Effect of CeO2 Content on Microstructure and Properties of SiCp/Al-Si Composites Prepared by Powder Metallurgy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
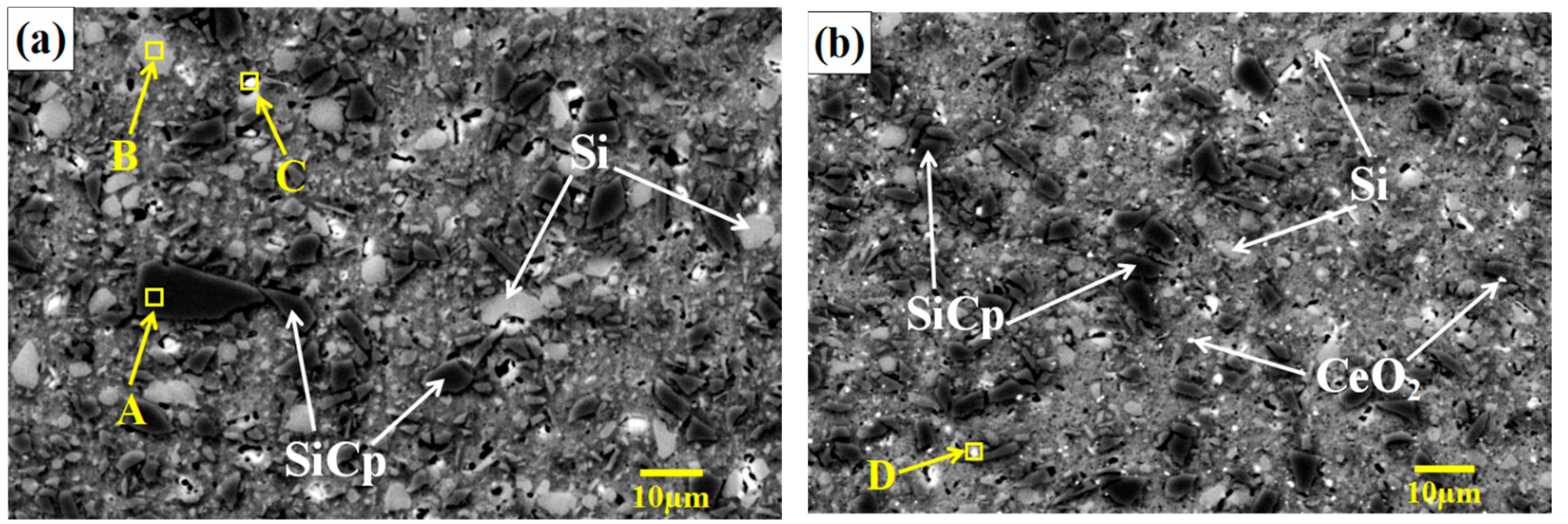
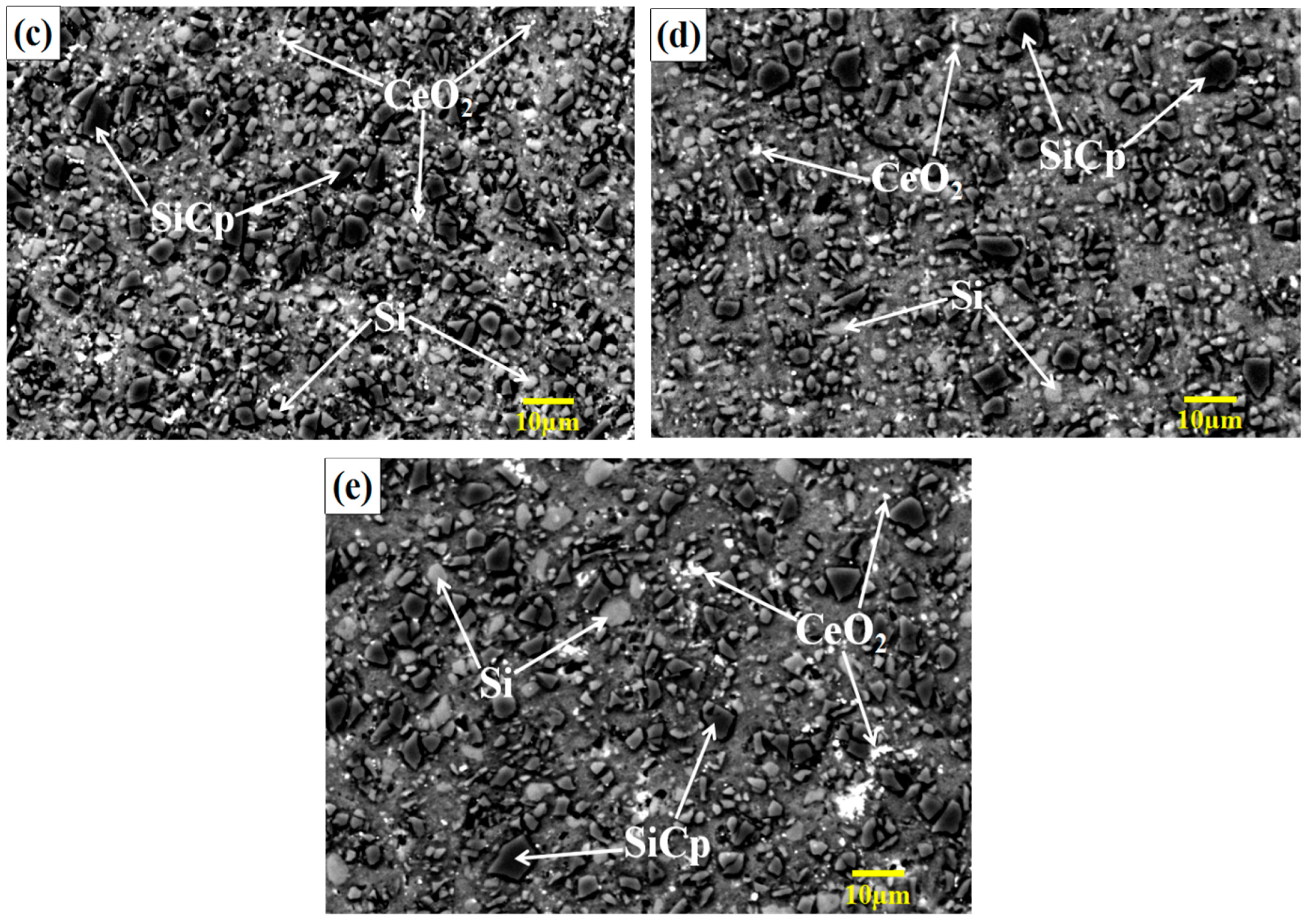
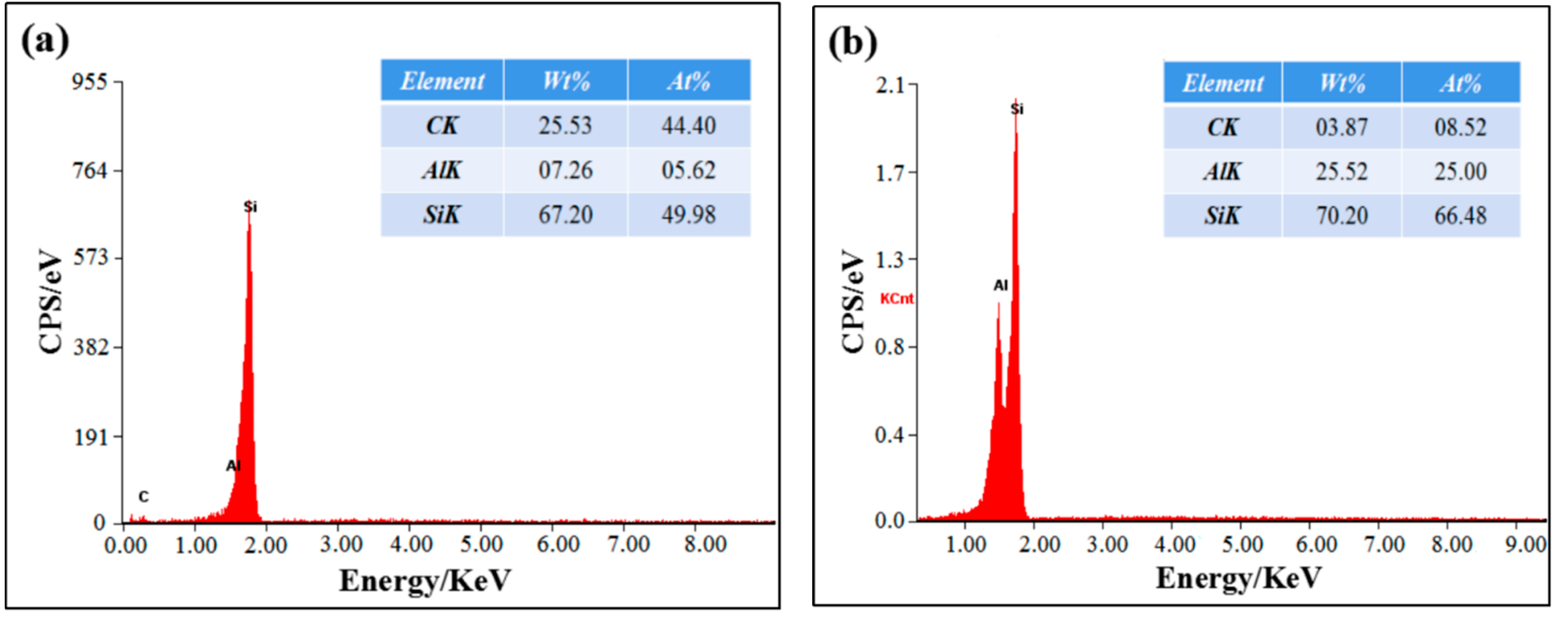
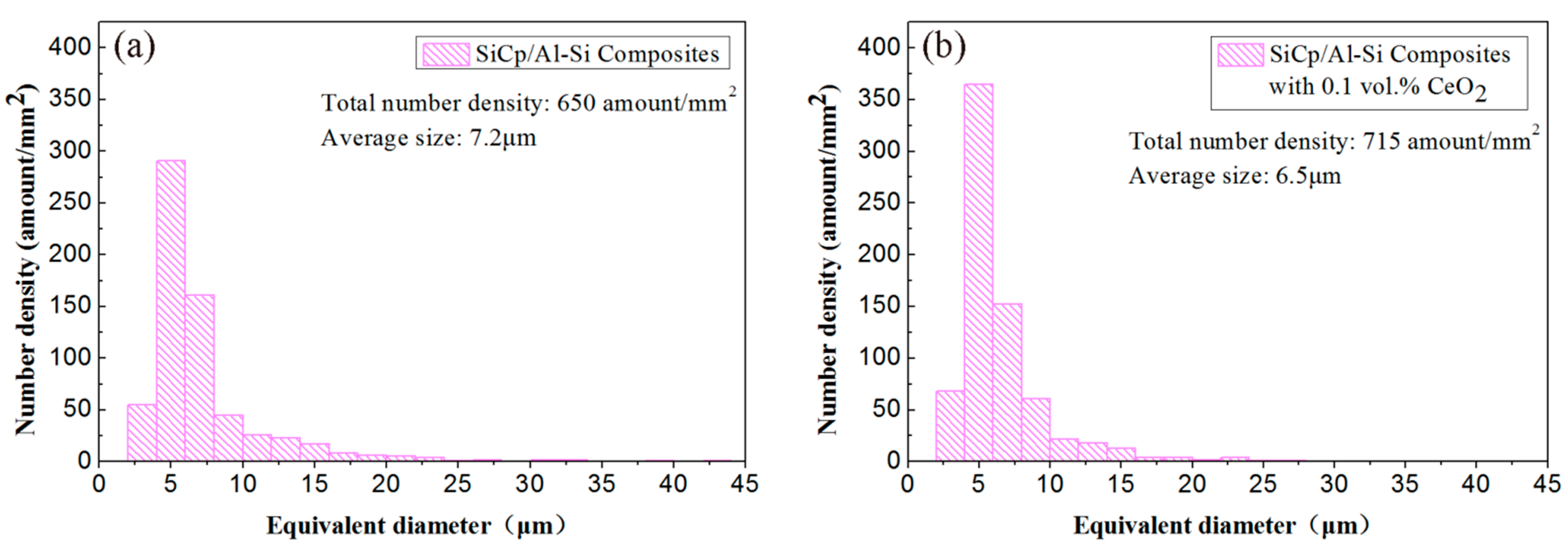
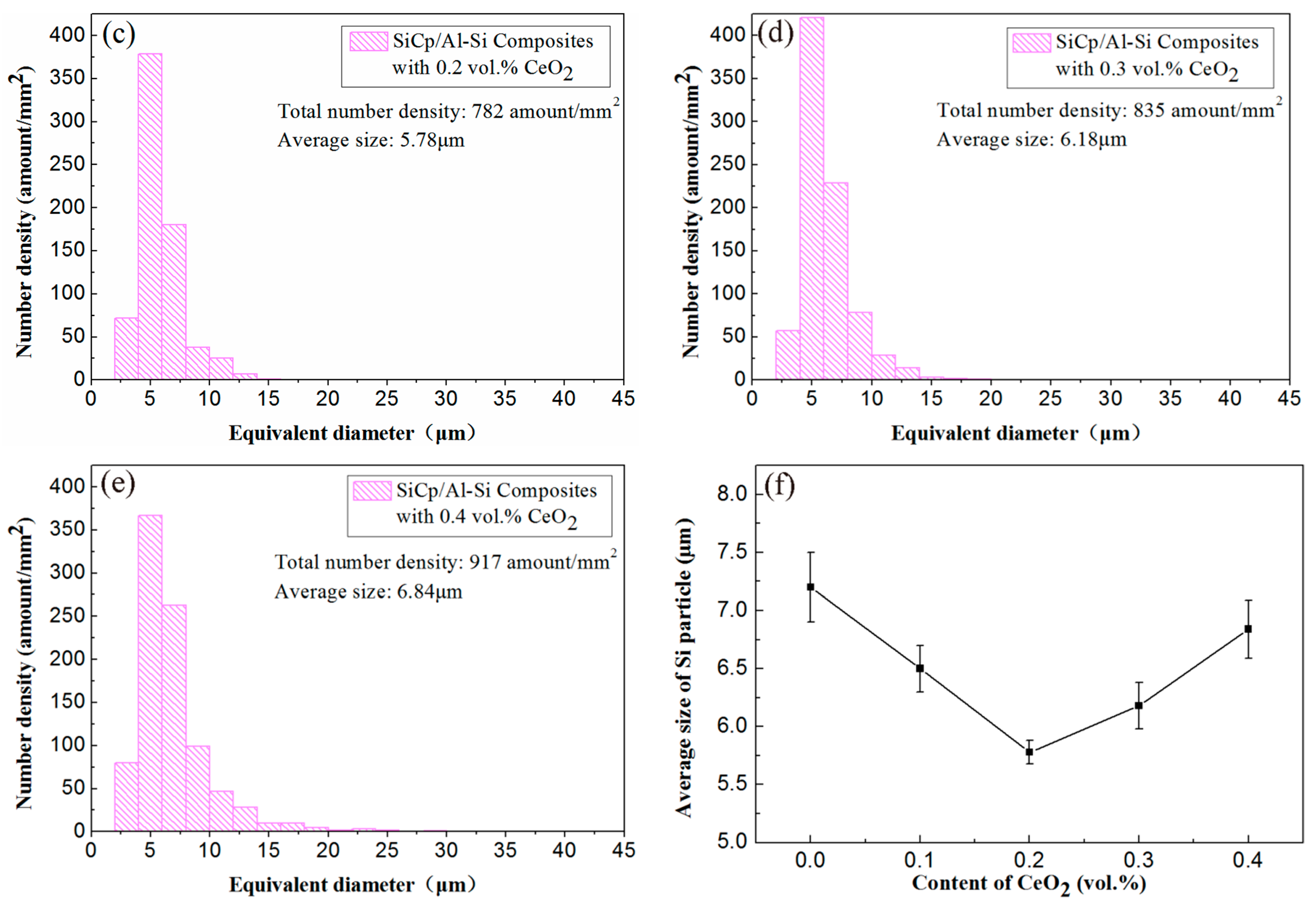
3.1. Effect of CeO2 Content on Microstructure of SiCp/Al-Si Composites
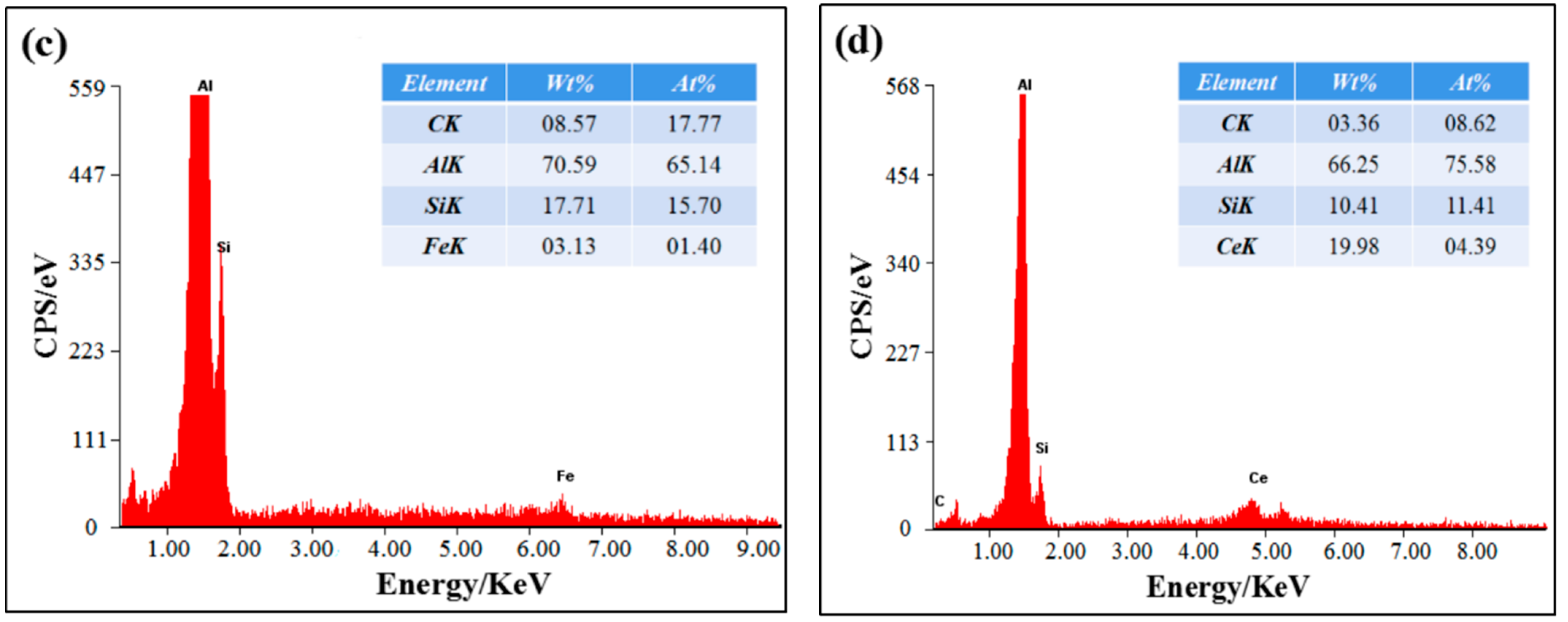
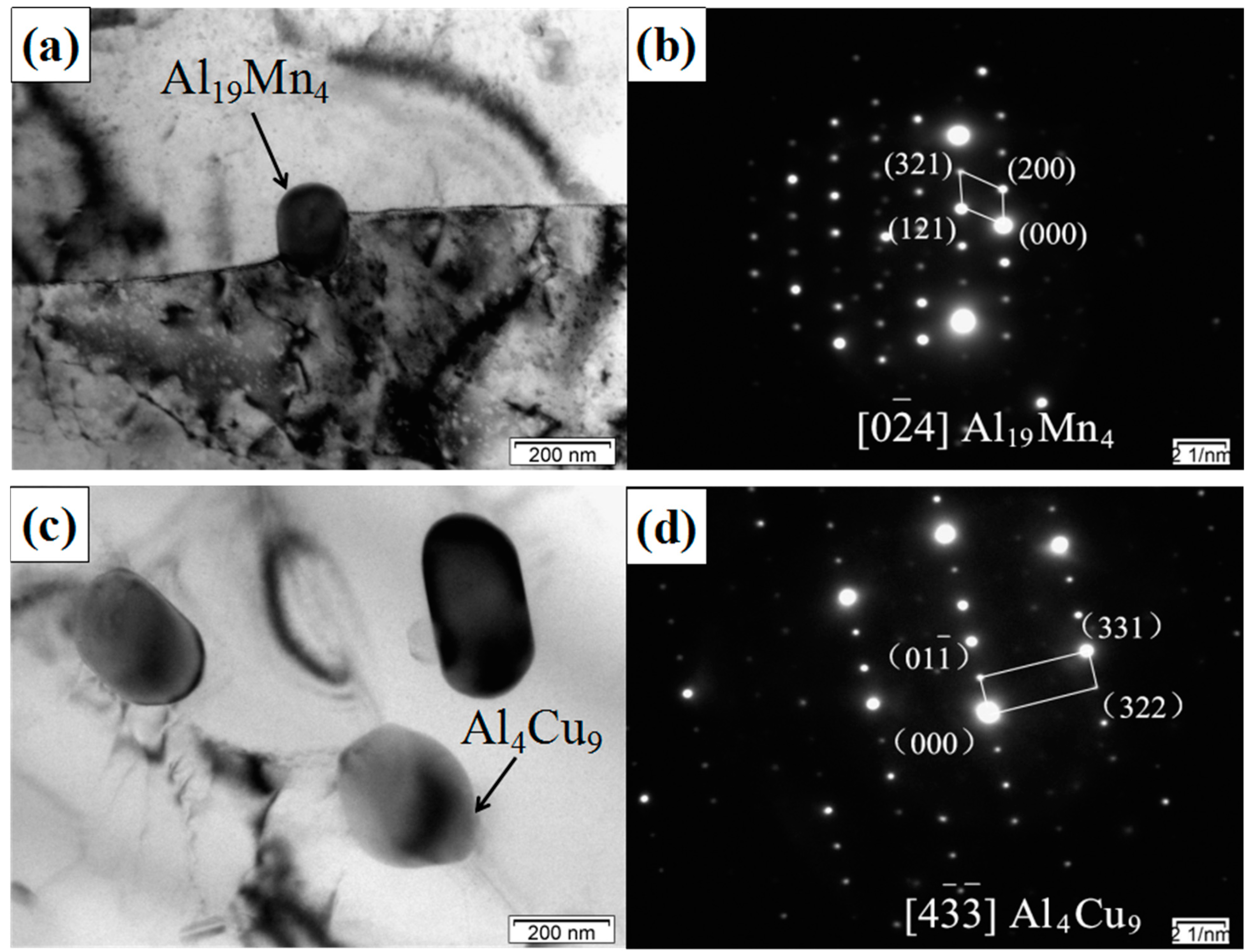
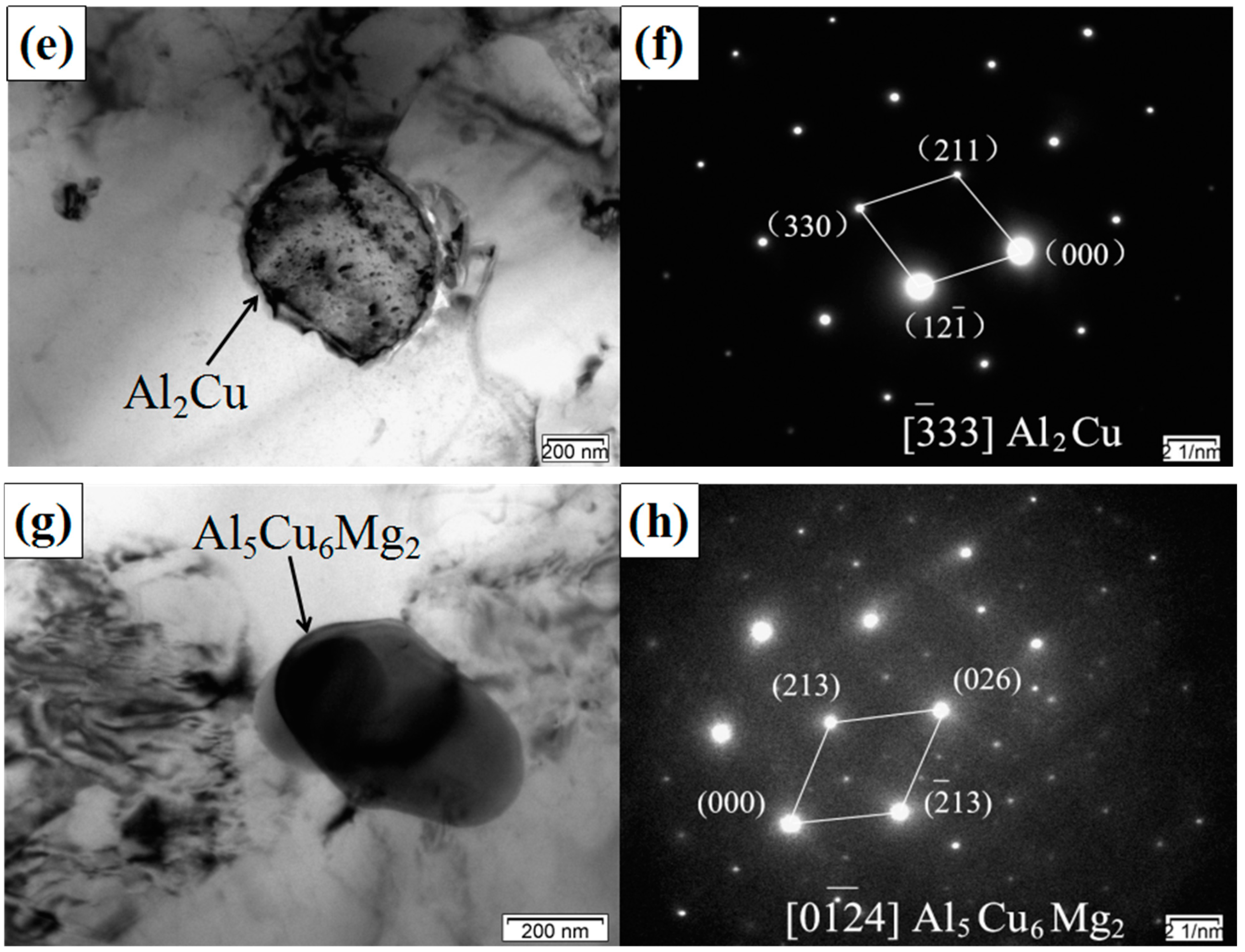
3.2. TEM Analysis of SiCp/Al-Si Composites
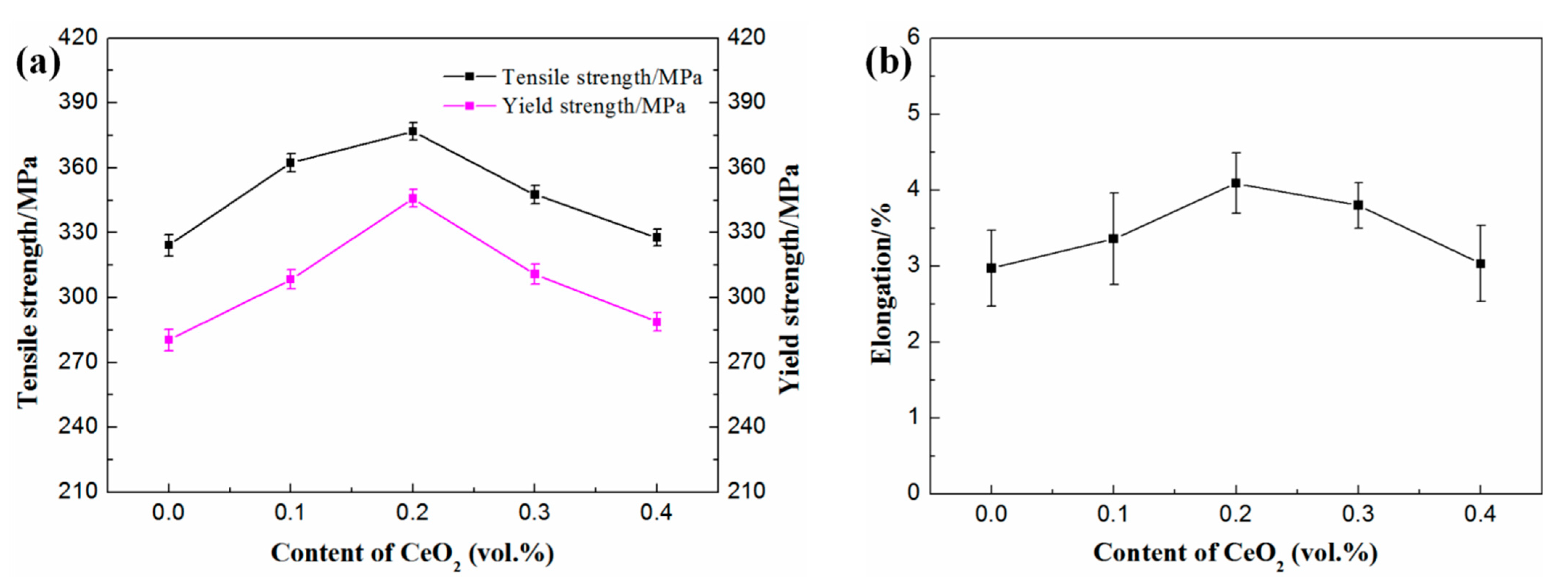
3.3. Effect of CeO2 Content on Tensile Properties of SiCp/Al-Si Composites
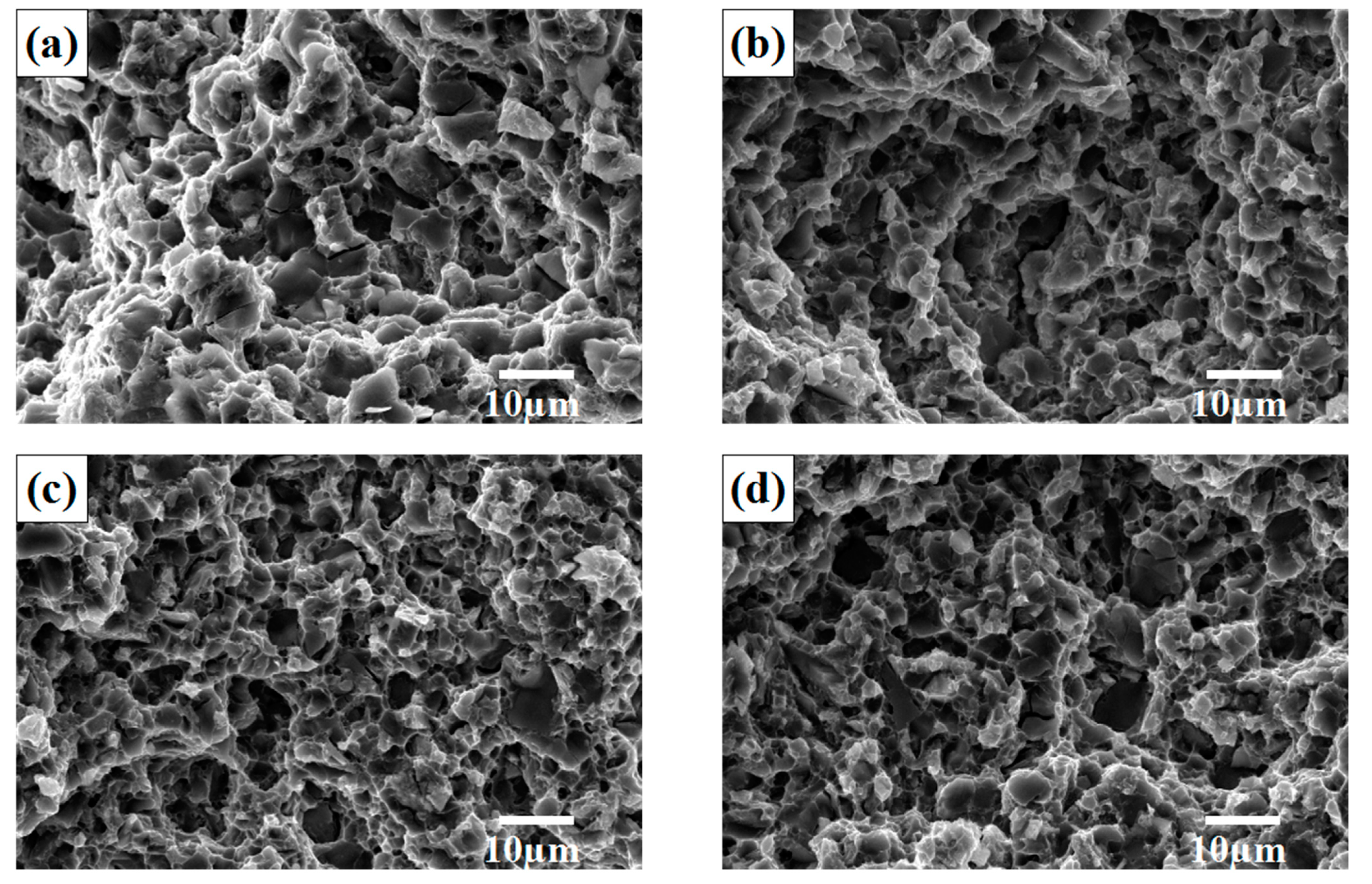
3.4. Fracture Behavior of SiCp/Al-Si Composites
3.5. Analysis of the Mechanism of CeO2 in SiCp/Al-Si Composites
4. Conclusions
- (1)
- The addition of an appropriate amount of CeO2 can refine the Si particles, reduce the agglomeration of Si phase, and improve its distribution uniformity in the SiCp/Al-Si composites. When the CeO2 volume fraction was 0.2%, the Si phase particle size was the smallest and the distribution uniformity was the best.
- (2)
- The main precipitates in SiCp/Al-Si composites without CeO2 were Al19Mn4, Al4Cu9, Al2Cu, Al5Cu6Mg2, and a new phase of CeCu2Si2 was formed after adding CeO2 in the composites. CeO2 was mainly located at the grain boundary or phase boundary of the composites.
- (3)
- The addition of appropriate content of CeO2 can improve the tensile properties of composites. When the CeO2 content was 0.2 vol%, the tensile properties of the composites were the best.
- (4)
- The fracture mode of the 20 vol% SiCp/Al-12Si composites with rare earth addition is a mixed fracture: brittle cleavage fracture of the Si phase and a few SiC particles, the tearing of Si and SiC particles with the matrix at the matrix interface, and ductile fracture in the Al matrix far away from Si and SiC particles.
- (5)
- There are three main mechanisms of CeO2 in SiCp/Al-Si composites. Firstly, CeO2 serves as the nucleation substrate of Si phase to refine Si particles; secondly, CeO2 reacts with the alloying elements in the aluminum matrix to form a new phase CeCu2Si2, which can exert a certain dispersion strengthening effect and improve the bonding strength between Al and Si particles; thirdly, is the pinning effect of CeO2 and CeCu2Si2 particles on grain boundaries or phase boundaries to refine aluminum grains.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, B.; Shi, Z.; Wang, C. Crystallization behavior, Al-Ce intermetallic formation, and microstructure refinement of near-eutectic Al–Si alloys by rare-earth element additions. Int. J. Mater. Res. 2020, 111, 938–952. [Google Scholar] [CrossRef]
- Liu, X.; Sekizawa, K.; Suzuki, A. Compressive Properties of Al-Si Alloy Lattice Structures with Three Different Unit Cells Fabricated via Laser Powder Bed Fusion. Materials 2020, 13, 2902. [Google Scholar] [CrossRef] [PubMed]
- Laghari, R.A.; Li, J.G.; Wu, Y. Study of Machining Process of SiCp/Al Particle Reinforced Metal Matrix Composite Using Finite Element Analysis and Experimental Verification. Materials 2020, 13, 5524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Z.; Ma, G.N.; Wang, D. Suppressed negative influence of natural aging in SiCp/6092Al composites. Mater. Sci. Eng. 2019, 767, 138422. [Google Scholar] [CrossRef]
- Yang, H.; She, X.W.; Tang, B.B. Study of the Microstructure and Ring Element Segregation Zone of Spray Deposited SiCp/7055Al. Materials 2019, 12, 1299. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, G.; Jiang, W. Effects of Cu-Coated SiC Content on Microstructure and Properties of Laser Cladding SiCp/Al–Si Composite Coatings. Materials 2019, 12, 1537. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, J.; He, J.J. Effect of the Sic Particle Orientation Anisotropy on the Tensile Properties of a Spray-Formed Sicp/Al-Si Composite. Strength Mater. 2014, 46, 221–228. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zou, A.H.; Hua, X.Z. Influence of Mg and Si in the Aluminum on the Thermo-Physical Properties of Pressureless Infiltrated SiCp/Al Composites. Mater. Sci. Forum 2009, 610, 546–553. [Google Scholar] [CrossRef]
- Gu, T.; Pan, Y.; Lu, T. Effects of boron addition on the morphology of silicon phases in Al-Si casting alloys. Mater. Charact. 2018, 141, 115–119. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N.; Song, D. Effects of La and Ce Mixed Rare Earth on Microstructure and Properties of Al-Mg-Si Aluminum Alloy. Mater. Sci. Forum 2017, 898, 367–371. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Li, J. Effect of yttrium addition on the microstructures and mechanical properties of hypereutectic Al-20Si alloy. Mater. Sci. Eng. A 2018, 722, 47–57. [Google Scholar] [CrossRef]
- Hu, G.Y.; Zhu, C.J.; Xu, D.F.; Dong, P.X.; Chen, K.H. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earths 2021, 39, 208–216. [Google Scholar] [CrossRef]
- Guan, L.Y.; Li, B.L.; Qi, P. Effect of Heat Treatment on the Microstructure and Property of Al-Si-Mg Alloy. Mater. Sci. Forum 2016, 850, 768–772. [Google Scholar] [CrossRef]
- Han, H.; Wang, A.; Xie, J. Effects of CeO2 on microstructure, friction and wear properties of SiCp/Al-Si composites. Emerg. Mater. Res. 2016, 5, 88–94. [Google Scholar] [CrossRef]
- Lu, J.; Yang, B.; Li, H. The effects of addition of La2O3 on the microstructure and mechanical properties of carbon/carbon composites. Mater. Sci. Eng. A 2014, 610, 350–354. [Google Scholar] [CrossRef]
- Sharma, A.; Roh, M.H.; Jung, J.P. Effect of La2O3 Nanoparticles on the Brazeability, Microstructure, and Mechanical Properties of Al-11Si-20Cu Alloy. J. Mater. Eng. Perform. 2016, 25, 1–8. [Google Scholar] [CrossRef]
- Elgallad, E.M.; Doty, H.W.; Alkahtani, S.A. Effects of La and Ce Addition on the Modification of Al-Si Based Alloys. Adv. Mater. Sci. Eng. 2016, 2016, 5027243. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, Z.; Luan, X. Effects of CeO2 and Y2O3 on the interfacial diffusion of Ti/Al2O3 composites. J. Alloys Compd. 2016, 656, 929–935. [Google Scholar] [CrossRef]
- Christensen, B.D.; Donaldson, I.W.; Bishop, D.P. Effects of process variables on the mechanical and physical properties of an Al–Cu–Mg powder metallurgy alloy. SN Appl. Sci. 2019, 1, 511. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.; Wu, M.; Du, Z. Microstructure evolution and densification behaviour of powder metallurgy Al–Cu–Mg–Si alloy. Powder Metall. 2020, 63, 54–63. [Google Scholar] [CrossRef]
- Zare, R.; Sharifi, H.; Saeri, M.R. Investigating the effect of SiC particles on the physical and thermal properties of Al6061/SiCp composite. J. Alloys Compd. 2019, 801, 520–528. [Google Scholar] [CrossRef]
- Toschi, S. Optimization of A354 Al-Si-Cu-Mg alloy heat treatment: Effect on microstructure, hardness, and tensile properties of peak aged and overaged alloy. Metals 2018, 8, 961. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, I.; Wells, M.A.; Esmaeili, S. Effect of particle shape and size distribution on the dissolution behavior of Al2Cu particles during homogenization in aluminum casting alloy Al-Si-Cu-Mg. J. Mater. Process. Technol. 2018, 251, 232–240. [Google Scholar] [CrossRef]
- Chen, Z.W.; Abraham, F.; Walker, J. Tensile Fracture Behavior of Friction Stir Processed Al-7Si-0.3Mg Cast Alloy. Mater. Sci. Forum 2012, 706–709, 971–976. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, D.Z.; Yao, C.K. Nucleation and growth behavior of primary silicon in alumina fiber reinforced hypereutectic Al-Si composite. J. Mater. Sci. Lett. 2002, 21, 921–922. [Google Scholar] [CrossRef]
- StJohn, D.; McDonald, S.; Darlapudi, A. A New Perspective on the Nucleation, Growth Morphology and Modification of the Silicon Phase During the Formation of Eutectic Al-Si Grains. JOM 2019, 71, 391–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Liu, Y. Cluster-assisted nucleation of silicon phase in hypoeutectic Al–Si alloy with further inoculation. Acta Mater. 2014, 70, 162–173. [Google Scholar] [CrossRef]
- Toth, G.I.; Tegze, G.; Pusztai, T. Heterogeneous Crystal Nucleation: The Effect of Lattice Mismatch. Phys. Rev. Lett. 2012, 108, 025502. [Google Scholar] [CrossRef]



| Component | Si | Cu | Mg | Mn | Al |
|---|---|---|---|---|---|
| Content | 12.0 | 1.4 | 0.7 | 0.6 | Bal. |
| Interface Structure | Lattice Mismatch Degree | Interface Energy |
|---|---|---|
| Coherent Interface | δ ≤ 0.05 | 0.1 J/m2 |
| Semi-coherent Interface | 0.05 ≤ δ ≤ 0.25 | 0.5 J/m2 |
| Non-coherent Interface | δ ≥ 0.25 | 1.0 J/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, A.; Xie, J.; Zhu, P. Effect of CeO2 Content on Microstructure and Properties of SiCp/Al-Si Composites Prepared by Powder Metallurgy. Materials 2021, 14, 4685. https://doi.org/10.3390/ma14164685
Dong X, Wang A, Xie J, Zhu P. Effect of CeO2 Content on Microstructure and Properties of SiCp/Al-Si Composites Prepared by Powder Metallurgy. Materials. 2021; 14(16):4685. https://doi.org/10.3390/ma14164685
Chicago/Turabian StyleDong, Xuedan, Aiqin Wang, Jingpei Xie, and Pengfei Zhu. 2021. "Effect of CeO2 Content on Microstructure and Properties of SiCp/Al-Si Composites Prepared by Powder Metallurgy" Materials 14, no. 16: 4685. https://doi.org/10.3390/ma14164685
APA StyleDong, X., Wang, A., Xie, J., & Zhu, P. (2021). Effect of CeO2 Content on Microstructure and Properties of SiCp/Al-Si Composites Prepared by Powder Metallurgy. Materials, 14(16), 4685. https://doi.org/10.3390/ma14164685





