An Innovative Digestion Method: Ultrasound-Assisted Electrochemical Oxidation for the Onsite Extraction of Heavy Metal Elements in Dairy Farm Slurry
Abstract
:1. Introduction
2. Materials and Method
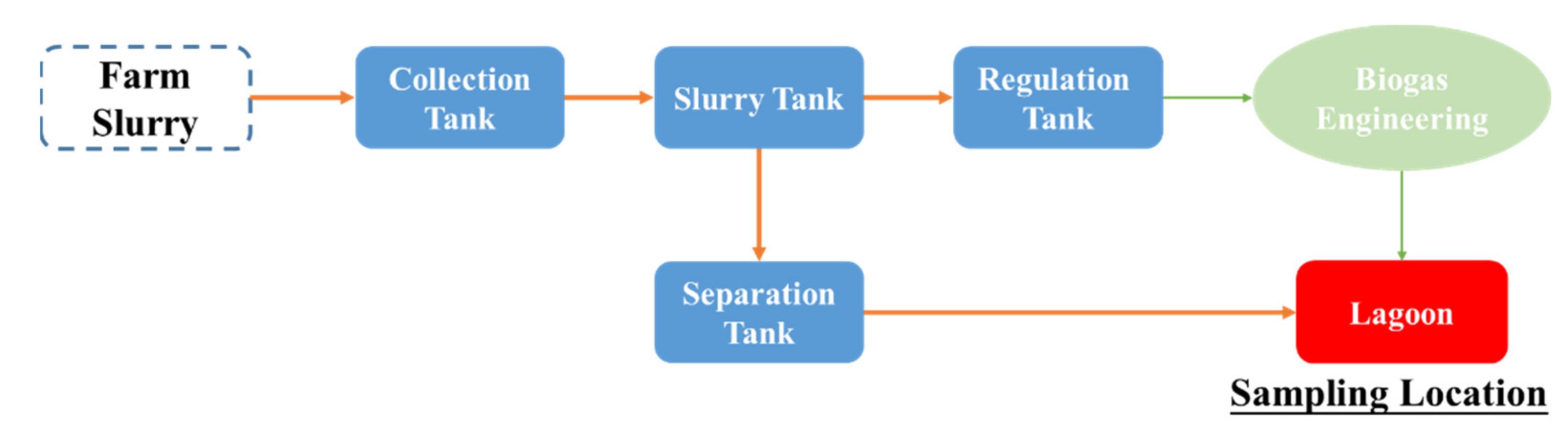
2.1. Reagents, Standards, and Samples
2.2. Construction of the UAEO Digestion Apparatus
2.3. UAEO Digestion Method and Zn and Cu Digestion Efficiency Test
2.4. Digestion Recovery Rate
2.5. Chlorine Quantitative Analysis
2.6. Particle Size Distribution and Micromorphology
2.7. Statistical Analysis
3. Results
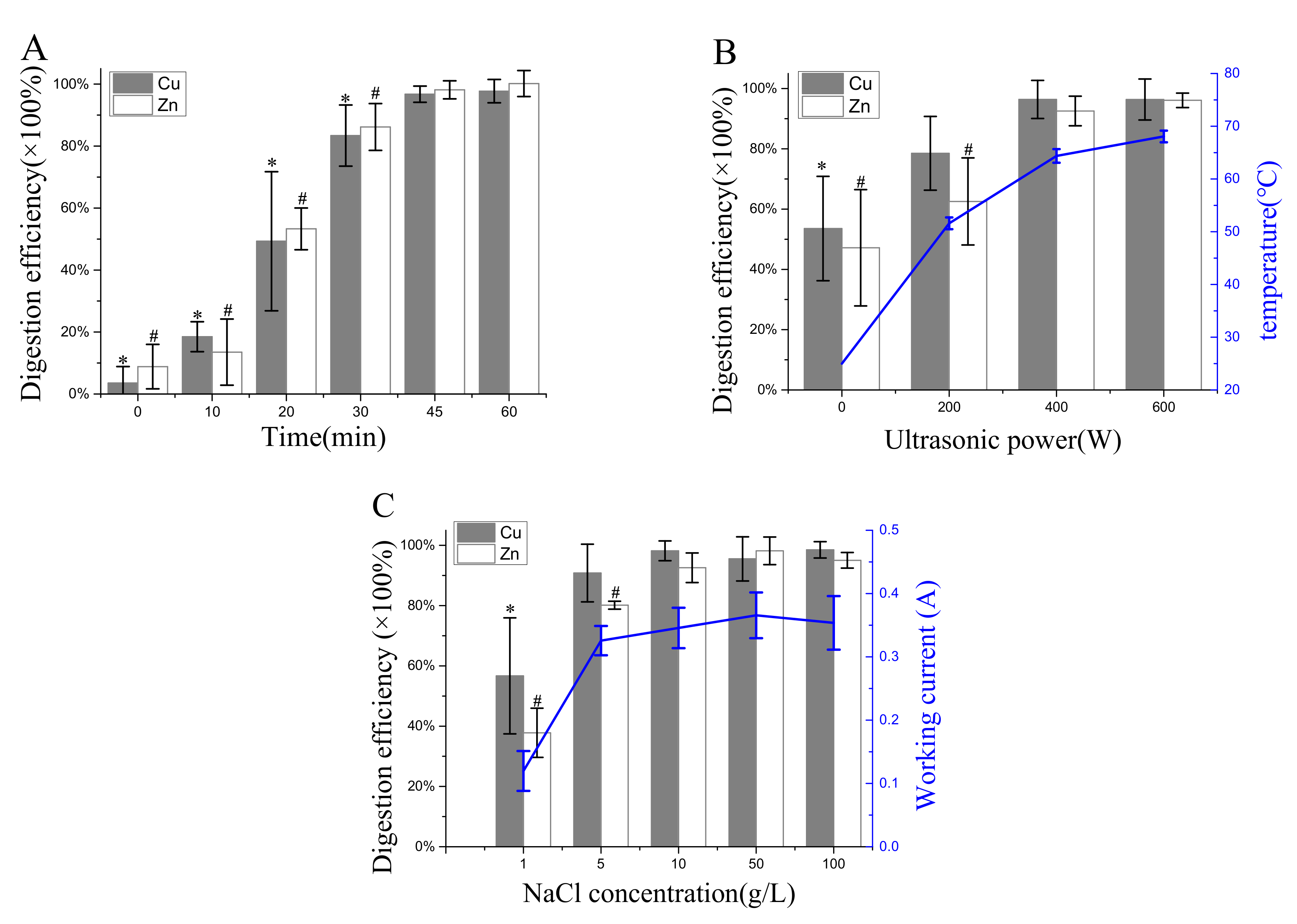
3.1. Effects of UAEO Operating Parameters on Digestion Efficiency
3.2. UAEO Digestion Recovery Rate
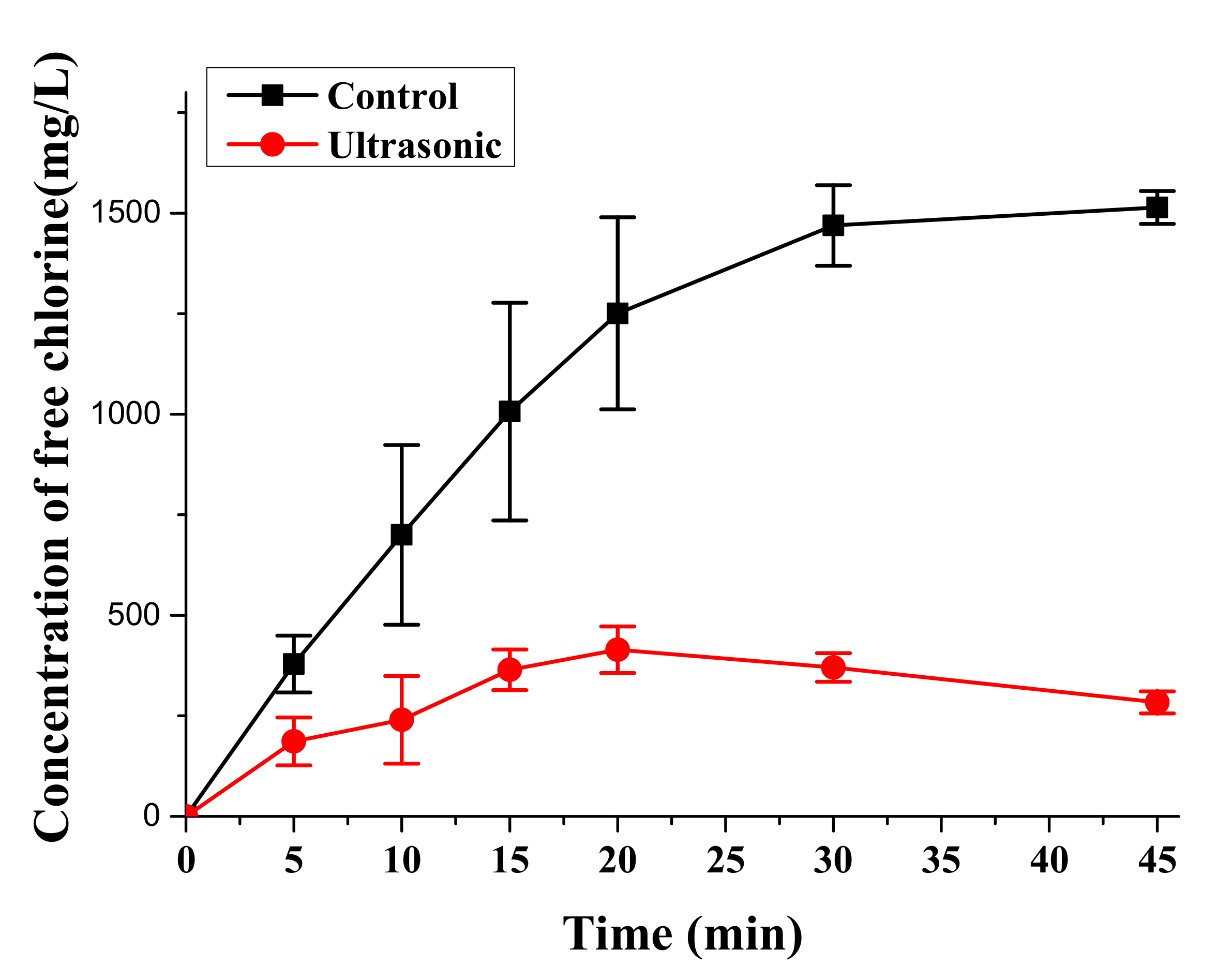
3.3. Free Chlorine
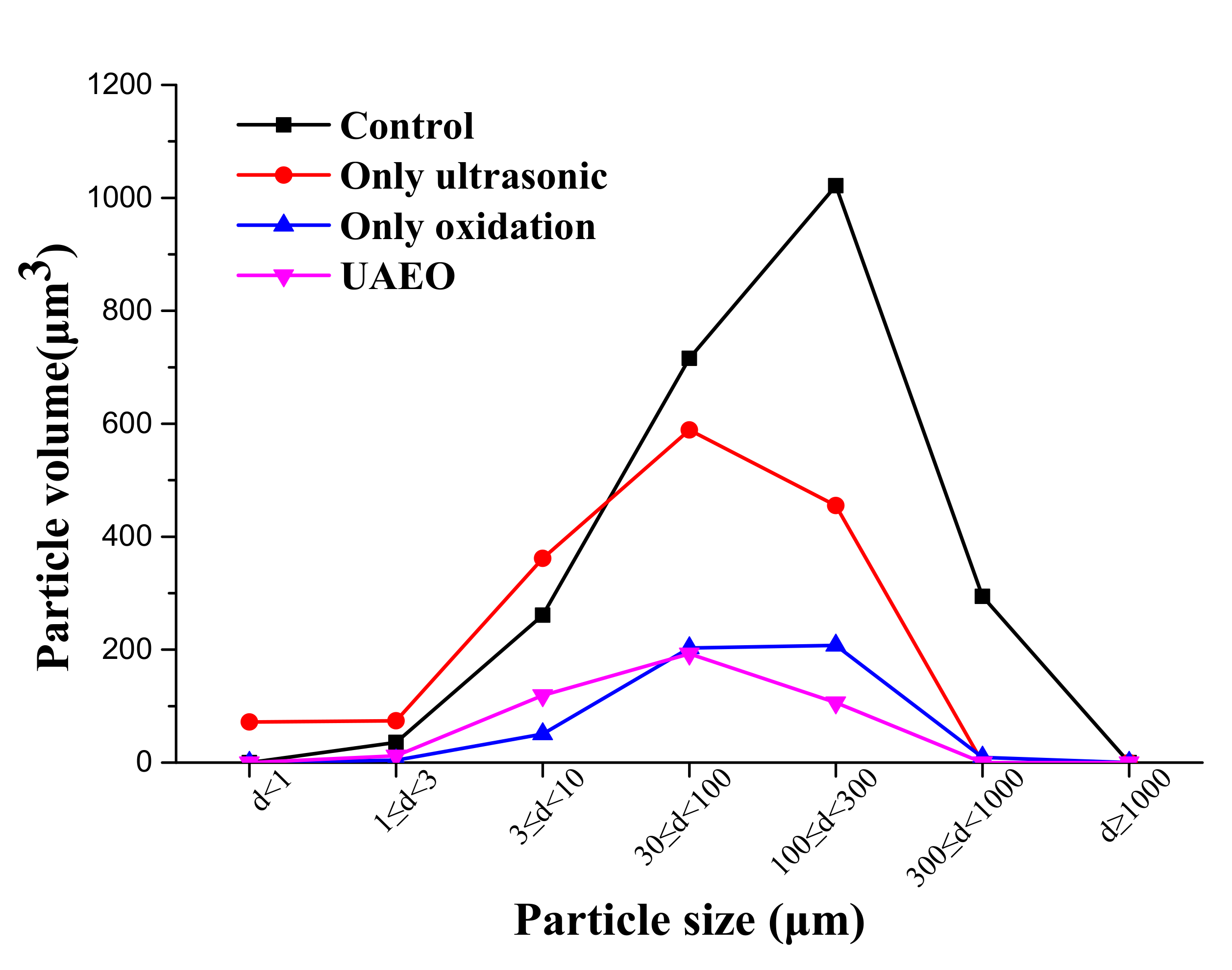
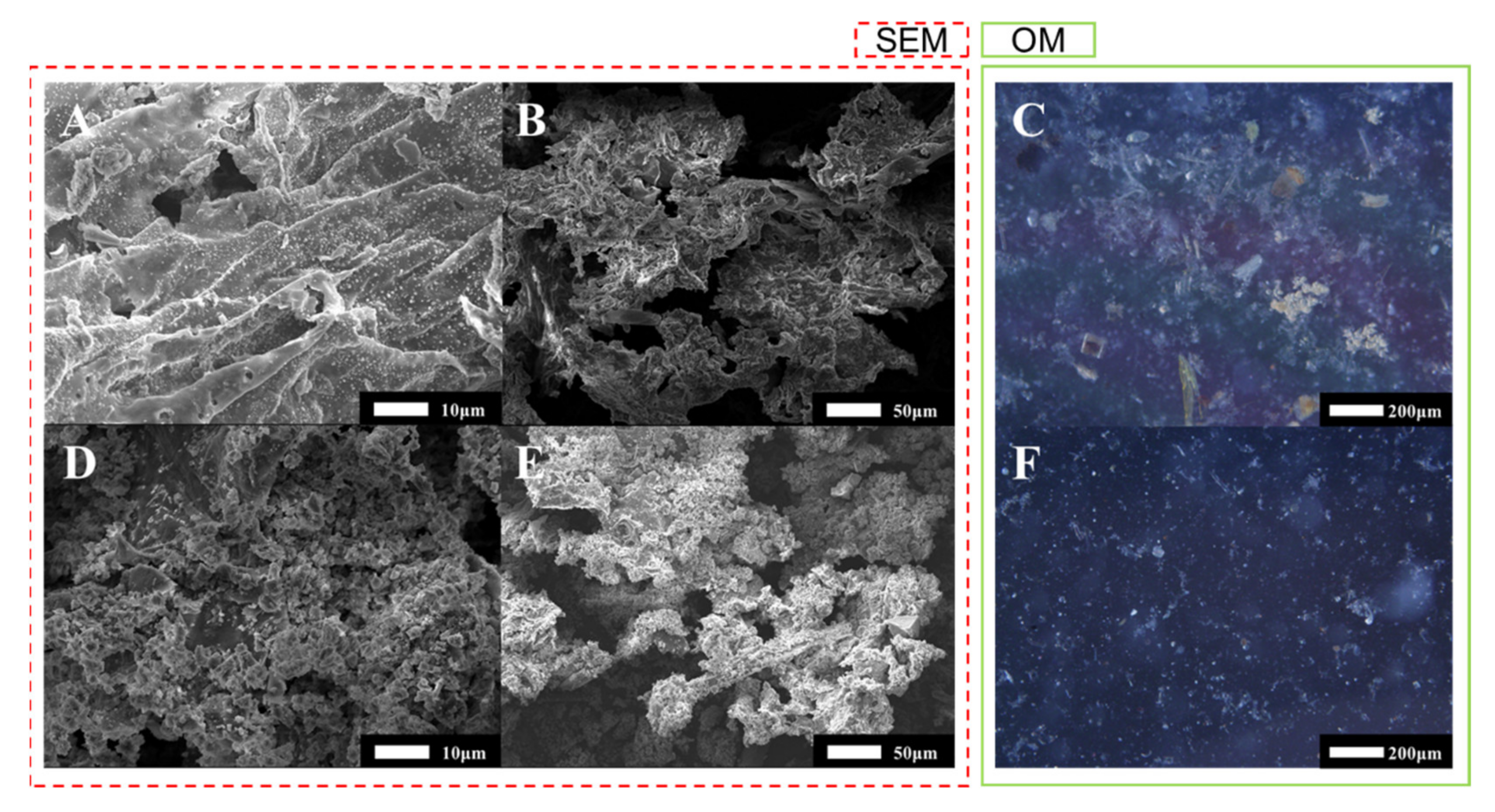
3.4. Effect of the UAEO Process on Particle Size Distribution and Micromorphology
4. Discussion
4.1. Digestion Theory of the UAEO Method
4.2. Digestion Efficiency of the UAEO Method
4.3. Digestion Mechanism of the UAEO Method
4.4. Advantages of the UAEO Digestion Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.Z.; Zhuo, C.; Li, Y.J.; Li, H.S.; Yang, M.Y.; Xu, D.N.; He, H.Z. Evaluation of filamentous heterocystous cyanobacteria for integrated pig-farm biogas slurry treatment and bioenergy production. Bioresour. Technol. 2020, 297, 9. [Google Scholar] [CrossRef]
- Muhammad, L.; Tareq, A. A novel solution towards zero waste in dairy farms: A thermodynamic study of an integrated polygeneration approach. Energy Convers. Manag. 2021, 230, 113753. [Google Scholar]
- Tethi, B.; Shashi, B.; Kumar, P.S.; Shaon, R.C. An eco-friendly strategy for dairy wastewater remediation with high lipid microalgae-bacterial biomass production. J. Environ. Manag. 2021, 286, 112196. [Google Scholar]
- Wang, X.; Ledgard, S.; Luo, J.; Guo, Y.; Zhao, Z.; Guo, L.; Liu, S.; Zhang, N.; Duan, X.; Ma, L. Environmental impacts and resource use of milk production on the North China Plain, based on life cycle assessment. Sci. Total Environ. 2018, 625, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wei, Y.; Zhao, Y.; Wang, Q.; Han, J. Heavy Metal Pollution and Ecological Risk Assessment of the Agriculture Soil in Xunyang Mining Area, Shaanxi Province, Northwestern China. Bull. Environ. Contam. Toxicol. 2018, 101, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jean, H.; Eléonore, L.; Carole, S.; Arnaud, H. Identifying the resource use and circularity in farm systems: Focus on the energy analysis of agroecosystems. Resour. Conserv. Recycl. 2021, 169, 105502. [Google Scholar]
- Li, J.; Akdeniz, N.; Kim, H.H.M.; Gates, R.S.; Wang, X.; Wang, K. Optimal manure utilization chain for distributed animal farms: Model development and a case study from Hangzhou, China. Agric. Syst. 2021, 187, 102996. [Google Scholar] [CrossRef]
- Phitsanu, T.; Alongkot, B.; Suwicha, K.; Srisamai, W.; Juree, P.; Suwanna, T.; Hathairad, H.; Ratchaneekorn, M.; Ramnaree, N.; Sutha, K. Comparative study of heavy metal and pathogenic bacterial contamination in sludge and manure in biogas and non-biogas swine farms. J. Environ. Sci. 2011, 23, 991–997. [Google Scholar]
- Roa Engel Carol, A.; Van Gulik Walter, M.; Leonie, M.; Van Der Wielen Luuk, A.M.; Straathof Adrie, J.J. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzym. Microb. Technol. 2011, 48, 39–47. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, W.; Chen, S. Production of cellulase by Trichoderma reesei from dairy manure. Bioresour. Technol. 2005, 96, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland–A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, X.; Xi, Z.; Lin, S.; Sun, X.; Jiang, X.; Tian, H. Individual heavy metal exposure and birth outcomes in Shenqiu county along the Huai River Basin in China. Toxicol. Res. 2018, 7, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Xiaodong, W.; Yu, Z.; Qingwen, D.; Shoulian, J.; Xia, Z.; Jie, G. Investigation of novel rapidly synergistic cloud point extraction pattern for bismuth in water and geological samples coupling with flame atomic absorption spectrometry determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 1–6. [Google Scholar]
- Chen, L.; Lei, Z.; Yang, S.; Wen, X. Application of portable tungsten coil electrothermal atomic absorption spectrometer for the determination of trace cobalt after ultrasound-assisted rapidly synergistic cloud point extraction. Microchem. J. 2017, 130, 452–457. [Google Scholar] [CrossRef]
- Wen, X.; Yang, S.; Zhang, H.; Deng, Q. Combination of knotted reactor with portable tungsten coil electrothermal atomic absorption spectrometer for on-line determination of trace cadmium. Microchem. J. 2016, 124, 60–64. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Li, C.; He, X.; Liu, G. On-site detection of heavy metals in agriculture land by a disposable sensor based virtual instrument. Comput. Electron. Agric. 2016, 123, 176–183. [Google Scholar] [CrossRef]
- Ajay, P.V.S.; Printo, J.; Kiruba, D.S.C.G.; Susithra, L.; Takatoshi, K.; Sivakumar, M. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar]
- Santos Daniele, C.M.B.; Carvalho Larissa, S.B.; Lima Daniel, C.; Leão Danilo, J.; Teixeira Leonardo, S.G.; Korn Maria Graças, A. Determination of micronutrient minerals in coconut milk by ICP OES after ultrasound-assisted extraction procedure. J. Food Compos. Anal. 2014, 34, 75–80. [Google Scholar] [CrossRef]
- Luisa, A.M.; Elisabetta, M.; Carmela, P.; Matteo, V.; Elisa, S.; Paola, M.; Silvia, C. Optimization and validation of a fast digestion method for the determination of major and trace elements in breast milk by ICP-MS. Anal. Chim. Acta 2018, 1040, 49–62. [Google Scholar]
- Fernandes, D.O.A.; Santos, C.; Rossana, B.S.; Araujo, N.A.R. The use of diluted formic acid in sample preparation for macro- and microelements determination in foodstuff samples using ICP OES. J. Food Compos. Anal. 2018, 66, 7–12. [Google Scholar] [CrossRef]
- Krishna, M.V.B.; Chandrasekaran, K.; Venkateswarlu, G.; Karunasagar, D. Development of a simple and rapid microwave-assisted extraction method using very dilute solutions of perchloric acid and hydrogen peroxide for the multi-elemental analysis of food materials by ICP-OES: A green analytical method. Microchem. J. 2019, 146, 807–817. [Google Scholar]
- Kuznetsova, O.V.; Burmii, Z.P.; Orlova, T.V.; Sevastyanov, V.S.; Timerbaev, A.R. Quantification of the diagenesis-designating metals in sediments by ICP-MS: Comparison of different sample preparation methods. Talanta 2019, 200, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Rovasi, A.F.; Cícero, D.N.P.; Camera, L.G.; Denise, B.; Carine, V.; Machado, L. Simultaneous determination of Fe and Ni in guarana (Paullinia cupana Kunth) by HR-CS GF AAS: Comparison of direct solid analysis and wet acid digestion procedures. J. Food Compos. Anal. 2020, 88, 103459. [Google Scholar]
- Da Silva, I.J.S.; Lavorante André, F.; Paim, A.P.S.; Da Silva, M.J. Microwave-assisted digestion employing diluted nitric acid for mineral determination in rice by ICP OES. Food Chem. 2020, 319, 126435. [Google Scholar] [CrossRef]
- Vimlesh, C.; Surendra, P. ICP-OES assessment of heavy metal contamination in tropical marine sediments: A comparative study of two digestion techniques. Microchem. J. 2013, 111, 53–61. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Water Works Association: Washington, DC, USA, 2012. [Google Scholar]
- Ge, B.; Hunter, J.; Hunter, W. Satistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Tadeo, J.L.; Sánchez-Brunete, C.; Albero, B.; García-Valcárcel, A.I. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]
- Musah, S.; Schlueter, C.F.; Humphrey, D.M.; Powell, K.S.; Roberts, A.M.; Hoyle, G.W. Acute lung injury and persistent small airway disease in a rabbit model of chlorine inhalation. Toxicol. Appl. Pharmacol. 2017, 315, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]

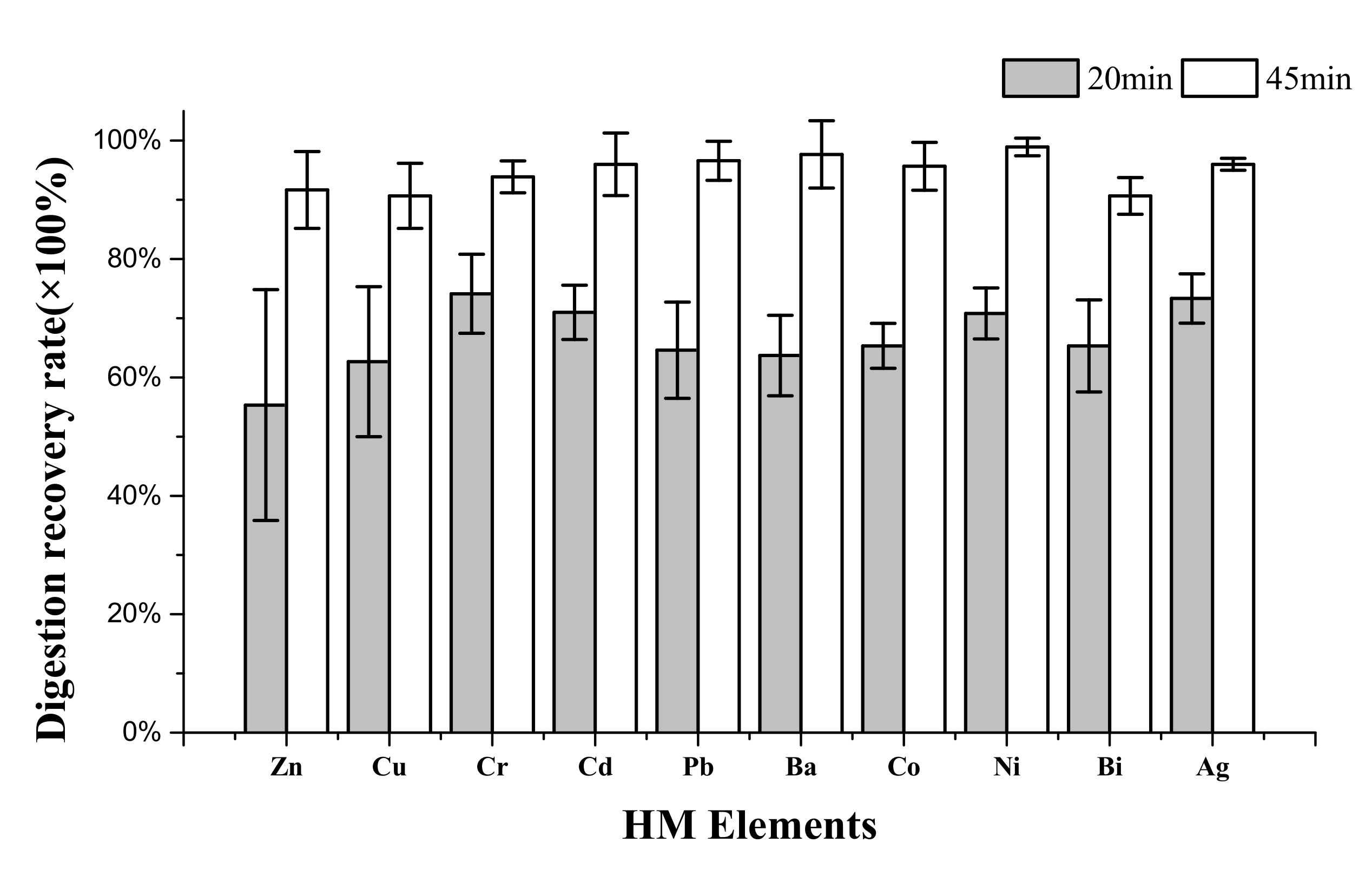
| Element | Certified Values (mg/L) | Found Values (mg/L) and Recovery Rates (%) | |||
|---|---|---|---|---|---|
| 20 min | 45 min | ||||
| Zn | 0 a | 1.25 ± 0.28 | 55.3% | 2.24 ± 0.45 | 91.7% |
| 1 b | 1.8 ± 0.09 | 3.16 ± 0.44 | |||
| Cu | 0 | 0.29 ± 0.05 | 62.7% | 0.54 ± 0.07 | 90.7% |
| 1 | 0.92 ± 0.17 | 1.45 ± 0.09 | |||
| Cr | 0 | 0.06 ± 0.03 | 74.1% | 0.11 ± 0.03 | 93.9% |
| 5 | 3.77 ± 0.35 | 4.81 ± 0.16 | |||
| Cd | 0 | ND c | 71% | ND | 96% |
| 1 | 0.71 ± 0.05 | 0.96 ± 0.05 | |||
| Pb | 0 | ND | 64.6% | 0.08 ± 0.04 | 96.6% |
| 10 | 6.46 ± 0.81 | 9.74 ± 0.36 | |||
| Ba | 0 | ND | 63.7% | ND | 97.7% |
| 1 | 0.64 ± 0.07 | 0.98 ± 0.06 | |||
| Co | 0 | ND | 65.3% | ND | 95.7% |
| 1 | 0.65 ± 0.04 | 0.96 ± 0.04 | |||
| Ni | 0 | ND | 70.8% | ND | 98.9% |
| 5 | 3.54 ± 0.22 | 4.95 ± 0.08 | |||
| Bi | 0 | ND | 65.3% | ND | 97.8% |
| 10 | 6.53 ± 0.78 | 9.78 ± 0.31 | |||
| Ag | 0 | ND | 73.3% | ND | 96% |
| 1 | 0.73 ± 0.04 | 0.96 ± 0.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xue, B.; Wang, S.; Zhang, X.; Zhao, C.; Yang, X.; Zhao, R.; Dai, L.; Su, S.; Xu, H.; et al. An Innovative Digestion Method: Ultrasound-Assisted Electrochemical Oxidation for the Onsite Extraction of Heavy Metal Elements in Dairy Farm Slurry. Materials 2021, 14, 4562. https://doi.org/10.3390/ma14164562
Li C, Xue B, Wang S, Zhang X, Zhao C, Yang X, Zhao R, Dai L, Su S, Xu H, et al. An Innovative Digestion Method: Ultrasound-Assisted Electrochemical Oxidation for the Onsite Extraction of Heavy Metal Elements in Dairy Farm Slurry. Materials. 2021; 14(16):4562. https://doi.org/10.3390/ma14164562
Chicago/Turabian StyleLi, Chenyu, Bin Xue, Shang Wang, Xi Zhang, Chen Zhao, Xiaobo Yang, Run Zhao, Lin Dai, Shengqi Su, Haoqi Xu, and et al. 2021. "An Innovative Digestion Method: Ultrasound-Assisted Electrochemical Oxidation for the Onsite Extraction of Heavy Metal Elements in Dairy Farm Slurry" Materials 14, no. 16: 4562. https://doi.org/10.3390/ma14164562
APA StyleLi, C., Xue, B., Wang, S., Zhang, X., Zhao, C., Yang, X., Zhao, R., Dai, L., Su, S., Xu, H., Shen, Z., Qiu, Z., & Wang, J. (2021). An Innovative Digestion Method: Ultrasound-Assisted Electrochemical Oxidation for the Onsite Extraction of Heavy Metal Elements in Dairy Farm Slurry. Materials, 14(16), 4562. https://doi.org/10.3390/ma14164562







