Effect of Heat Treatment Temperature on Microstructure and Properties of PM Borated Stainless Steel Prepared by Hot Isostatic Pressing
Abstract
:1. Introduction
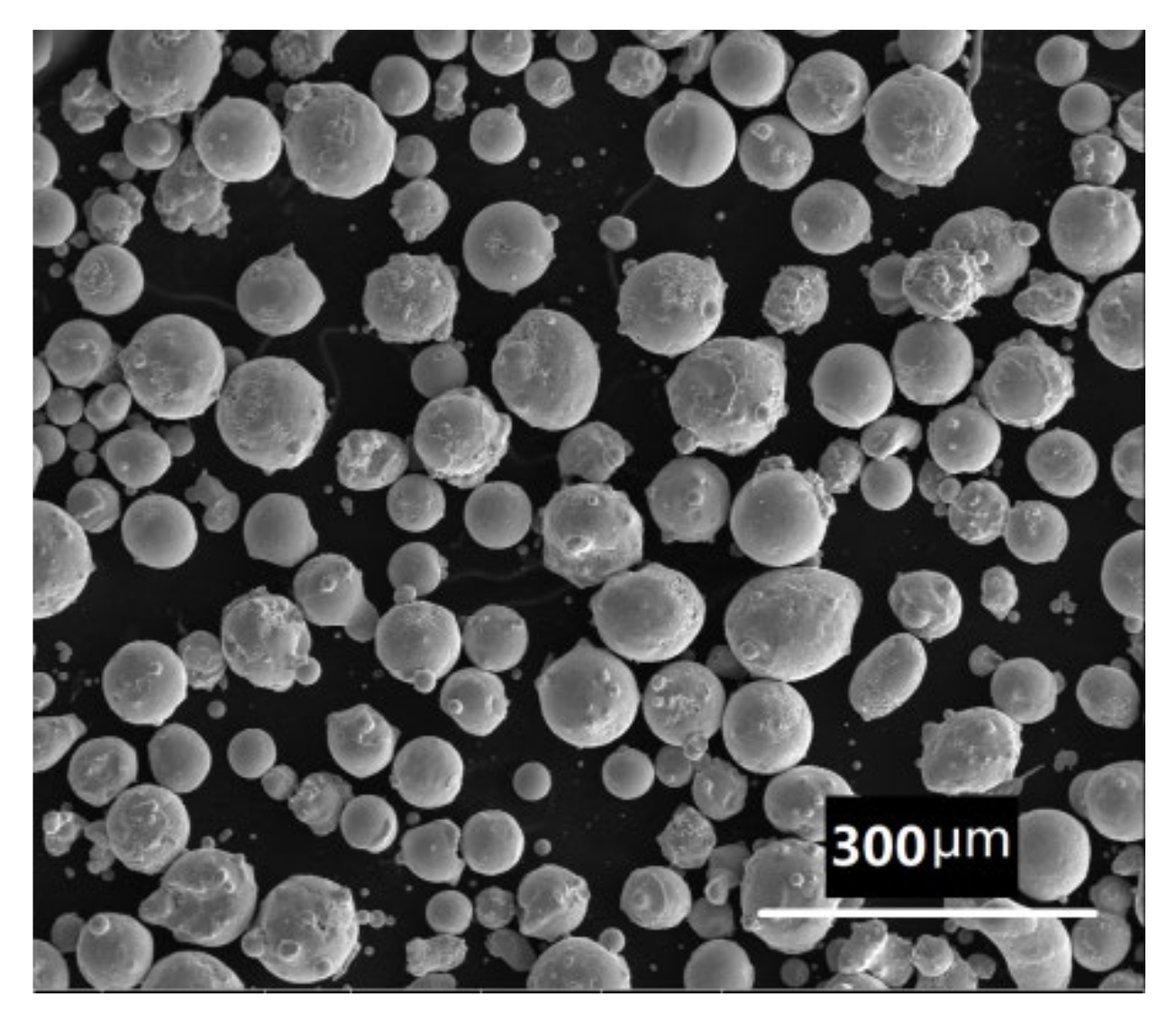
2. Materials and Methods
3. Results
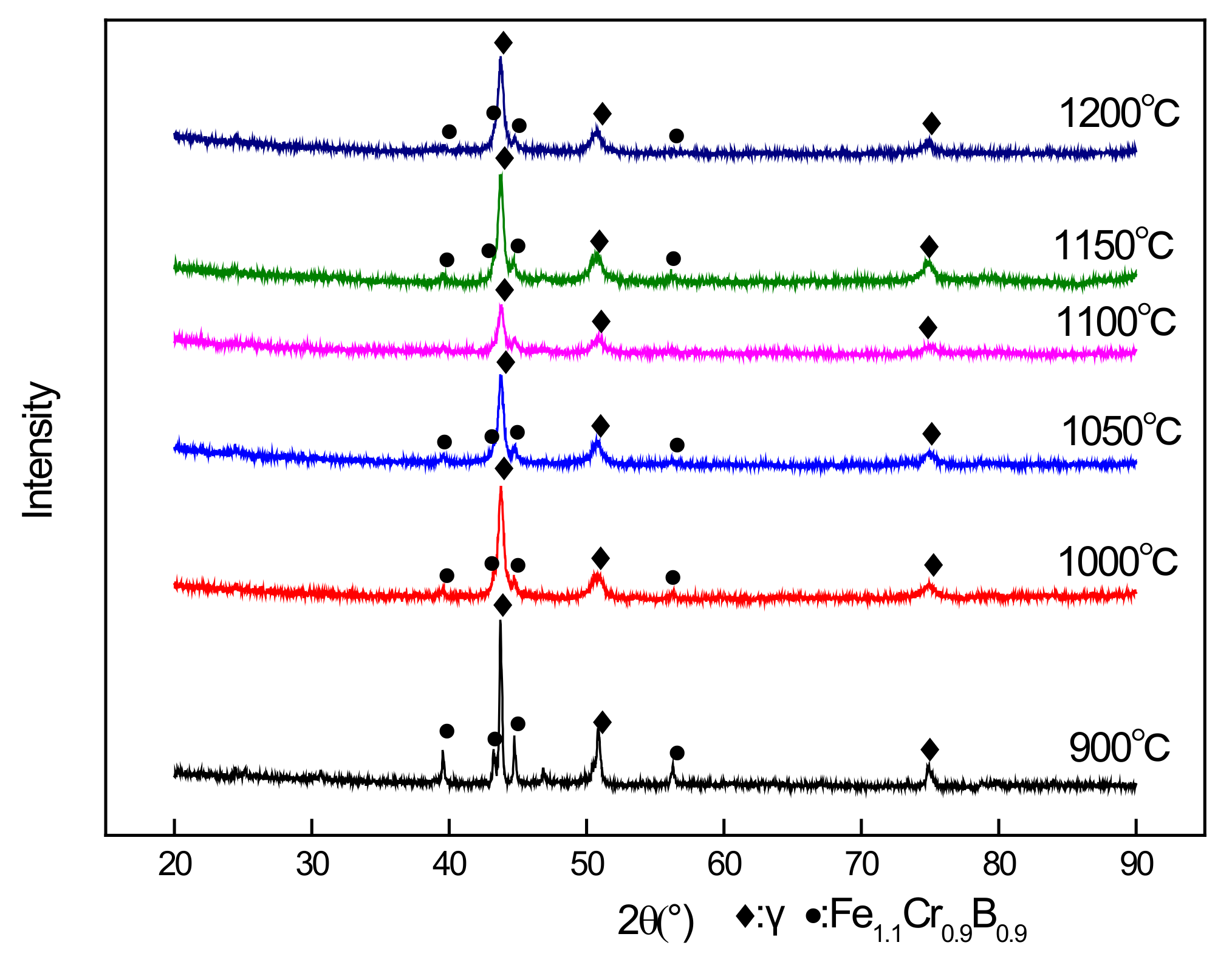
3.1. Quantitative X-ray Diffraction Phase Analysis
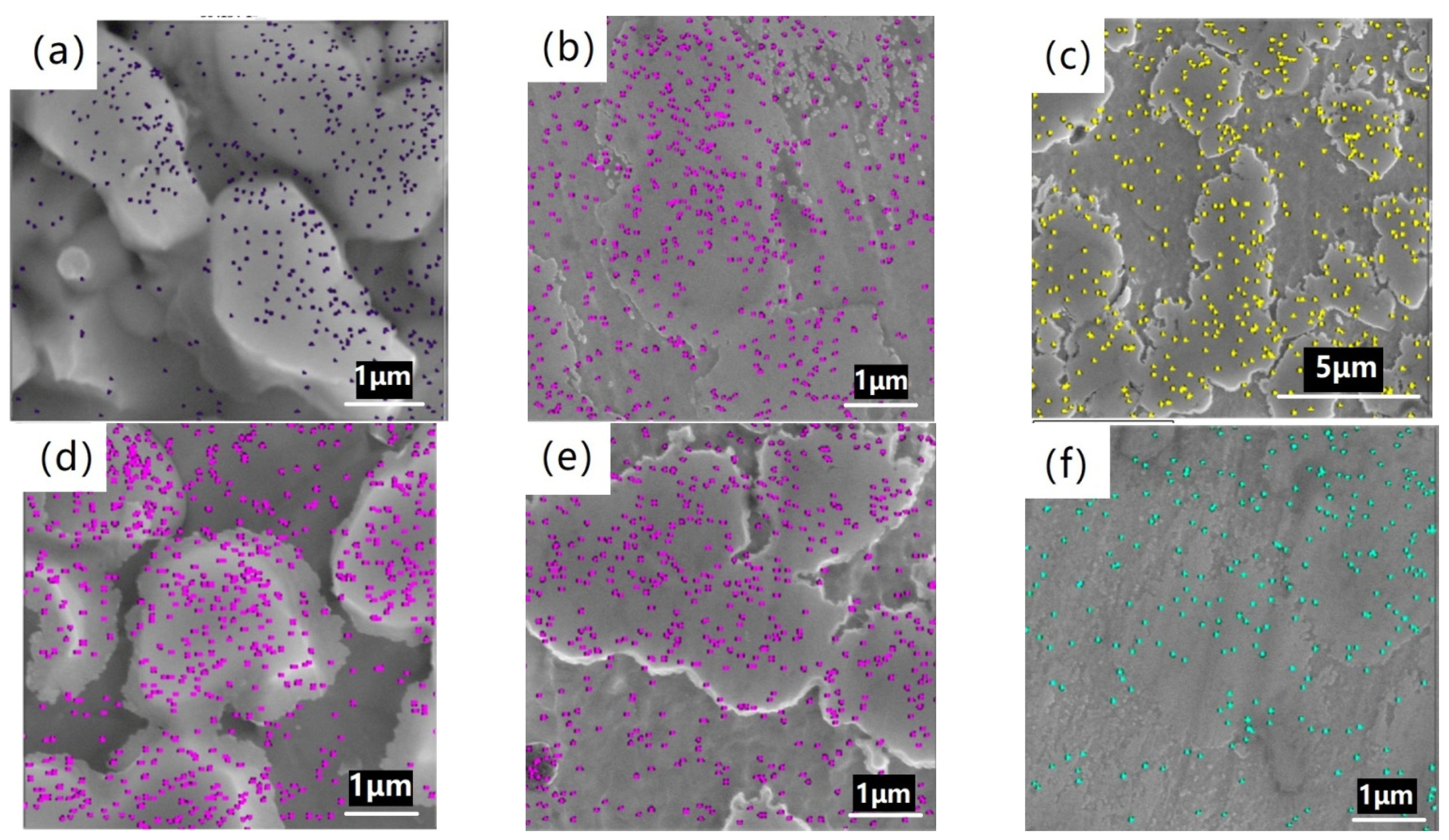
3.2. Metallographic Structure
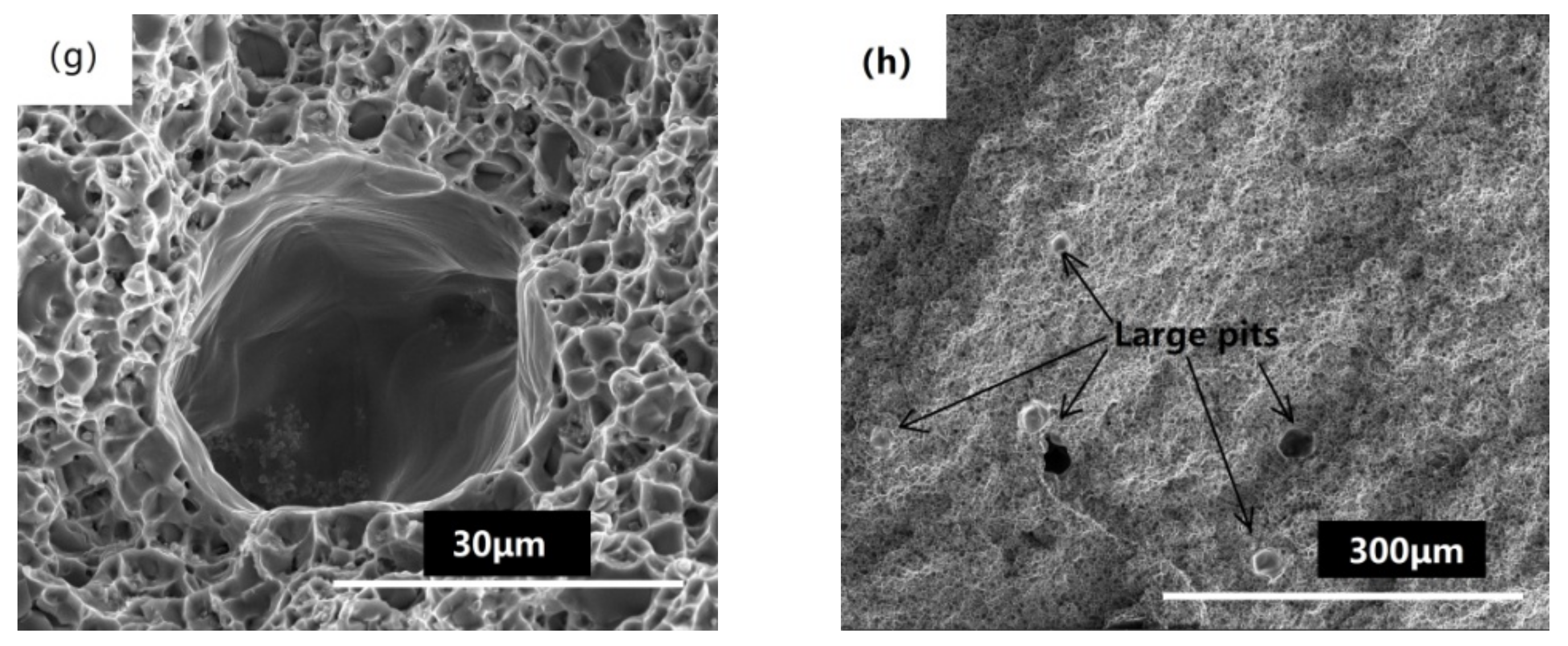
3.3. Mechanical Properties
4. Discussion
5. Conclusions
- Borated stainless steel prepared by PM consists of two phases: an austenite matrix and a hard boron phase (Fe1.1Cr0.9B0.9) distributed in chromium-rich austenite after heat treatment at different temperatures.
- Due to the diffusion coefficient and binding force between the elements varying at different temperatures; it leads to a different distribution of each element in the boron phase and austenite matrix.
- Alloy with good strength and plasticity can be obtained when the heat treatment temperature of high boron stainless steel reaches 1000–1150 °C. The tensile strength is approximately 800 MPa, and the elongation is approximately 20%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivai Bharasi, N.; Pujar, M.G.; Nirmal, S.; Mallika, C.; Kamachi Mudali, U.; Angelo, P.C. Comparison of SCC behavior of 304L stainless steels with and without boron addition in acidic chloride environment. J. Mater. Eng. Perform. 2016, 25, 2786–2798. [Google Scholar] [CrossRef]
- Moon, J.; Jang, J.H.; Kim, S.-D.; Lee, T.-H.; Ha, H.-Y.; Lee, C.-H.; Hong, H.-U. Different aspect of solidification cracking susceptibility and hot ductility behavior of borated stainless steels and the effects of boron content. Mater. Charact. 2020, 164, 110319. [Google Scholar] [CrossRef]
- Won, C.-H.; Jang, J.H.; Kim, S.-D.; Moon, J.; Ha, H.-Y.; Kang, J.-Y.; Lee, C.-H.; Lee, T.-H.; Kang, N. Effect of annealing on mechanical properties and microstructure evolution of borated stainless steels. J. Nucl. Mater. 2019, 515, 206–214. [Google Scholar] [CrossRef]
- Li, Y.W.; Wang, Z.J.; Fu, D.G.; Li, G.; Liu, H.T.; Zhang, X.M. Fabrication of high borated austenitic stainless steel thick plates with enhanced ductility and toughness using a hot-roll-bonding method. Mater. Sci. Eng. A 2021, 799, 140212. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, M.; Fu, Y.; Wang, Z.; Li, Y.; Yang, S.; Zhao, H.; Li, H. Effect of borides on hot deformation behavior and microstructure evolution of powder metallurgy high borated stainless steel. Mater. Charact. 2017, 124, 182–191. [Google Scholar] [CrossRef]
- Stinner, C. The Application and Properties of ATI NuShield™ Borated Stainless Steels Properties of ATI NuShield™ Borated Stainless Steels. In Proceedings of the Corrosion Conference and Expo 2011, Houston, TX, USA, 13–17 March 2011; pp. 297–305. [Google Scholar]
- Serafini, F.L.; Peruzzo, M.; Krindges, I.; Ordoñez, M.F.C.; Rodrigues, D.; Souza, R.M.; Farias, M.C.M. Microstructure and mechanical behavior of 316L liquid phase sintered stainless steel with boron addition. Mater. Charact. 2019, 152, 253–264. [Google Scholar] [CrossRef]
- Busby, P.E.; Warga, M.E.; Wells, C. Diffusion and Solubility of Boron in Iron and Steel. JOM 1953, 5, 1463–1468. [Google Scholar] [CrossRef]
- Guo, C.; Kelly, P.M. Boron solubility in Fe-Cr-B cast irons. Mater. Sci. Eng. 2003, A352, 40–45. [Google Scholar] [CrossRef]
- Shigesato, G.; Fujishiro, T.; Hara, T. Grain Boundary Segregation Behavior of Boron in Low-Alloy Steel. Met. Mater. Trans. A 2014, 45, 1876–1882. [Google Scholar] [CrossRef]
- Bondar, A. Boron—Chromium—Iron; Landolt-Börnstein, Group IV Physical Chemistry: Berlin/Heidelberg, Germany, 2008; pp. 403–432. [Google Scholar]
- Zhang, X.; Li, X.; Wu, P.; Chen, S.; Zhang, S.; Chen, N.; Huai, X. First principles calculation of boron diffusion in fcc-Fe. Curr. Appl. Phys. 2018, 18, 1108–1112. [Google Scholar] [CrossRef]
- Campos, I.; Ramirez, G.; Figueroa, U.; Martinez, J.; Morales, O. Evaluation of boron mobility on the phases FeB, Fe2B and diffusion zone in AISI 1045 and M2 steels. Appl. Surf. Sci. 2007, 253, 3469–3475. [Google Scholar] [CrossRef]
- Miyamoto, G.; Goto, A.; Takayama, N.; Furuhara, T. Three-dimensional atomprobe analysis of boron segregation at austenite grain boundary in a low carbon steel—Effects of boundarymisorientation and quenching temperature. Scr. Mater. 2018, 154, 168–171. [Google Scholar] [CrossRef]
- Park, D.Y.; Shin, D.S.; Park, H.J.; Baik, Y.-J.; Oh, Y.J.; Park, S.J. Sintering behavior of addictive boron in stainless steel via master sintering curves and microstructural verification using image processing. Mater. Charact. 2019, 149, 63–73. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kim, S.-D.; Jang, J.H.; Lee, T.-H.; Lee, C.-H.; Moon, J. Pitting Corrosion and Passive Behavior of Type AISI 304-based Borated Stainless Steels in a Boric Acid Solution. J. Electrochem. Soc. 2020, 167, 101506. [Google Scholar] [CrossRef]
- Loria, E.A.; Isaacs, H.S. Type 304 Stainless Steel With 0.5% Boron for Storage of Spent Nuclear Fuel. JOM 1980, 32, 10–17. [Google Scholar] [CrossRef]
- Robino, C.V.; Cieslak, M.J. Effect of annealing on mechanical properties and microstructure evolution of borated stainless steels. Metall. Mater. Trans. A 1995, 26A, 1674–1685. [Google Scholar]
- Kudin, V.G.; Makara, V.A. Thermodynamic Properties of Metal–Boron Alloys. Inorg. Mater. 2002, 38, 216–219. [Google Scholar] [CrossRef]
- Perkins, R.A.; Padgett, R.A.; Tunali, N.K. Tracer diffusion of 59Fe and 51Cr in Fe-17 Wt Pct Cr-12 Wt Pct Ni austenitic alloy. Metall. Trans. 1973, 4, 2535–2540. [Google Scholar] [CrossRef]



| Element | B | C | Cr | Ni | Mn | Si | Ti | P | S |
|---|---|---|---|---|---|---|---|---|---|
| mass% | 1.86 | 0.020 | 19.30 | 14.10 | 2.00 | 0.66 | — | 0.0070 | 0.0051 |
| Spectrum | B | Fe | Cr | Ni | Mn | Si | |
|---|---|---|---|---|---|---|---|
| Figure 3a | 1 | - | 62.6 | 21.9 | 12.5 | 2.2 | 0.8 |
| 2 | - | 46.9 | 50.1 | 3.0 | - | - | |
| 3 | - | 64.8 | 16.5 | 15.9 | 1.9 | 0.8 | |
| Figure 3b | 4 | - | 65.4 | 21.6 | 12.6 | - | 0.4 |
| 5 | 23.2 | 42.0 | 27.1 | 6.2 | - | - | |
| 6 | 17.6 | 40.6 | 35.3 | 4.5 | - | - | |
| 7 | 23.2 | 33.7 | 38.8 | 2.3 | - | - | |
| 8 | 27.4 | 30.2 | 38.9 | 1.7 | - | - | |
| Figure 3c | 9 | - | 68.5 | 13.2 | 15.3 | 1.9 | 1.0 |
| 10 | 5.7 | 60.4 | 16.6 | 14.2 | 2.1 | 0.3 | |
| 11 | 5.3 | 56.7 | 24.4 | 11.1 | 1.9 | 0.6 | |
| 12 | 5.8 | 48.7 | 38.8 | 4.7 | 2.1 | - | |
| 13 | - | 48.6 | 44.6 | 4.1 | 2.3 | 0.5 | |
| 14 | - | 50.3 | 45.7 | 4.0 | - | - | |
| Figure 3d | 15 | - | 66.7 | 14.0 | 16.5 | 1.9 | 0.9 |
| 16 | 17.7 | 49.6 | 20.4 | 9.8 | 1.8 | 0.8 | |
| 17 | 17.7 | 49.6 | 21.0 | 8.9 | 2.0 | 0.8 | |
| 18 | 15.5 | 49.8 | 22.3 | 9.6 | 1.9 | 0.9 | |
| 19 | 18.4 | 48.9 | 21.2 | 9.0 | 1.7 | 0.7 | |
| 20 | 17.4 | 47.0 | 27.9 | 7.2 | - | 0.5 | |
| Figure 3e | 21 | 64.5 | 16.7 | 15.5 | 2.5 | 0.8 | |
| 22 | - | 65.3 | 16.0 | 15.7 | 1.8 | 1.2 | |
| 23 | - | 64.2 | 18.3 | 14.3 | 2.3 | 0.9 | |
| 24 | 60.8 | 24.5 | 12.1 | 1.9 | 0.7 | ||
| Figure 3f | 25 | - | 65.6 | 16.5 | 14.9 | 2.2 | 0.8 |
| 26 | 11.8 | 47.2 | 33.0 | 5.6 | 2.1 | 0.5 | |
| 27 | - | 48.4 | 46.2 | 2.9 | 2.5 | - | |
| 28 | - | 45.3 | 49.8 | 2.3 | 2.5 | - | |
| 29 | 8.3 | 40.2 | 49.7 | 1.7 | - | - | |
| 30 | - | 43.4 | 52.7 | 1.2 | 2.7 | - | |
| 31 | 6.2 | 41.4 | 51.2 | 1.2 | - | - |
| Heat Temperature (°C) | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Reduction of Area (%) |
|---|---|---|---|---|
| 900 | 804 | 398 | 18 | 14 |
| 1000 | 802 | 396 | 20 | 18 |
| 1050 | 798 | 390 | 20 | 20 |
| 1100 | 792 | 406 | 23 | 22 |
| 1150 | 776 | 380 | 20 | 23 |
| 1200 | 745 | 352 | 21 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Qu, X.; Ge, Q.; Wang, T. Effect of Heat Treatment Temperature on Microstructure and Properties of PM Borated Stainless Steel Prepared by Hot Isostatic Pressing. Materials 2021, 14, 4646. https://doi.org/10.3390/ma14164646
Pei Y, Qu X, Ge Q, Wang T. Effect of Heat Treatment Temperature on Microstructure and Properties of PM Borated Stainless Steel Prepared by Hot Isostatic Pressing. Materials. 2021; 14(16):4646. https://doi.org/10.3390/ma14164646
Chicago/Turabian StylePei, Yanbin, Xuanhui Qu, Qilu Ge, and Tiejun Wang. 2021. "Effect of Heat Treatment Temperature on Microstructure and Properties of PM Borated Stainless Steel Prepared by Hot Isostatic Pressing" Materials 14, no. 16: 4646. https://doi.org/10.3390/ma14164646







