch Bread with BCS and BCW
3.5. Phenolic Acid Content in Starch Bread with BCS and BCW Detected by GC-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Canary, J.C. Effect of starch structure on glucose and insulin responses in adults. Am. J. Clin. Nutr. 1988, 47, 428–432. [Google Scholar] [CrossRef]
- Special Report Committee, Canadian Diabetes Association. Guidelines for the nutritional management of diabetes mellitus: 1980. Beta Release 1989, 13, 8–17. [Google Scholar]
- Sierra, M.; Hernanz, N.; Alonso, I.G.y.L. Celiac disease. Medicine 2020, 13, 9–15. [Google Scholar]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. european society for the study of coeliac disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Mroczek, B.; Karakiewicz, B.; Bardadyn, E. Evaluation of effectiveness of health education in phenylketonuria. Fam. Med. Prim. Care Rev. 2007, 9, 535–537. [Google Scholar]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Matejova, S.; Fikselova, M.; Curlej, J.; Czako, P. Application of by-products in the development of foodstuffs for particular nutritional uses. J. Cent. Eur. Agric. 2016, 17, 1306–1319. [Google Scholar] [CrossRef][Green Version]
- Pandey, A.; Kumar, A.; Mishra, A.A. Development and qualitative estimation of high fibre enriched bread fortified with carrot pomace. Food Sci. Res. J. 2016, 7, 51–56. [Google Scholar] [CrossRef]
- Hsu, C.-T.; Chang, Y.-H.; Shiau, S.-Y. Color, antioxidation, and texture of dough and Chinese steamed bread enriched with pitaya peel powder. Cereal Chem. J. 2019, 96, 76–85. [Google Scholar] [CrossRef]
- Plazzotta, S.; Sillani, S.; Manzocco, L. Exploitation of lettuce waste flour to increase bread functionality: Effect on physical, nutritional, sensory properties and on consumer response. Int. J. Food Sci. Technol. 2018, 53, 2290–2297. [Google Scholar] [CrossRef]
- Zdybel, B.; Różyło, R.; Sagan, A. Use of a waste product from the pressing of chia seed oil in wheat and gluten-free bread processing. J. Food Process Preserv. 2019, 43, e14002. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.-C.; Tania, M.; Zhang, D.-Z. Anticancer activities of Nigella sativa (Black Cumin). Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 226–232. [Google Scholar] [CrossRef]
- Mohtashami, A. Effects of bread with Nigella sativa on blood glucose, blood pressure and anthropometric indices in patients with metabolic syndrome. Clin. Nutr. Res. 2019, 8, 138–147. [Google Scholar] [CrossRef]
- Thippeswamy, N.B.; Naidu, K.A. Antioxidant potency of cumin varieties-cumin, black cumin and bitter cumin-on antioxidant systems. Eur. Food Res. Technol. 2005, 220, 472–476. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (black seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Ikhsan, M.; Hiedayati, N.; Maeyama, K.; Nurwidya, F. Nigella sativa as an anti-inflammatory agent in asthma. BMC Res. Notes 2018, 11, 744. [Google Scholar] [CrossRef]
- Abduallah, A.M.; Rashed, A.A.; Gamaleldeen, A.K.; Sayed, S.R.M. The effect of Nigella Sativa extract (thymoquinone) on glucose insulin levels and body weight of induced diabetic female rats. Am. J. Life Sci. 2017, 5, 52–56. [Google Scholar] [CrossRef][Green Version]
- Hamdan, A.; Idrus, R.H.; Mokhtar, M.H. Effects of nigella sativa on type-2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 4911. [Google Scholar] [CrossRef]
- Osman, M.; Alamri, M.; Mohamed, A.; Hussain, S.; Gassem, M.; Rahman, I.A. Black cumin-fortified flat bread: Formulation, processing, and quality. Qual. Assur. Saf. Crop. Foods 2015, 7, 233–238. [Google Scholar] [CrossRef]
- Debonne, E.; De Leyn, I.; Verwaeren, J.; Moens, S.; Devlieghere, F.; Eeckhout, M.; Van Bockstaele, F. The influence of natural oils of blackcurrant, black cumin seed, thyme and wheat germ on dough and bread technological and microbiological quality. LWT 2018, 93, 212–219. [Google Scholar] [CrossRef]
- Al-Subhi, F.M.M. Supplementation of pan bread with some cereals gluten free to decrease risk of celiac diseases. Life Sci. J. 2014, 11, 347–353. [Google Scholar]
- Coşkun, Ö.; Pehlivanoğlu, H.; Gülseren, İ. Pilot scale assessment for seed protein enrichment of gluten-free breads at varying water content levels and after protein modification treatments. J. Food Process Preserv. 2020, 44, e14512. [Google Scholar] [CrossRef]
- Ahlborn, G.J.; Pike, O.A.; Hendrix, S.B.; Hess, W.M.; Huber, C.S. Sensory, mechanical, and microscopic evaluation of staling in low-protein and gluten-free breads. Cereal Chem. J. 2005, 82, 328–335. [Google Scholar] [CrossRef]
- ISO Standard 20483:2013. Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; ISO International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- ISO Standard 659:2009. Oilseeds—Determination of Oil Content (Reference Method); ISO International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- ISO 2171:2007. Cereals, Pulses and by-Products—Determination of Ash Yield by incineration; ISO International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- ISO 1666:1996. Starch—Determination of Moisture Content—Oven-Drying Method; ISO International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljestróm, M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef]
- Costantini, L.; Lukšič, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef]
- ISO Standard 12966-2:2017. Animal and Vegetable Fats and Oils Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids; ISO International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Różyło, R.; Rudy, S.; Krzykowski, A.; Dziki, D. Novel application of freeze-dried amaranth sourdough in gluten-free bread production. J. Food Process Eng. 2015, 38, 135–143. [Google Scholar] [CrossRef]
- Ziemichód, A.; Wójcik, M.; Różyło, R. Seeds of plantago psyllium and plantago ovata: Mineral composition, grinding, and use for gluten-free bread as substitutes for hydrocolloids. J. Food Process Eng. 2019, 42, e12931. [Google Scholar] [CrossRef]
- Witczak, M.; Korus, J.; Ziobro, R.; Juszczak, L. Waxy starch as dough component and anti-staling agent in gluten-free bread. LWT 2019, 99, 476–482. [Google Scholar] [CrossRef]
- Kumar, R.K.; Bejkar, M.; Du, S.; Serventi, L. Flax and wattle seed powders enhance volume and softness of gluten-free bread. Food Sci. Technol. Int. 2019, 25, 66–75. [Google Scholar] [CrossRef]
- Mariotti, M.; Lucisano, M.; Pagani, M.A.; Ng, P.K.W. The role of corn starch, amaranth flour, pea isolate, and psyllium flour on the rheological properties and the ultrastructure of gluten-free doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar] [CrossRef]
- Aprodu, I.; Banu, I. Influence of dietary fiber, water, and glucose oxidase on rheological and baking properties of maize based gluten-free bread. Food Sci. Biotechnol. 2015, 24, 1301–1307. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, T.; Ziobro, R.; Juszczak, L. Linseed (Linum usitatissimum L.) mucilage as a novel structure forming agent in gluten-free bread. LWT 2015, 62, 257–264. [Google Scholar] [CrossRef]
- Gularte, M.A.; de la Hera, E.; Gómez, M.; Rosell, C.M. Effect of different fibers on batter and gluten-free layer cake properties. LWT-Food Sci. Technol. 2012, 48, 209–214. [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophysics 2019, 33, 217–225. [Google Scholar] [CrossRef]
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvia hispanica L.) addition on quality and nutritional value of wheat bread. J. Food Qual. 2017, 2017, e7352631. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011, 124, 1577–1582. [Google Scholar] [CrossRef]
- Kang, O.-J. Distribution of free, esterified, and insoluble bound forms of phenolics in tea seeds and their antioxidant activity. Food Sci. Biotechnol. 2017, 26, 121–127. [Google Scholar] [CrossRef]
- Topcagic, A.; Zeljkovic, S.C.; Karalija, E.; Galijasevic, S.; Sofic, E. Evaluation of phenolic profile, enzyme inhibitory and antimicrobial activities of Nigella sativa L. Seed extracts. Bosn. J. Basic Med. Sci. 2017, 17, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Smolewska, M.; Purzyńska-Pugacewicz, A.; Tyszkiewicz, Z.E. Chemical composition of volatile and extractive compounds of pine and spruce leaf litter. Biomolecules 2010, 7, 2785–2794. [Google Scholar]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical composition of cumin seeds, and biorefining study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. sativa) flour and protein preparation as natural nutrients and structure forming agents in starch based gluten-free bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and tiger nut flours as alternatives to emulsifier and shortening in gluten-free bread. LWT 2015, 62, 225–232. [Google Scholar] [CrossRef]
- Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Cacak-Pietrzak, G.; Miś, A.; Rudy, S. Physical properties of gluten-free bread caused by water addition. Int. Agrophysics. 2015, 29, 353–364. [Google Scholar] [CrossRef]

| Fatty Acids | Black Cumin Seeds (BCS) (g/100 g) Mean ± SD * | Black Cumin Pressing Waste (BCW) (g/100 g) Mean ± SD * |
|---|---|---|
| C 12:0 lauric acid | 0.048 ± 0.006 a | 0.026 ± 0.004 b |
| C 14:0 myristic acid | 0.006 ± 0.002 a | 0.003 ± 0.001 b |
| C 16:0 palmitic acid | 4.522 ± 0.156 a | 2.392 ± 0.110 b |
| C 16:1 palmitoleic acid | 0.074 ± 0.011 a | 0.039 ± 0.005 b |
| C 18:0 stearic acid | 1.453 ± 0.103 a | 0.769 ± 0.043 b |
| C 18:1 oleic (n − 9) acid | 8.389 ± 0.130 a | 4.437 ± 0.112 b |
| C 18:2 linoleic (n − 6) acid | 18.954 ± 0.244 a | 10.025 ± 0.201 b |
| C 18:3 α-linolenic (n − 3) acid | 0.096 ± 0.011 a | 0.051 ± 0.003 b |
| C 20:0 arachidic acid | 0.063 ± 0.004 a | 0.034 ± 0.001 b |
| C 20:1 eicosenoic acid | 0.123 ± 0.011 a | 0.065 ± 0.004 b |
| C 20:2 eicosadienic acid | 0.871 ± 0.090 a | 0.461 ± 0.022 b |
| Kind of Sample | Specific Volume (cm3/g) | pH-Value | Crumb Color Values | |||
|---|---|---|---|---|---|---|
| L *-Value | A *-Value | B *-Value | ΔE | |||
| C | 1.56 ± 0.06 a | 6.09 ± 0.31 a | 50.9 ± 0.3 a | 5.07 ± 0.04 a | 7.62 ± 0.06 a | - |
| 2% BCS | 1.60 ± 0.07 a | 5.75 ± 0.29 ab | 41.1 ± 0.4 c | 3.87 ± 0.12 b | 6.90 ± 0.09 c | 9.93 |
| 4% BCS | 1.65 ± 0.07 a | 5.68 ± 0.22 ab | 39.2 ± 0.2 d | 2.83 ± 0.06 d | 6.40 ± 0.11 d | 12.05 |
| 6% BCS | 1.76 ± 0.07 b | 5.52 ± 0.24 b | 37.4 ± 0.3 e | 2.31 ± 0.04 e | 6.46 ± 0.03 d | 13.86 |
| 8% BCS | 1.74 ± 0.08 b | 5.46 ± 0.21 b | 35.8 ± 0.2 f | 1.50 ± 0.02 h | 6.82 ± 0.11 c | 15.51 |
| 10% BCS | 1.72 ± 0.07 b | 5.31 ± 0.25 b | 34.1 ± 0.2 g | 1.41 ± 0.01 i | 6.88 ± 0.09 c | 17.20 |
| 2% BCW | 1.70 ± 0.08 ab | 5.67 ± 0.27 ab | 44.1 ± 0.3 b | 3.58 ± 0.01 c | 6.98 ± 0.06 bc | 6.97 |
| 4% BCW | 1.78 ± 0.08 b | 5.49 ± 0.25 b | 39.8 ± 0.5 d | 2.99 ± 0.05 d | 7.26 ± 0.07 b | 11.30 |
| 6% BCW | 1.82 ± 0.07 b | 5.39 ± 0.26 b | 37.1 ± 0.4 e | 2.11 ± 0.05 f | 7.20 ± 0.09 b | 14.15 |
| 8% BCW | 1.79 ± 0.08 b | 5.33 ± 0.29 b | 35.6 ± 0.2 f | 1.71 ± 0.01 g | 7.63 ± 0.07 a | 15.66 |
| 10% BCW | 1.76 ± 0.09 b | 5.25 ± 0.21 b | 34.0 ± 0.3 g | 1.45 ± 0.02 hi | 7.52 ± 0.09 a | 16.85 |
| Kind of Sample | Protein (%) | Fat (%) | Fiber (%) | Carbohydrates (%) | Calorific Value kcal/100 g |
|---|---|---|---|---|---|
| C | 0.15 ± 0.005 a | 0.10 ± 0.002 a | 2.03 ± 0.17 a | 44.91 | 185.2 |
| 2% BCS | 0.37 ± 0.007 b | 0.47 ± 0.003 c | 2.16 ± 0.18 ab | 44.26 | 187.1 |
| 4% BCS | 0.58 ± 0.015 c | 0.84 ± 0.026 e | 2.29 ± 0.19 b | 43.60 | 188.9 |
| 6% BCS | 0.79 ± 0.022 d | 1.21 ± 0.009 f | 2.43 ± 0.16 bc | 42.95 | 190.8 |
| 2% BCW | 0.43 ± 0.006 e | 0.30 ± 0.006 b | 2.20 ± 0.17 ab | 44.34 | 186.2 |
| 4% BCW | 0.71 ± 0.012 f | 0.50 ± 0.011 c | 2.38 ± 0.18 bc | 43.77 | 187.1 |
| 6% BCW | 0.98 ± 0.018 g | 0.68 ± 0.017 d | 2.55 ± 0.19 c | 43.20 | 188.0 |
| 8% BCW | 1.27 ± 0.025 h | 0.89 ± 0.023 e | 2.73 ± 0.21 c | 45.45 | 200.3 |
| µg/g d.m. | C | 2% BCS | 4% BCS | 6% BCS | BCS | 2% BCW | 4% BCW | 6% BCW | 8% BCW | BCW |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenoxyacetic acid | 0.1163 d ± 0.0021 | 0.1029 c ± 0.0097 | 0.1044 c ± 0.0099 | 0.1031 c ± 0.0077 | 0.0230 a ± 0.0013 a | 0.1098 cd ± 0.0098 | 0.1080 cd ± 0.0102 | 0.1146 cd ± 0.0113 | 0.1153 cd ± 0.0088 | 0.0774 b ± 0.0055 b |
| 2-Methoxybenzoic acid (Anisic) | 0.0048 abc ± 0.0013 | 0.0042 ab ± 0.0004 | 0.0047 bc ± 0.0005 | 0.0039 abc ± 0.0011 | 0.0039 a ± 0.0004 | 0.0046 abc ± 0.0012 | 0.0046 bc ± 0.0004 | 0.0051 c ± 0.0006 | 0.0051abc ± 0.0010 | 0.0043 abc ± 0.0013 |
| 2-Hydroxybenzoic acid (Salicylic) | 0.2004 a ± 0.0017 | 0.3326 b ± 0.0086 | 0.3767 c ± 0.0303 | 0.5254 d ± 0.0029 | 7.7710 h ± 0.5983 | 0.6096 e ± 0.0553 | 0.7210 f ± 0.0284 | 0.9193 g ± 0.1040 | 1.0903 g ± 0.0992 | 9.5139 i ± 0.2078 |
| Cinnamic acid | 1.4404 c ± 0.0697 | 1.4516 c ± 0.1024 | 1.4919 c ± 0.0861 | 1.5073 c ± 0.2044 | 0.0771 a ± 0.0066 | 1.4475 c ± 0.1055 | 1.4480 c ± 0.0997 | 1.4593 c ± 0.1144 | 1.4626 c ± 0.1206 | 0.1098 b ± 0.0082 |
| 2-Hydroxyphenylacetic acid | 0.2545 a ± 0.0203 | 0.2999 ab ± 0.0311 | 0.2996 b ± 0.0212 | 0.3422 c ± 0.0099 | 0.4510 de ± 0.0408 | 0.3750 d ± 0.0321 | 0.3837 d ± 0.0299 | 0.5146 e ± 0.0358 | 0.9769 f ± 0.0881 | 2.2954 g ± 0.2246 |
| 4-Hydroxyphenylacetic acid | 0.7185 b ± 0.0351 | 0.7242 b ± 0.0460 | 0.7846 bc ± 0.0594 | 0.8355 c ± 0.0701 | 0.3552 a ± 0.0299 | 0.7521 b ± 0.0078 | 0.8564 c ± 0.0630 | 0.9255 c ± 0.1061 | 0.9649 c ± 0.1022 | 1.8789 d ± 0.1605 |
| o-Coumaric acid | 0.0097 d ± 0.0004 | 0.0071 b ± 0.0015 | 0.0088 c ± 0.0008 | 0.0095 cd ± 0.0008 | 0.0055 a ± 0.0011 | 0.0122 de ± 0.0021 | 0.0146 e ± 0.0009 | 0.0150 e ± 0.0012 | 0.0152 e ± 0.0011 | 0.0756 f ± 0.0063 |
| p-Coumaric acid | 0.0386 bc ± 0.0022 | 0.0410 bc ± 0.0042 | 0.0419 bc ± 0.0043 | 0.0490 d ± 0.0024 | 0.0218 a ± 0.0018 | 0.0376 bc ± 0.0032 | 0.0385 b ± 0.0012 | 0.0425 c ± 0.0029 | 0.0455 cd ± 0.0028 | 0.0425 bcd ± 0.0054 |
| Ferulic acid | - | - | - | - | 0.0051 a ± 0.0007 | - | - | - | 0.0046 b ± 0.0012 | 0.0395 c ± 0.0064 |
| Sinapinic acid | - | - | - | - | - | - | - | - | - | 0.0015 a ± 0.0006 |
| Chlorogenic acid | - | - | - | 0.0784 a ± 0.0072 | 0.4241 b ± 0.0398 | - | - | - | 0.1113 a ± 0.0431 | 0.5561 b ± 0.1588 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różyło, R.; Piekut, J.; Wójcik, M.; Kozłowicz, K.; Smolewska, M.; Krajewska, M.; Szmigielski, M.; Bourekoua, H. Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread. Materials 2021, 14, 4560. https://doi.org/10.3390/ma14164560
Różyło R, Piekut J, Wójcik M, Kozłowicz K, Smolewska M, Krajewska M, Szmigielski M, Bourekoua H. Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread. Materials. 2021; 14(16):4560. https://doi.org/10.3390/ma14164560
Chicago/Turabian StyleRóżyło, Renata, Jolanta Piekut, Monika Wójcik, Katarzyna Kozłowicz, Marzena Smolewska, Marta Krajewska, Marek Szmigielski, and Hayat Bourekoua. 2021. "Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread" Materials 14, no. 16: 4560. https://doi.org/10.3390/ma14164560
APA StyleRóżyło, R., Piekut, J., Wójcik, M., Kozłowicz, K., Smolewska, M., Krajewska, M., Szmigielski, M., & Bourekoua, H. (2021). Black Cumin Pressing Waste Material as a Functional Additive for Starch Bread. Materials, 14(16), 4560. https://doi.org/10.3390/ma14164560









