Study on Structure and Properties of La2O3-Doped Basaltic Glasses for Immobilizing Simulated Lanthanides
Abstract
:1. Introduction
2. Experimental Method
2.1. Fabrication of Simulated Waste Glass
2.2. Characterization
2.3. Chemical Stability
3. Results and Discussion
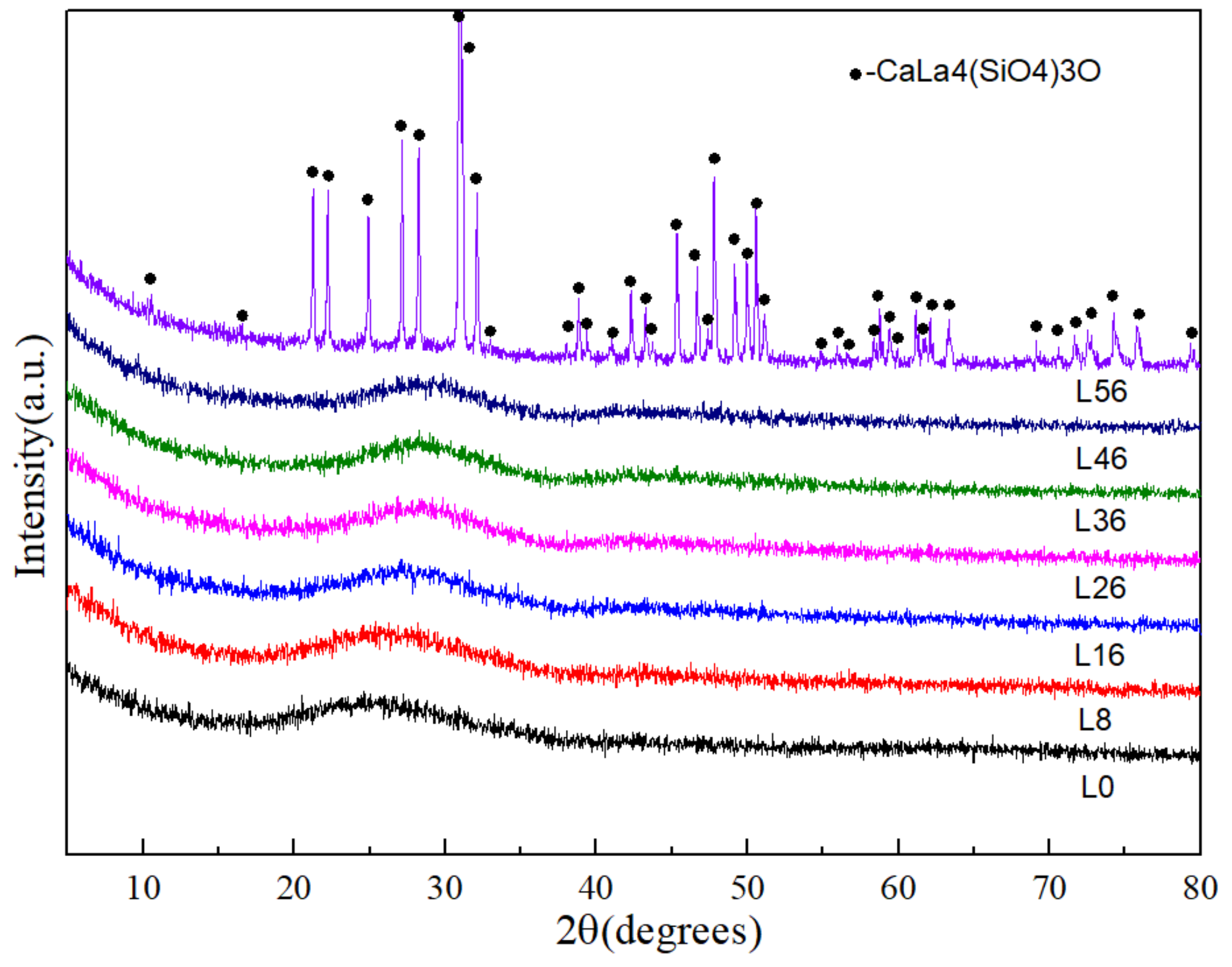
3.1. Glass and Crystallization Behavior
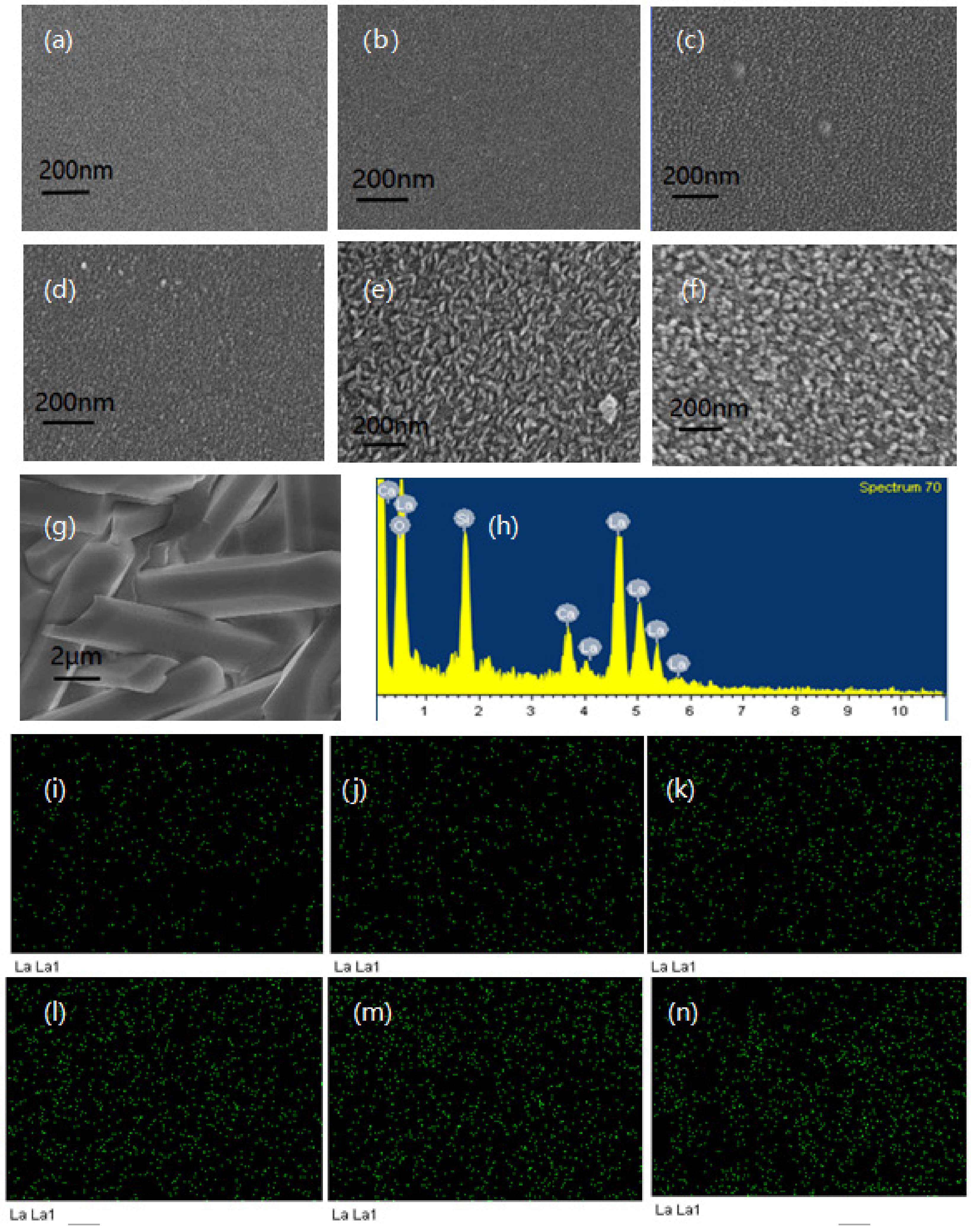
3.2. SEM and EDX Analysis
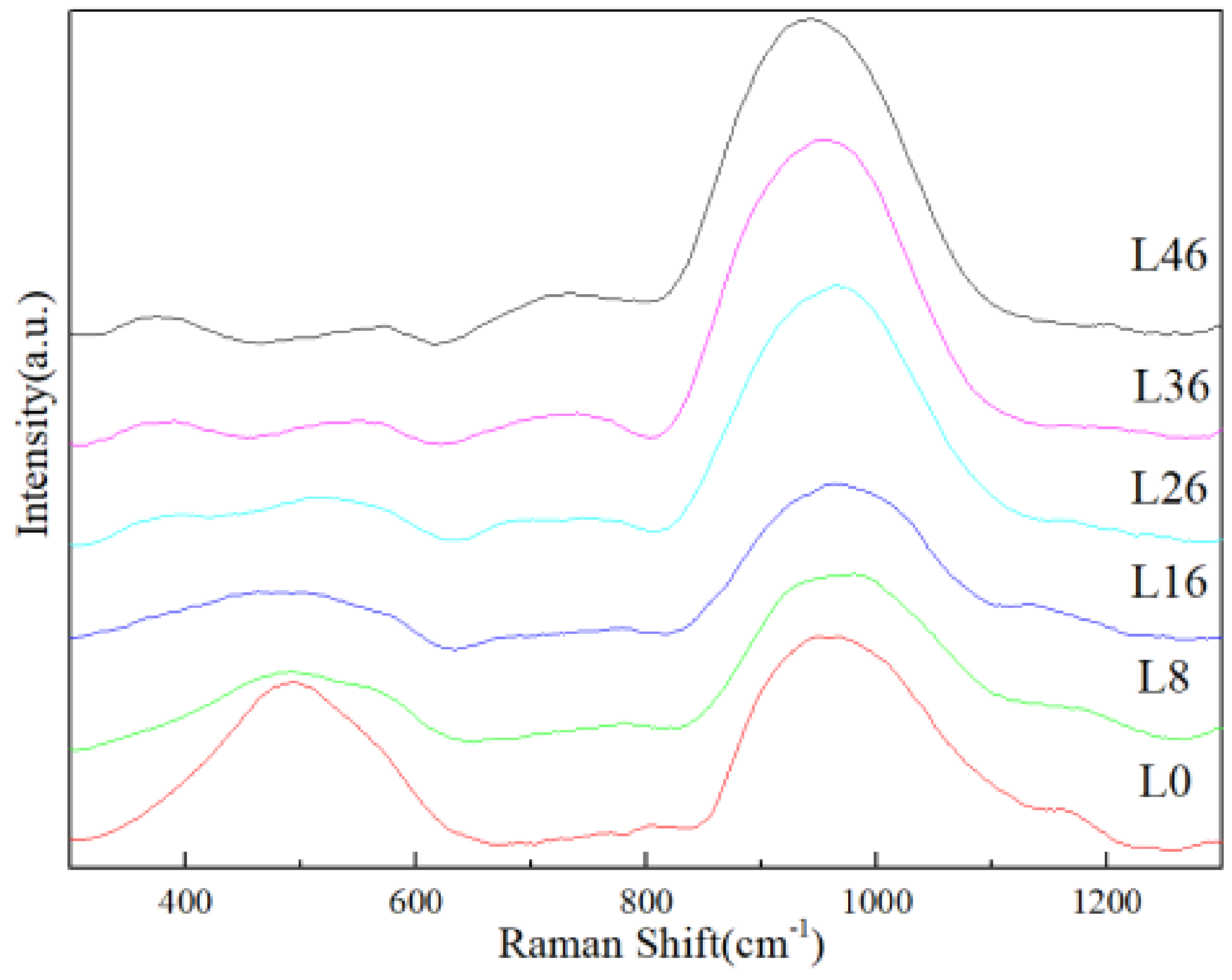
3.3. Raman Analysis
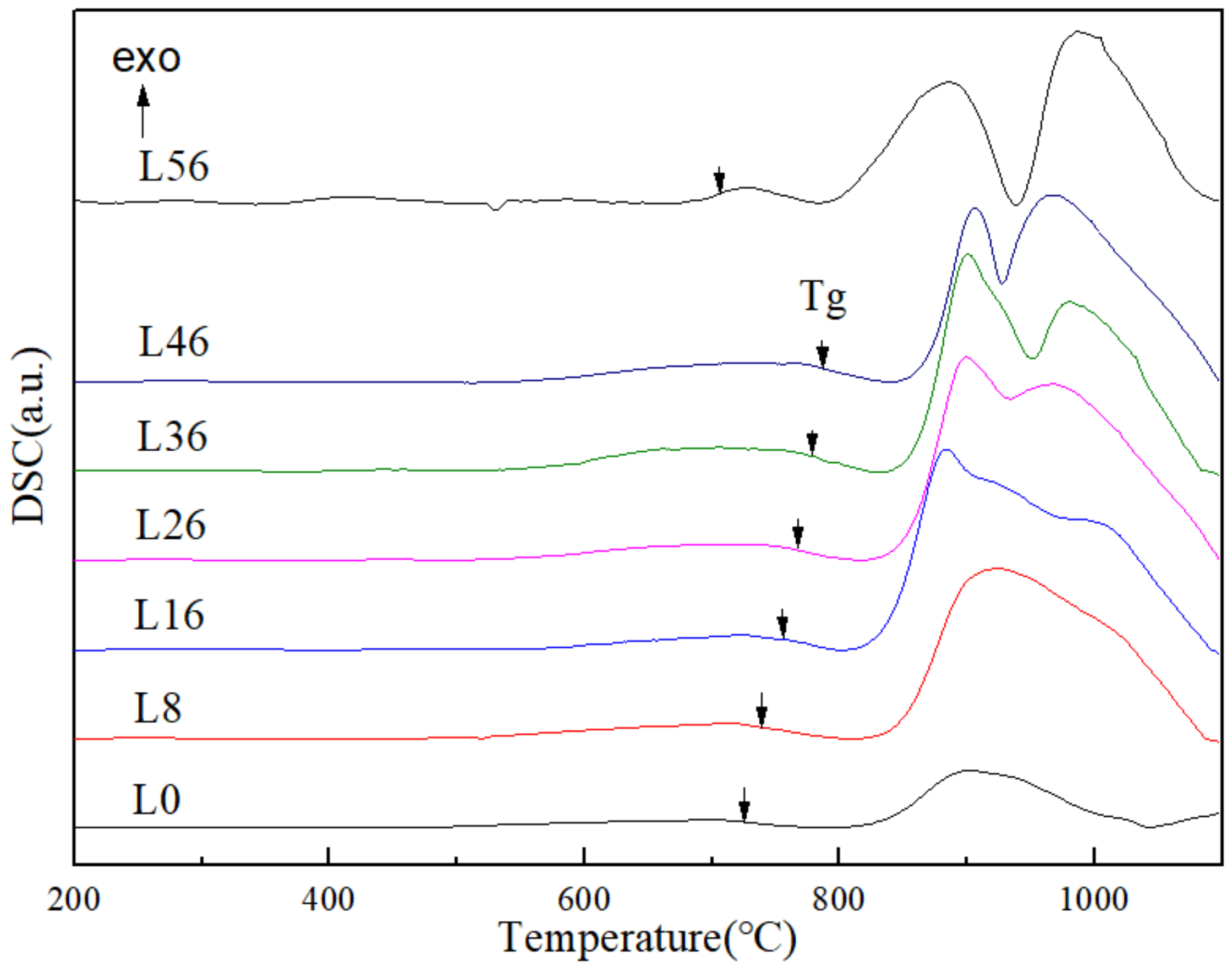
3.4. DSC Curves
3.5. Density and Hardness
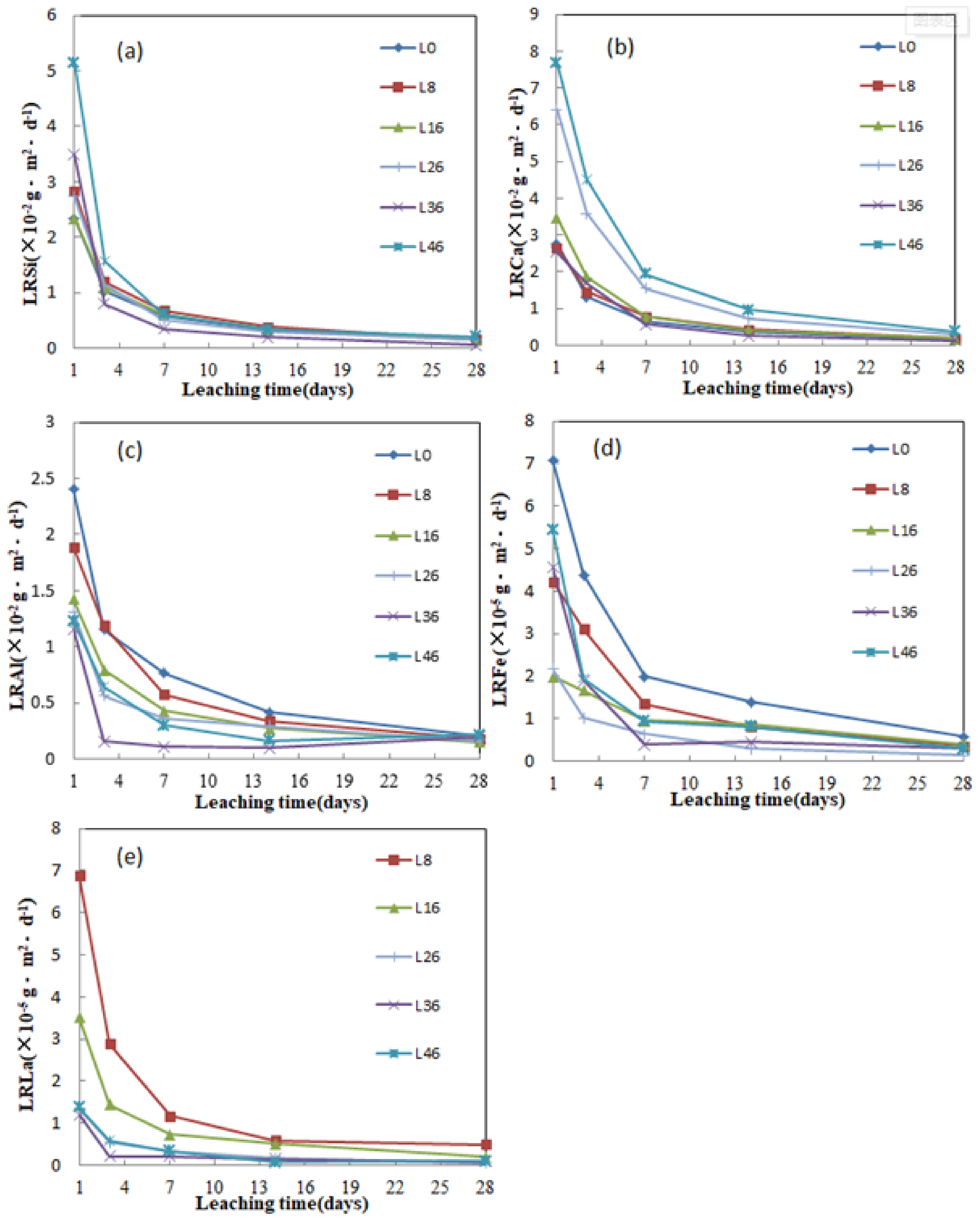
3.6. Leaching Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, F.; Wang, Q.; Zhu, H.; Xiang, G.; Liao, Q.; Zhu, Y. Effect of neodymium on the glass formation, dissolution rate and crystallization kinetic of borophosphate glasses containing iron. J. Non- Cryst. Solids 2019, 526, 119726. [Google Scholar] [CrossRef]
- Gin, S.; Abdelouas, A.; Criscenti, L.; Ebert, W.; Ferrand, K.; Geisler, T.; Harrison, M.; Inagaki, Y.; Mitsui, S.; Mueller, K.; et al. An international initiative on long-term behavior of high-level nuclear waste glass. Mater. Today 2013, 16, 243–248. [Google Scholar] [CrossRef]
- Day, D.E.; Wu, Z.; Ray, C.S.; Hrma, P. Chemically durable iron phosphate glass waste forms. J. Non- Cryst. Solids 1998, 241, 1–12. [Google Scholar] [CrossRef]
- Donald, I.W.; Metcalfe, B.L.; Taylor, R.N.J. The immobilization of high level radioactive wastes using ceramics and glasses. J. Mater. Sci. 1997, 32, 5851–5887. [Google Scholar] [CrossRef]
- He, Y.; Lu, Y.; Zhang, Q. Characterization of monazite glass–ceramics as wasteform for simulated α-HLLW. J. Nucl. Mater. 2008, 376, 201–206. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Wang, M.; Fang, L.; Li, M.; Cheng, J.; He, F.; Deng, W.; Dongol, R. Dependence of Gd2O3 containing silicate glass workability and fragility on structure. Mater. Chem. Phys. 2016, 179, 304–309. [Google Scholar] [CrossRef]
- Xiao, L.; Xiao, Q.; Liu, Y.; Ai, P.; Li, Y.; Wang, H. A transparent surface-crystallized Eu2+, Dy3+ co-doped strontium aluminate long-lasting phosphorescent glass-ceramic. J. Alloy. Compd. 2010, 495, 72–75. [Google Scholar] [CrossRef]
- Lai, S.C. Petrology of Magmatic Rocks, 2nd ed.; Higher Education Press: Beijing, China, 2016. [Google Scholar]
- Liu, J.; Cui, Y.; Yang, J.; Wu, Z.; Amp, N. Effect of basalt composition and mineral on high temperature melting process. J. Yanshan Univ. 2017, 41, 323–328. [Google Scholar]
- Shi, F.; Hu, L.; Cui, Y.; Ren, J. Revealing the Structures in Short- and Middle-Order of Lanthanum-Doped Al2O3–NaPO3 Glasses by Solid State NMR Spectroscopy. J. Phys. Chem. C 2021, 125, 2097–2110. [Google Scholar] [CrossRef]
- Qian, B.; Liang, X.; Yang, S.; Gao, L.; Guo, X. Raman spectra study on the structure of lanthanum-iron phosphate glasses. Chin. J. Inorg. Chem. 2013, 29, 314–318. [Google Scholar]
- Li, S.; Lu, Y.; Qu, Y.; Kang, J.; Yue, Y.; Liang, X. Dielectric and thermal properties of aluminoborosilicate glasses doped with mixed rare-earth oxides. J. Non- Cryst. Solids 2020, 556, 120550. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Qu, Y.; Lu, H.; Huang, S.; Yue, Y. Influence of La2O3 and Ce2O3 additions on structure and properties of aluminoborosilicate glasses. J. Mater. Sci. Mater. Electron. 2016, 28, 2716–2722. [Google Scholar] [CrossRef]
- Wang, M.; Fang, L.; Li, M.; Liu, Z.; Yan, Y.; Zhang, X. Effect of rare earth dopant on thermal stability and structure of ZnO-B2O3-SiO2 glass. J. Inorg. Mater. 2017, 32, 643–648. [Google Scholar]
- Xu, K.; Yan, Y.; Zhang, L.; Pan, H.; Zhao, J.; Jia, X.; Wang, G.; Wu, H. Study on structural, dielectric, thermal and chemical characteristics of aluminoborosilicate glasses doped by rare earth oxides. Mater. Technol. 2014, 29, A40–A43. [Google Scholar] [CrossRef]
- ASTM C1285-14. Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Ahmadzadeh, M.; Scrimshire, A.; Mottram, L.; Stennett, M.C.; Hyatt, N.C.; Bingham, P.A.; McCloy, J.S. Structure of NaFeSiO4, NaFeSi2O6, and NaFeSi3O8 glasses and glass-ceramics. Am. Miner. 2020, 105, 1375–1384. [Google Scholar] [CrossRef]
- Kline, J.; Tangstad, M.; Tranell, G. Raman spectroscopic study of the structural modifications associated with the addition of calcium oxide and boron oxide to silica. Metall. Mater. Trans. B 2015, 46B, 62–73. [Google Scholar] [CrossRef]
- Lu, P.; Xia, W.; Jiang, H.; Zhao, H. Analysis of high alumina silicate glass with infrared and raman spectroscopy. Bull. Chin. Ceram. Soc. 2015, 34, 878–887. [Google Scholar]
- Iguchi, Y.; Kashio, S.; Goto, T.; Nishina, Y.; Fuwa, T. Raman spectroscopic study on the structure of silicate slags. Can. Metall. Q. 1981, 20, 51–56. [Google Scholar] [CrossRef]
- Zheng, K.; Liao, J.; Wang, X.; Zhang, Z. Raman spectroscopic study of the structural properties of CaO-MgO-SiO2-TiO2 slags. J. Non- Cryst. Solids 2013, 376, 209–215. [Google Scholar] [CrossRef]
- Bechgaard, T.K.; Mauro, J.; Bauchy, M.; Yue, Y.; Lamberson, L.A.; Jensen, L.R.; Smedskjaer, M.M. Fragility and configurational heat capacity of calcium aluminosilicate glass-forming liquids. J. Non- Cryst. Solids 2017, 461, 24–34. [Google Scholar] [CrossRef]
- Tarrago, M.; Royo, I.; Martínez, S.; Garcia-Valles, M.; Neuville, D.R. Incorporation of calcium in glasses: A key to understand the vitrification of sewage sludge. Int. J. Appl. Glas. Sci. 2021, 12, 367–380. [Google Scholar] [CrossRef]
- Cui, J.; Cao, X.; Wang, P.; Shi, L.; Zhong, S.; Zhao, F.; Gao, Q.; Li, J. Infrared and raman spectroscopy analysis of substrate glass and cover glass in liquid crystal display. Bull. Chin. Ceram. Soc. 2020, 39, 3664–3668. [Google Scholar]
- Abo-Mosallam, H.; Park, H. Crystallization characteristics of La2O3-containing glasses for glass ionomer cement. Mater. Lett. 2012, 72, 137–140. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, A.; Han, L. Effects of doping La2O3 on crystaUization and mechanical properties of lithium disilicate gIass—Ceramics. Chin. J. Nonferrous Met. 2009, 19, 2018–2023. [Google Scholar]
- Bingham, P.; Hand, R.; Forder, S. Doping of iron phosphate glasses with Al2O3, SiO2 or B2O3 for improved thermal stability. Mater. Res. Bull. 2006, 41, 1622–1630. [Google Scholar] [CrossRef]
- Li, X.; Tao, X.; Xiao, Z.; Kong, L.; Liu, Y.; Zeng, X.; Yang, K.; Shi, J. Structure and properties of iron phosphate wasteforms loaded with simulated radioactive waste enriched in SrO and alkali metal oxides. J. Ceram. 2020, 41, 904–912. [Google Scholar]
- Lafi, O.A.; Imran, M.M.A. Compositional dependence of thermal stability, glass–forming ability and fragility index in some Se–Te–Sn glasses. J. Alloy. Compd. 2011, 509, 5090–5094. [Google Scholar] [CrossRef]
- Saad, M.; Poulain, M. Glass forming ability criteria. Mater. Sci. Forum 1987, 19, 11–18. [Google Scholar] [CrossRef]
- Novatski, A.; Steimacher, A.; Medina, A.N.; Bento, A.C.; Baesso, M.L.; Andrade, L.H.; Lima, S.M.; Guyot, Y.; Boulon, G. Relations among nonbridging oxygen, optical properties, optical basicity, and color center formation in CaO–MgO aluminosilicate glasses. J. Appl. Phys. 2008, 104, 094910. [Google Scholar] [CrossRef]
- Kang, U.; Zhilin, A.; Logvinov, D.P.; Onushchenko, A.A.; Savost’Yanov, V.A.; Chuvaeva, T.I.; Shashkin, A.V. Transparent Nd3+-Activated Glass-Ceramics in the Li2O–Al2O3–SiO2System: Physicochemical Aspects of Their Preparation and Optical Characteristics. Glas. Phys. Chem. 2001, 27, 344–352. [Google Scholar] [CrossRef]
- Lu, P.; Zheng, Y.; Cheng, J.; Guo, D. Effect of La2O3 addition on crystallization and properties of Li2O–Al2O3–SiO2 glass-ceramics. Ceram. Int. 2013, 39, 8207–8212. [Google Scholar] [CrossRef]
- Geisler, T.; Janssen, A.; Scheiter, D.; Stephan, T.; Berndt, J.; Putnis, A. Aqueous corrosion of borosilicate glass under acidi conditions a new corrosion mechanism. J. Non- Cryst. Solids 2010, 356, 1458–1465. [Google Scholar] [CrossRef]
- Ma, T.; Liang, W.; Xu, H.; Li, W.; Zhao, J.; Han, X. Research progresss on dissolution behavior and mechanisms of rasioactive waste glasses. J. Nucl. Radiochem. 2019, 41, 411–417. [Google Scholar]
- EJ 1186-2005. Characterization of Radioactive Waste Forms and Packages; Nuclear Industry Standard in China, Commission for Science, Technology and Industry for National Defense: Beijing, China, 2005. [Google Scholar]
| Raw Materials | Chemical Components, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O | La2O3 | |
| L0 | 54.97 | 14.24 | 7.38 | 15.63 | 2.80 | 2.29 | 1.24 | 0.77 | - |
| L8 | 50.57 | 13.10 | 6.79 | 14.38 | 2.58 | 2.11 | 1.14 | 0.71 | 8 |
| L16 | 46.17 | 11.96 | 6.20 | 13.13 | 2.35 | 1.92 | 1.04 | 0.65 | 16 |
| L26 | 40.68 | 10.54 | 5.46 | 11.57 | 2.07 | 1.69 | 0.92 | 0.57 | 26 |
| L36 | 35.18 | 9.11 | 4.72 | 10.00 | 1.79 | 1.47 | 0.79 | 0.49 | 36 |
| L46 | 29.68 | 7.69 | 3.99 | 8.44 | 1.51 | 1.24 | 0.67 | 0.42 | 46 |
| L56 | 24.19 | 6.27 | 3.25 | 6.88 | 1.23 | 1.01 | 0.55 | 0.34 | 56 |
| Sample | L0 | L8 | L16 | L26 | L36 | L46 |
|---|---|---|---|---|---|---|
| Tg (°C) | 725.836 | 738.402 | 745.46 | 766.88 | 778.716 | 787.449 |
| Tc (°C) | 803.836 | 819.402 | 811.46 | 823.88 | 835.716 | 843.449 |
| Tp (°C) | 901.803 | 922.402 | 884.46 | 898.88 | 899.716 | 905.449 |
| S | 17.26 | 18.74 | 9.96 | 9.52 | 7.36 | 6.90 |
| Sample | L0 | L8 | L16 | L26 | L36 | L46 |
|---|---|---|---|---|---|---|
| Density (g/cm3) | 2.5025 | 2.7504 | 2.9856 | 3.2029 | 3.3918 | 3.5034 |
| Vickers hardness | 898.52 | 825.80 | 775.84 | 716.42 | 623.04 | 521.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.; Huo, J.; Zhang, X.; Cui, Z.; Zhu, Y. Study on Structure and Properties of La2O3-Doped Basaltic Glasses for Immobilizing Simulated Lanthanides. Materials 2021, 14, 4709. https://doi.org/10.3390/ma14164709
Tong Q, Huo J, Zhang X, Cui Z, Zhu Y. Study on Structure and Properties of La2O3-Doped Basaltic Glasses for Immobilizing Simulated Lanthanides. Materials. 2021; 14(16):4709. https://doi.org/10.3390/ma14164709
Chicago/Turabian StyleTong, Qin, Jichuan Huo, Xingquan Zhang, Zhu Cui, and Yongchang Zhu. 2021. "Study on Structure and Properties of La2O3-Doped Basaltic Glasses for Immobilizing Simulated Lanthanides" Materials 14, no. 16: 4709. https://doi.org/10.3390/ma14164709
APA StyleTong, Q., Huo, J., Zhang, X., Cui, Z., & Zhu, Y. (2021). Study on Structure and Properties of La2O3-Doped Basaltic Glasses for Immobilizing Simulated Lanthanides. Materials, 14(16), 4709. https://doi.org/10.3390/ma14164709






