Polysaccharide-Based Bilayer Coatings for Biofilm-Inhibiting Surfaces of Medical Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Model PDMS Films: QCM-D Sensors and Modification
2.2.2. Adsorption of Chi-77KS, Chi-77KS/AMOX and HA onto PDMS Films
2.2.3. Real Samples: PDMS Discs
2.3. Characterization Methods
2.3.1. Surface Analyses
2.3.2. Potentiometric Titrations
2.3.3. Adsorption of Proteins
2.3.4. Biofilm Formation Assay
2.3.5. Biocompatibility Testing
3. Results and Discussion
3.1. Influence of the Additional HA Layer on Antifouling Properties
3.1.1. Adsorption and Characterization of the Additional HA Layer
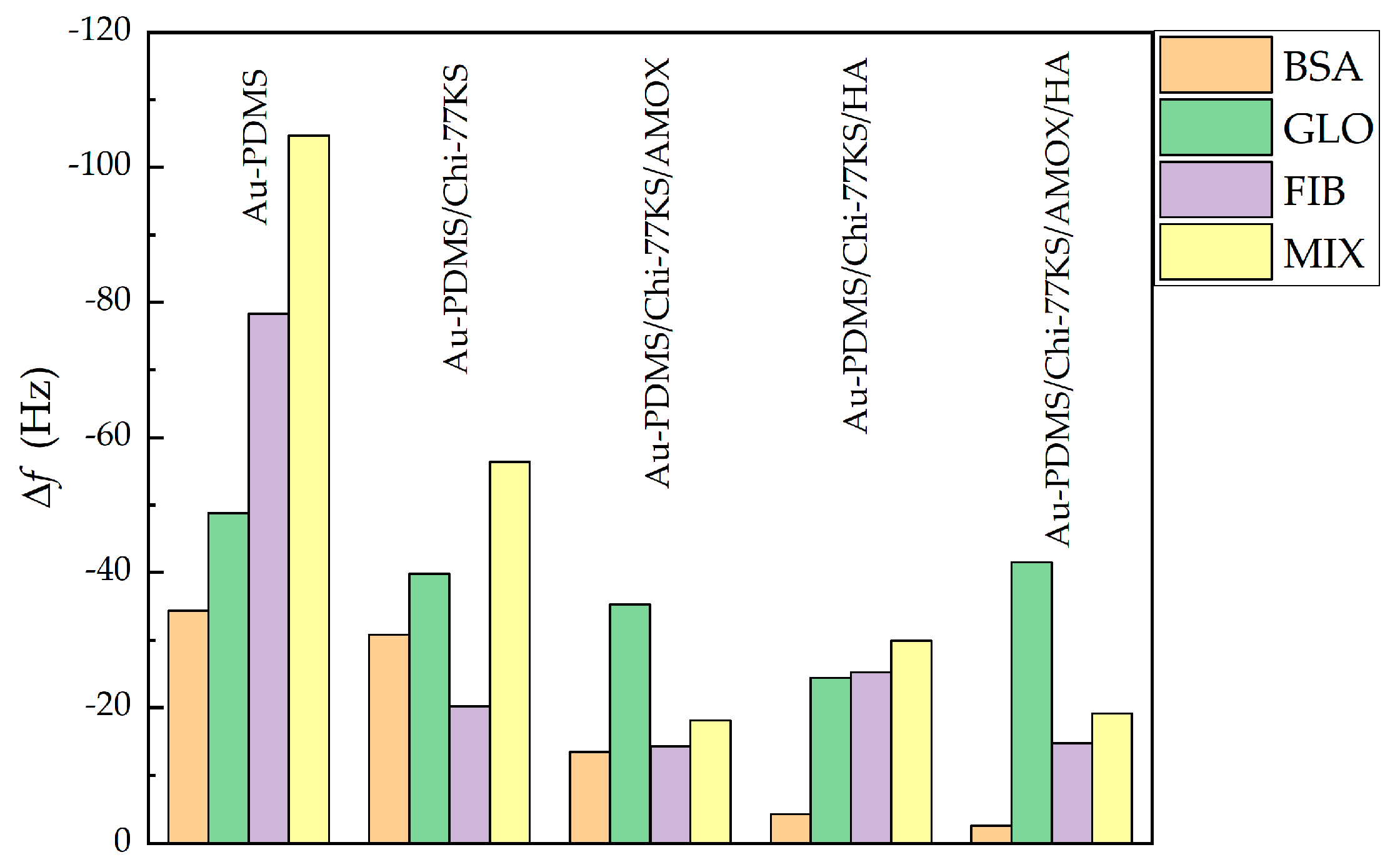
3.1.2. Adsorption of Mixed-Protein Solution
3.2. Real Samples
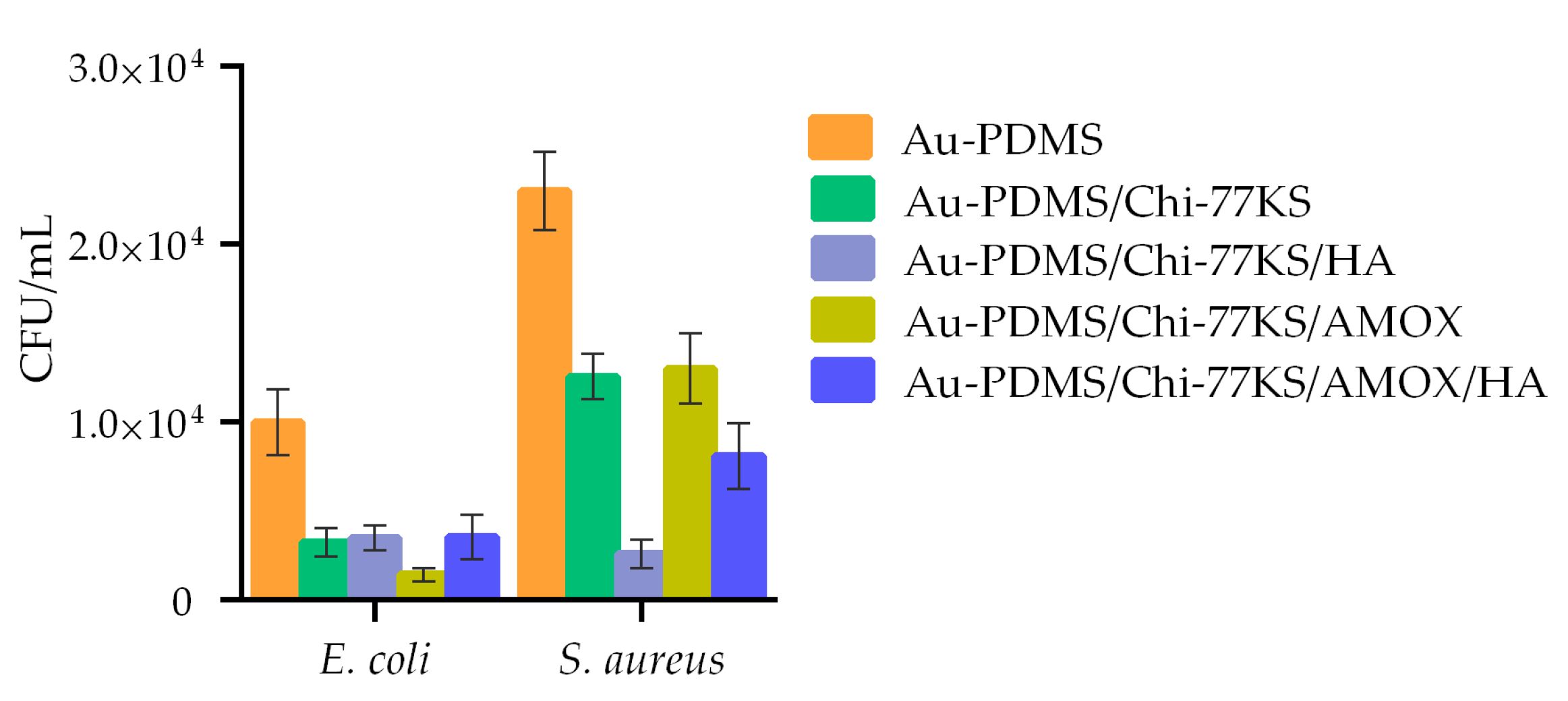
3.2.1. Anti-Biofilm Capacity of Coated Discs
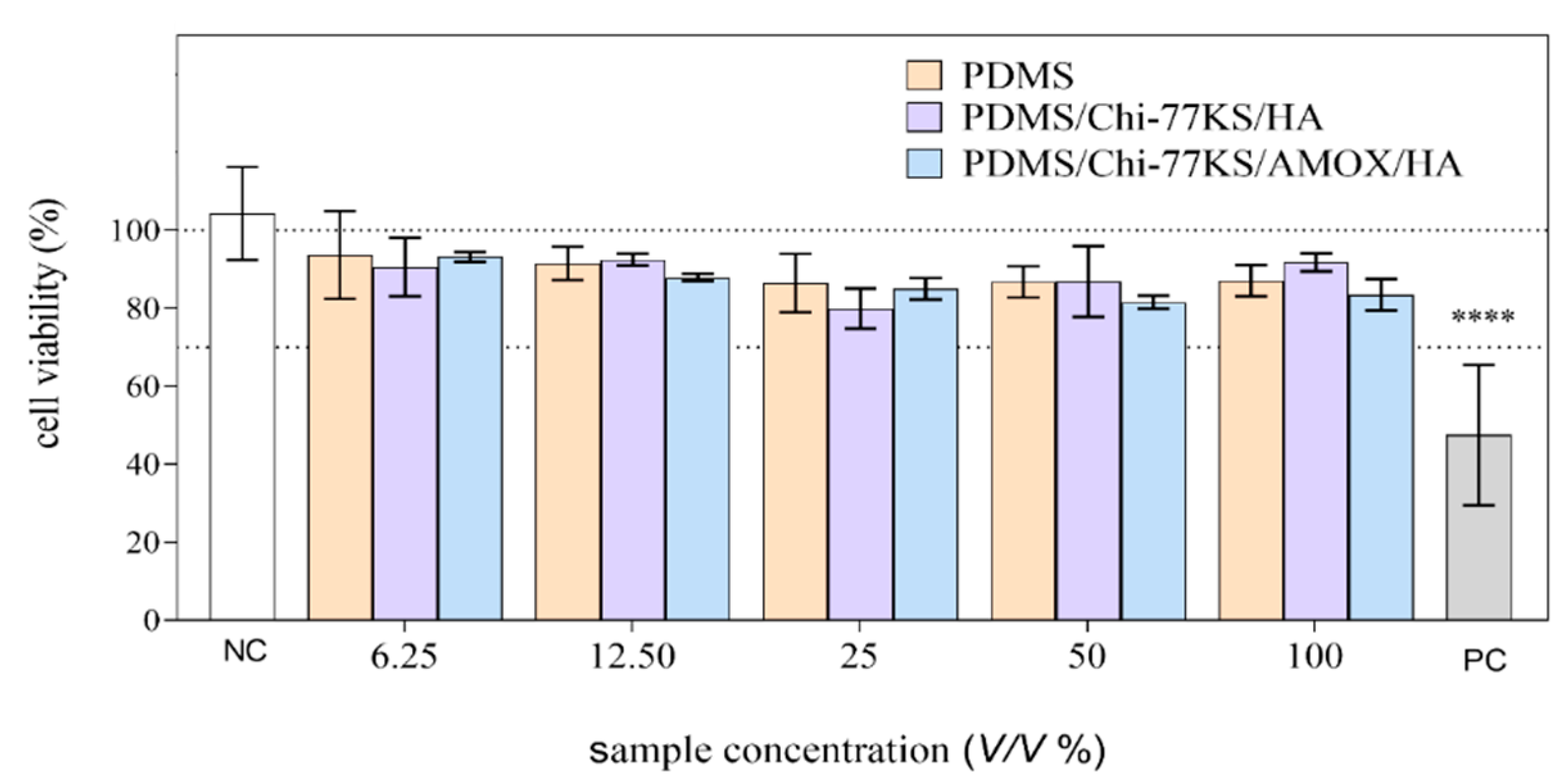
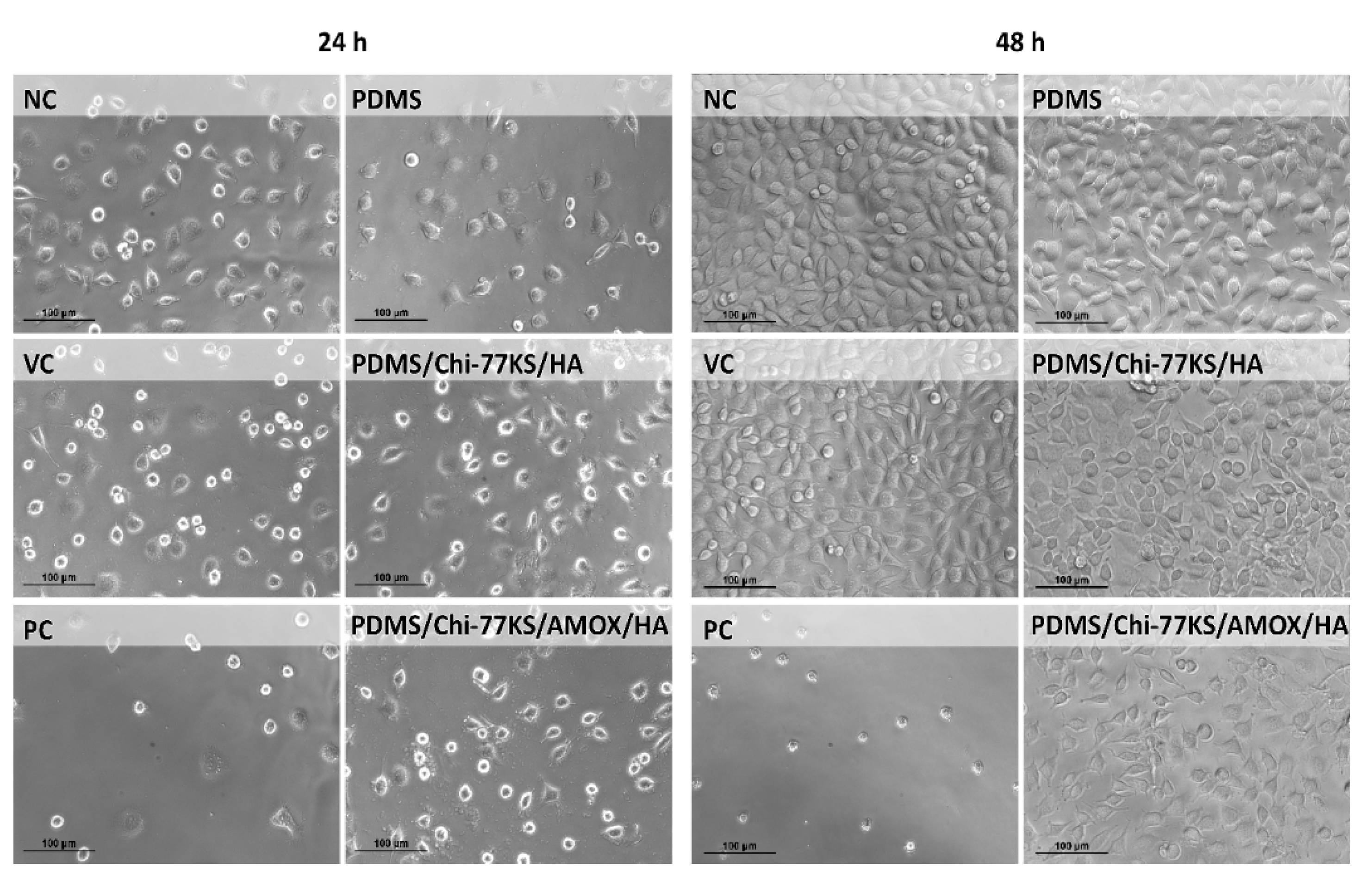
3.2.2. Biocompatibility Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Lv, W. Biofilms and Implantable Medical Devices: Infection and Control; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Junter, G.-A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical devices biomaterials—A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 234, 218–228. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Szarpak, A.; Pignot-Paintrand, I.; Varrot, A.; Boudou, T.; Detrembleur, C.; Jérôme, C.; Picart, C.; Auzély-Velty, R. Contact-Killing Polyelectrolyte Microcapsules Based on Chitosan Derivatives. Adv. Funct. Mater. 2010, 20, 3303–3312. [Google Scholar] [CrossRef]
- Stepnova, E.A.; Tikhonov, V.E.; Babushkina, T.A.; Klimova, T.P.; Vorontsov, E.V.; Babak, V.G.; Lopatin, S.A.; Yamskov, I.A. New approach to the quaternization of chitosan and its amphiphilic derivatives. Eur. Polym. J. 2007, 43, 2414–2421. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Neoh, K.G.; Shi, Z.; Kang, E.-T.; Tambyah, P.A.; Chiong, E. Inhibition of escherichia coli and proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2012, 109, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by Layer Buildup of Polysaccharide Films: Physical Chemistry and Cellular Adhesion Aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Jo, D.G.; Park, J.H. Polysaccharide-Based Nanoparticles: A Versatile Platform for Drug Delivery and Biomedical Imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.a.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Csaba, N.; Köping-Höggård, M.; Alonso, M.J. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int. J. Pharm. 2009, 382, 205–214. [Google Scholar] [CrossRef]
- Huang, M.; Vitharana, S.N.; Peek, L.J.; Coop, T.; Berkland, C. Polyelectrolyte Complexes Stabilize and Controllably Release Vascular Endothelial Growth Factor. Biomacromolecules 2007, 8, 1607–1614. [Google Scholar] [CrossRef]
- Bračič, M.; Strnad, S.; Fras Zemljič, L. Bioactive Functionalisation of Silicones with Polysaccharides, 1st ed.; SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2018; p. 75. [Google Scholar]
- Chiappisi, L.; Gradzielski, M. Co-assembly in chitosan–surfactant mixtures: Thermodynamics, structures, interfacial properties and applications. Adv. Colloid Interface Sci. 2015, 220, 92–107. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, R.S.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer-surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M. Recent progress of the characterization of oppositely charged polymer/surfactant complex in dilution deposition system. Adv. Colloid Interface Sci. 2017, 239, 146–157. [Google Scholar] [CrossRef]
- Bračič, M.; Hansson, P.; Pérez, L.; Zemljič, L.F.; Kogej, K. Interaction of Sodium Hyaluronate with a Biocompatible Cationic Surfactant from Lysine: A Binding Study. Langmuir 2015, 31, 12043–12053. [Google Scholar] [CrossRef]
- Nylander, T.; Samoshina, Y.; Lindman, B. Formation of polyelectrolyte–surfactant complexes on surfaces. Adv. Colloid Interface Sci. 2006, 123–126, 105–123. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, P.S.; Levin, Y.; Barbosa, M.C. Complex formation between polyelectrolytes and ionic surfactants. Chem. Phys. Lett. 1998, 298, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Goswami, M.; Borreguero, J.M.; Pincus, P.A.; Sumpter, B.G. Surfactant-Mediated Polyelectrolyte Self-Assembly in a Polyelectrolyte–Surfactant Complex. Macromolecules 2015, 48, 9050–9059. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte Complexes: A Review of their Applicability in Drug Delivery Technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Verma, A. Polyelectrolyte complex-an overview. Int. J. Pharm. Sci. Res. 2013, 4, 1684. [Google Scholar]
- Mirtič, J.; Paudel, A.; Laggner, P.; Hudoklin, S.; Kreft, M.E.; Kristl, J. Polyelectrolyte–surfactant–complex nanoparticles as a delivery platform for poorly soluble drugs: A case study of ibuprofen loaded cetylpyridinium-alginate system. Int. J. Pharm. 2020, 580, 119199. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Ohadi, M.; Shahravan, A.; Dehghannoudeh, N.; Eslaminejad, T.; Banat, I.M.; Dehghannoudeh, G. Potential use of microbial surfactant in microemulsion drug delivery system: A systematic review. Drug Des. Dev. Ther. 2020, 14, 541. [Google Scholar] [CrossRef] [Green Version]
- Bračič, M.; Pérez, L.; Martinez-Pardo, R.I.; Kogej, K.; Hribernik, S.; Šauperl, O.; Fras Zemljič, L. A novel synergistic formulation between a cationic surfactant from lysine and hyaluronic acid as an antimicrobial coating for advanced cellulose materials. Cellulose 2014, 21, 2647–2663. [Google Scholar] [CrossRef]
- Bračič, M.; Šauperl, O.; Strnad, S.; Kosalec, I.; Plohl, O.; Zemljič, L.F. Surface modification of silicone with colloidal polysaccharides formulations for the development of antimicrobial urethral catheters. Appl. Surf. Sci. 2019, 463, 889–899. [Google Scholar] [CrossRef]
- Ajdnik, U.; Finšgar, M.; Fras Zemljič, L. Characterization of chitosan-lysine surfactant bioactive coating on silicone substrate. Carbohydr. Polym. 2020, 232, 115817. [Google Scholar] [CrossRef] [PubMed]
- Ajdnik, U.; Zemljič, L.F.; Plohl, O.; Pérez, L.; Trček, J.; Bračič, M.; Mohan, T. Bioactive Functional Nanolayers of Chitosan–Lysine Surfactant with Single- and Mixed-Protein-Repellent and Antibiofilm Properties for Medical Implants. ACS Appl. Mater. Interfaces 2021, 13, 23352–23368. [Google Scholar] [CrossRef] [PubMed]
- Gaetano, G.; Pitaressi, G.; Palumbo, F.S.; Maraldi, S.; Scarponi, S.; Romano, C.L. Hyaluronic-Based Antibacterial Hydrogel Coating for Implantable Biomaterials in Orthopedics and Trauma: From Basic Research to Clinical Applications. In Hydrogels; IntehOpen: London, UK, 2018; p. 179. [Google Scholar]
- Bračič, M.; Mohan, T.; Kargl, R.; Griesser, T.; Hribernik, S.; Köstler, S.; Stana Kleinschek, K.; Fras Zemljič, L. Preparation of PDMS ultrathin films and patterned surface modification with cellulose. RSC Adv. 2014, 4, 11955–11961. [Google Scholar] [CrossRef]
- Čakara, D.; Fras, L.; Bračič, M.; Kleinschek, K.S. Protonation behavior of cotton fabric with irreversibly adsorbed chitosan: A potentiometric titration study. Carbohydr. Polym. 2009, 78, 36–40. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Čakara, D.; Michaelis, N.; Heinze, T.; Stana Kleinschek, K. Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: A potentiometric titration study. Cellulose 2011, 18, 33–43. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO 10993-5:2009; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO 10993-12:2021; ISO: Geneva, Switzerland, 2021. [Google Scholar]
- Mohan, T.; Čas, A.; Bračič, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljič, L.F.; Rebol, J. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Gupta, V.; Anselmo, A.C.; Muraski, J.A.; Mitragotri, S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J. Control. Release 2014, 173, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.-H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Suchánek, J.; Ivančaková, R.K.; Mottl, R.; Browne, K.Z.; Pilneyová, K.C.; Pilbauerová, N.; Schmidt, J.; Suchánková Kleplová, T. Hyaluronic Acid-Based Medical Device for Treatment of Alveolar Osteitis—Clinical Study. Int. J. Environ. Res. Public Health 2019, 16, 3698. [Google Scholar] [CrossRef] [Green Version]
- Schnyder, B.; Lippert, T.; Kötz, R.; Wokaun, A.; Graubner, V.-M.; Nuyken, O. UV-irradiation induced modification of PDMS films investigated by XPS and spectroscopic ellipsometry. Surf. Sci. 2003, 532–535, 1067–1071. [Google Scholar] [CrossRef]
- Mundry, T.; Surmann, P.; Schurreit, T. Surface characterization of polydimethylsiloxane treated pharmaceutical glass containers by X-ray-excited photo- and Auger electron spectroscopy. Fresenius J. Anal. Chem. 2000, 368, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Matsumoto, H.; Yako, H.; Minagawa, M.; Tanioka, A. Characterization of chitosan nanofiber fabric by electrospray deposition: Electrokinetic and adsorption behavior. J. Colloid Interface Sci. 2007, 310, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zemljič, L.F.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical Characterization of Packaging Foils Coated by Chitosan and Polyphenols Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altgärde, N.; Nilebäck, E.; de Battice, L.; Pashkuleva, I.; Reis, R.L.; Becher, J.; Möller, S.; Schnabelrauch, M.; Svedhem, S. Probing the biofunctionality of biotinylated hyaluronan and chondroitin sulfate by hyaluronidase degradation and aggrecan interaction. Acta Biomater. 2013, 9, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Pandey, L.M. Conformational and Organizational Insights into Serum Proteins during Competitive Adsorption on Self-Assembled Monolayers. Langmuir 2018, 34, 8178–8194. [Google Scholar] [CrossRef]
- Niemczyk, A.; El Fray, M.; Franklin, S.E. Friction behaviour of hydrophilic lubricious coatings for medical device applications. Tribol. Int. 2015, 89, 54–61. [Google Scholar] [CrossRef] [Green Version]
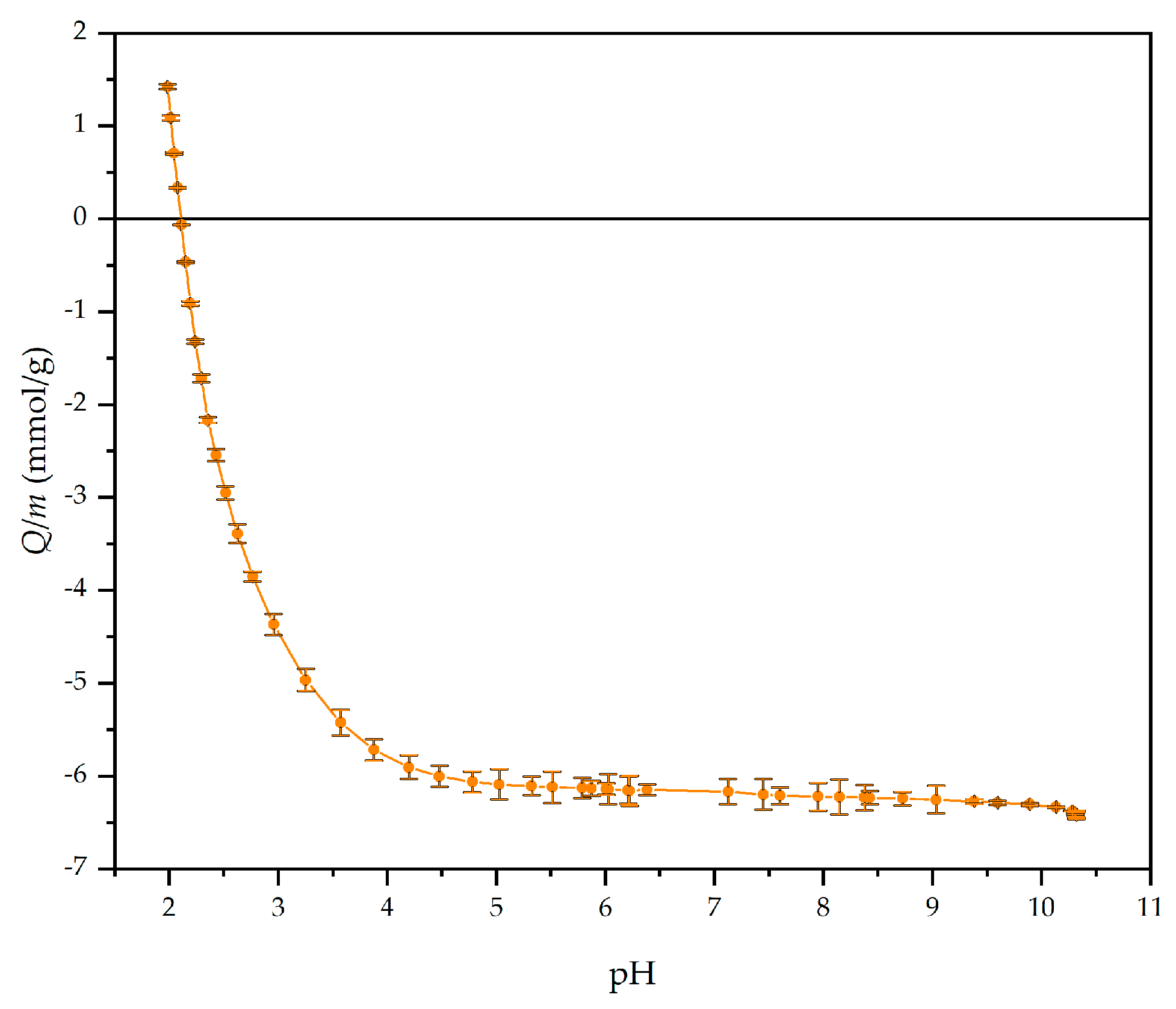
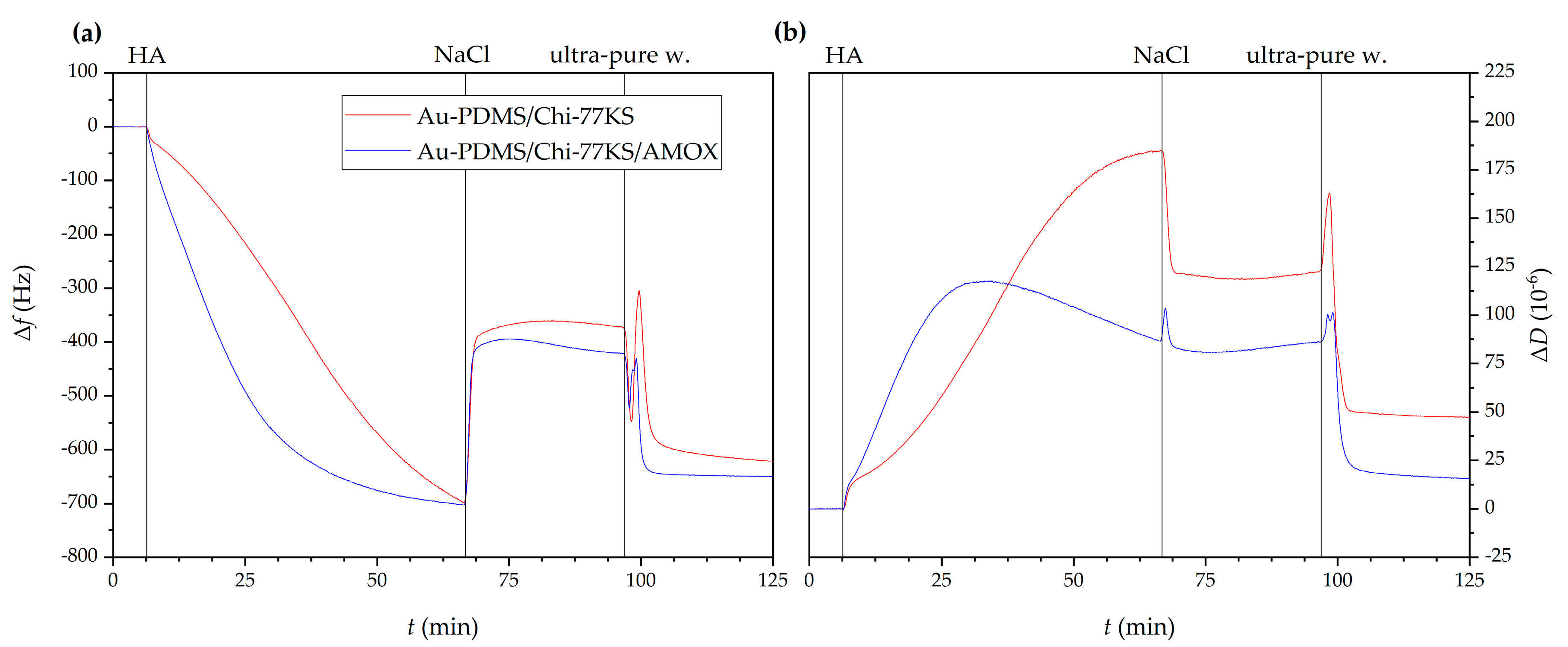
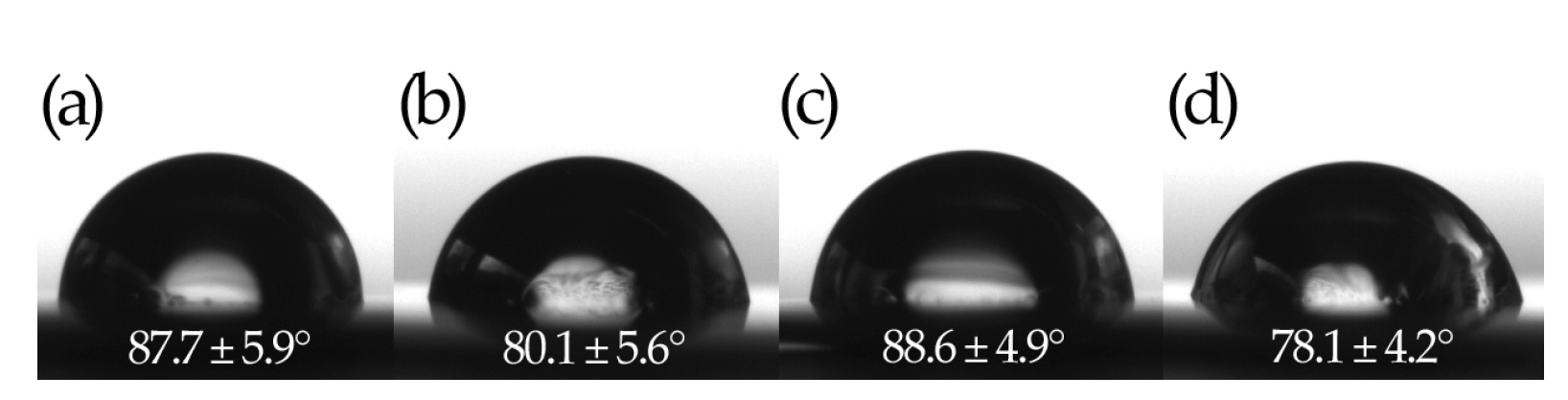
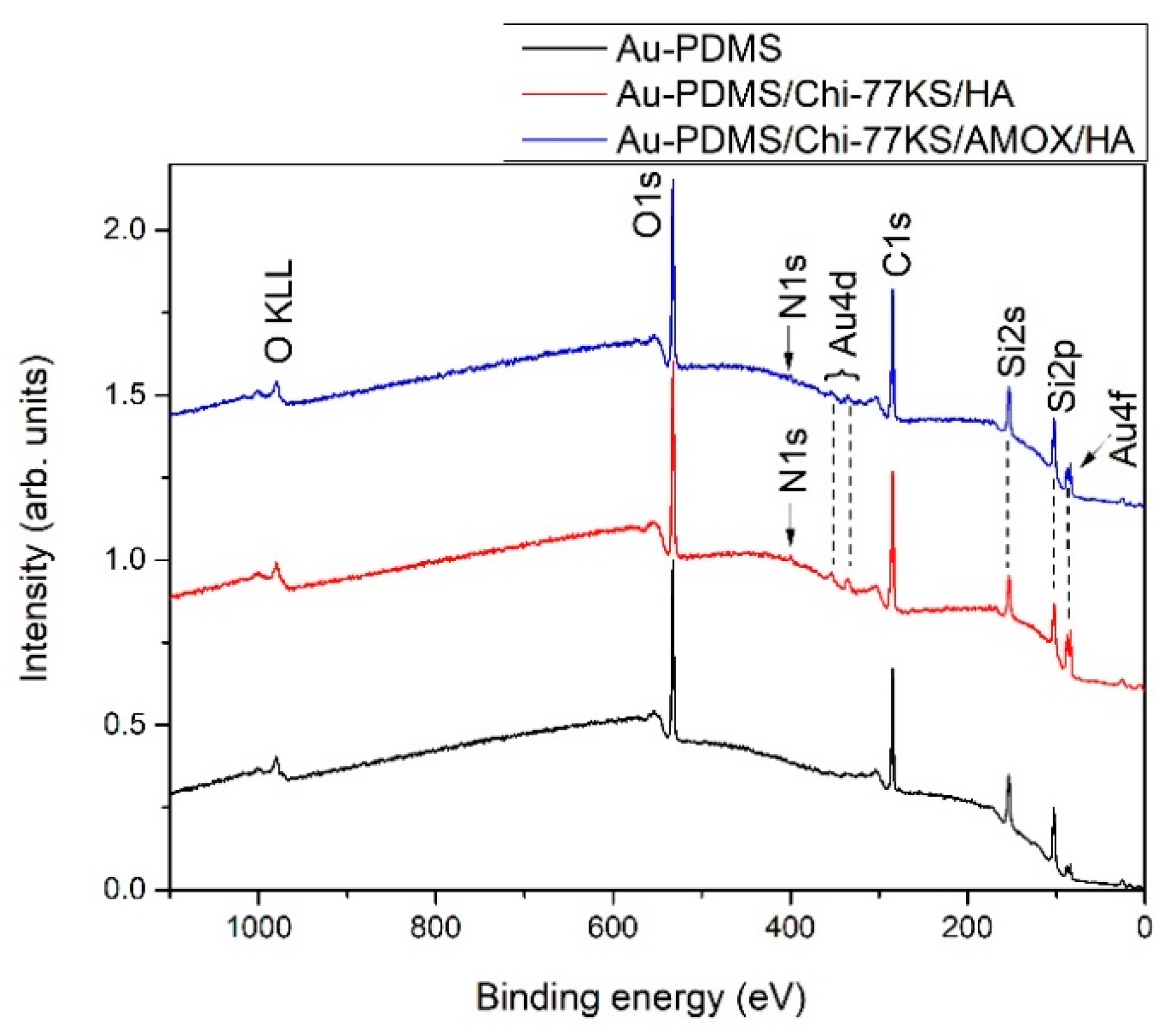
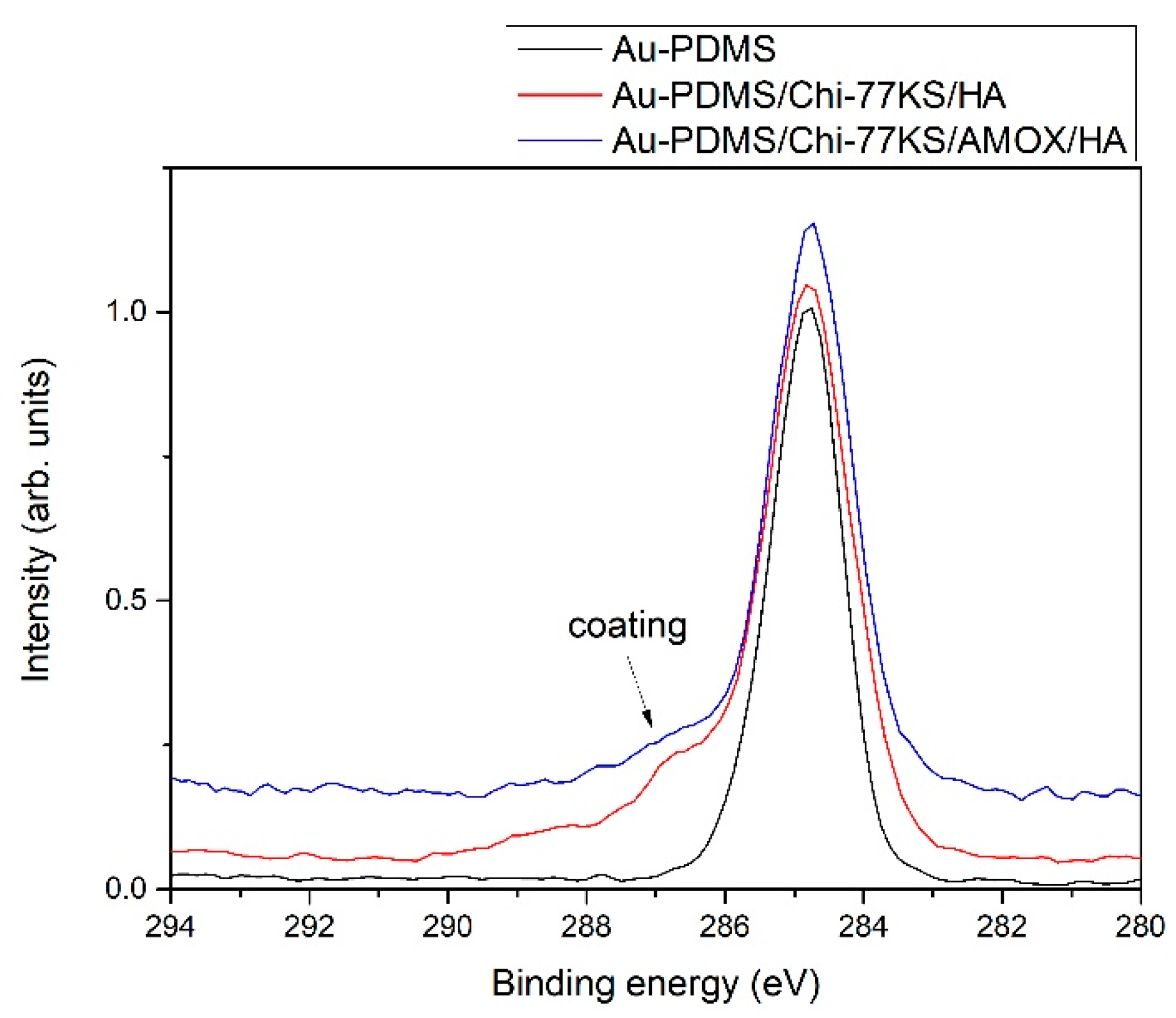
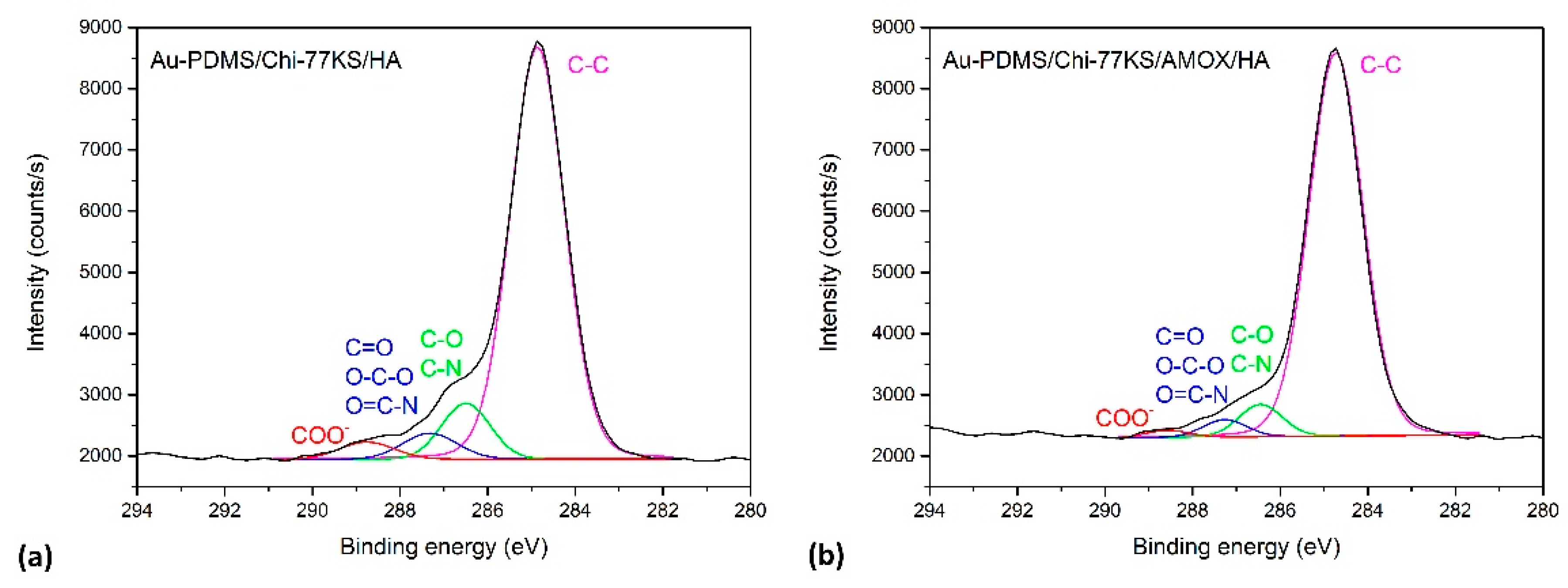

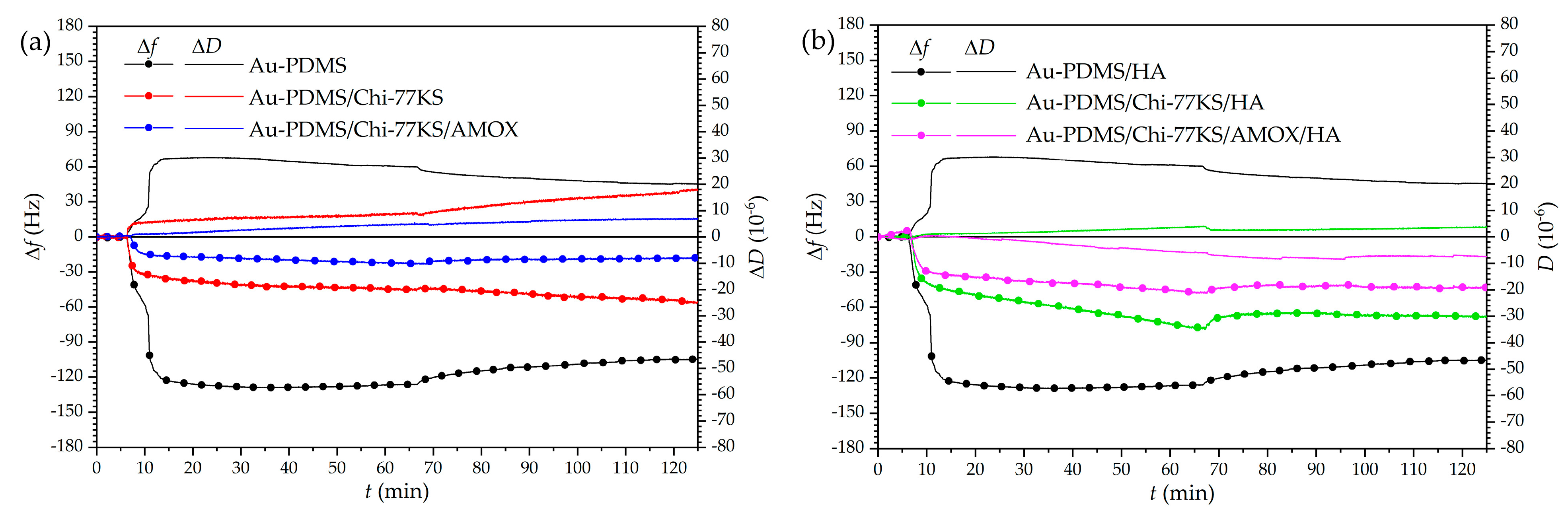
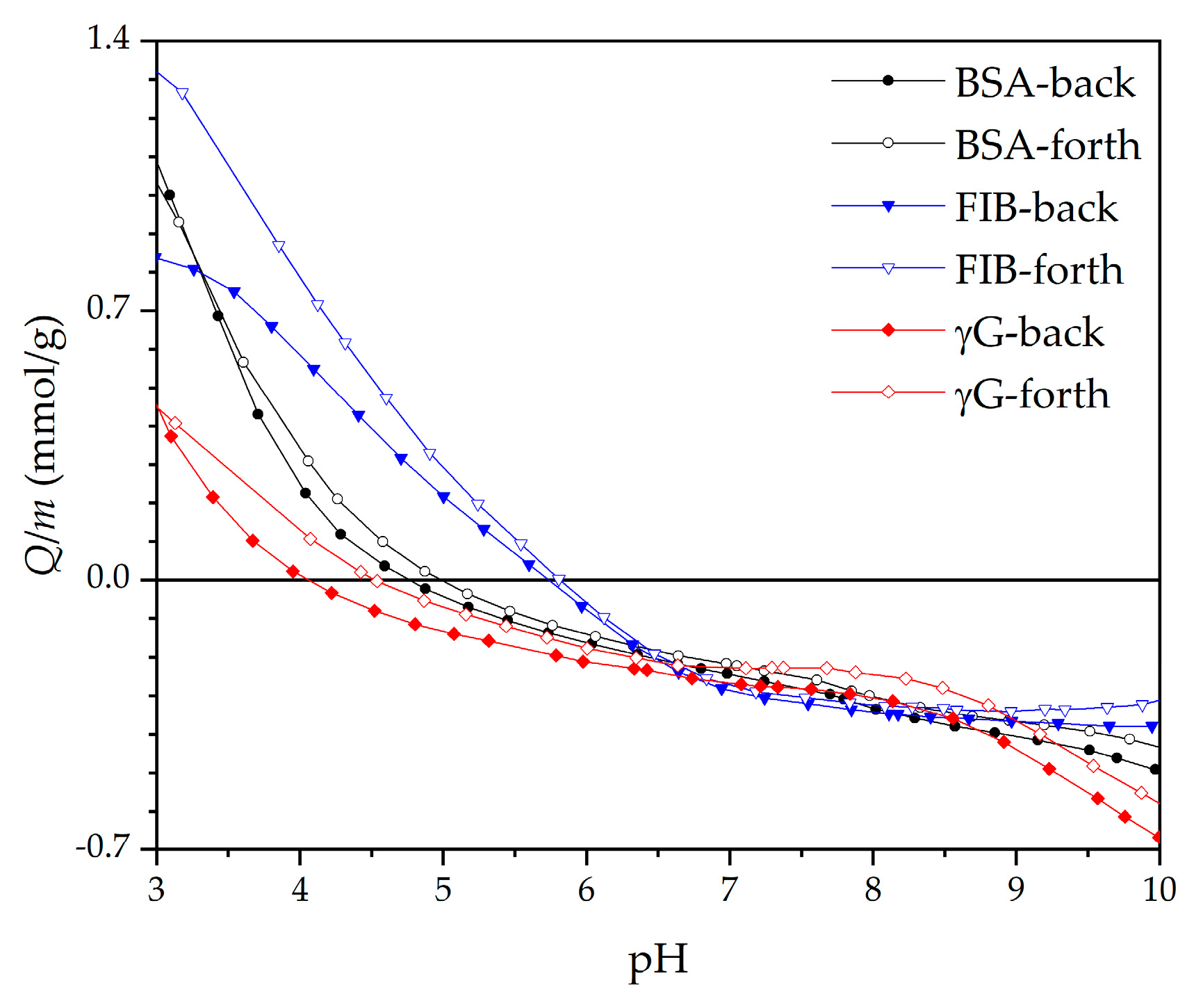
| Model Samples | |
|---|---|
| Au-PDMS | QSX301 coated with PDMS |
| Au-PDMS/Chi-77KS | Au-PDMS coated with Chi-77KS complex |
| Au-PDMS/Chi-77KS/AMOX | Au-PDMS coated with Chi-77KS/AMOX complex |
| Au-PDMS/Chi-77KS/HA | Au-PDMS coated with Chi-77KS complex and further by HA |
| Au-PDMS/Chi-77KS/AMOX/HA | Au-PDMS coated with Chi-77KS/AMOX complex and further by HA |
| Real Samples | |
|---|---|
| PDMS | Reference PDMS disc |
| PDMS/Chi-77KS | PDMS disc coated with Chi-77KS complex |
| PDMS/Chi-77KS/AMOX | PDMS disc coated with Chi-77KS/AMOX complex |
| PDMS/Chi-77KS/HA | PDMS disc coated with Chi-77KS complex and further by HA |
| PDMS/Chi-77KS/AMOX/HA | PDMS disc coated with Chi-77KS/AMOX complex and further by HA |
| Mix | Δf [Hz] | ΔfB/ΔfA [%] | ΔD [10−6] | ΔD/Δf [10−7 Hz−1] | |||
|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||
| Au-PDMS [35] | −126.2 ± 4.0 | −104.7 ± 6.2 | 83.0 | 26.7 ± 2.1 | 20.1 ± 1.3 | 2.1 | 1.9 |
| Au-PDMS/Chi-77KS [35] | −45.1 ± 3.4 | −56.4 ± 3.3 | 125.1 | 8.3 ± 2.0 | 18.1 ± 1.1 | 1.8 | 3.2 |
| Au-PDMS/Chi-77KS/AMOX [35] | −22.7 ± 2.2 | −18.1 ± 1.4 | 79.7 | 4.8 ± 1.2 | 7.1 ± 0.9 | 2.1 | 3.9 |
| Au-PDMS/Chi-77KS/HA | −34.8 ± 3.2 | −29.9 ± 2.9 | 85.9 | 3.9 ± 1.9 | 3.6 ± 1.5 | 1.1 | 1.2 |
| Au-PDMS/Chi-77KS/AMOX/HA | −21.3 ± 3.7 | −19.1 ± 3.8 | 89.7 | −6.4 ± 2.3 | −7.4 ± 2.2 | 3.0 | 3.9 |
| Sample | ΓQCM Wet [ng/m2] | hf [nm] | ηf × 10−3 [Ns/m2] | μf × 104 [N/m2] |
|---|---|---|---|---|
| Au-PDMS [35] | 2662.60 ± 25.89 | 26.63 ± 2.86 | 0.002 ± 0.000 | 13.69 ± 1.52 |
| Au-PDMS/Chi-77KS [35] | 2550.40 ± 20.67 | 25.50 ± 1.62 | 0.002 ± 0.000 | 7.26 ± 0.86 |
| Au-PDMS/Chi-77KS/AMOX [35] | 2307.60 ± 18.66 | 23.08 ± 2.08 | 0.001 ± 0.000 | 1.76 ± 0.05 |
| Au-PDMS/Chi-77KS/HA | 800.31 ± 10.05 | 8.00 ± 0.54 | 0.003 ± 0.000 | 14.91 ± 0.91 |
| Au-PDMS/Chi-77KS/AMOX/HA | 341.61 ± 8.82 | 3.42 ± 0.12 | 0.715 ± 0.004 | 12.01 ± 1.35 |
| Sample | Cell Reactivity Grade | Biological Reactivity | ||
|---|---|---|---|---|
| 24 h | 48 h | |||
| NC | 0 | 0 | none | |
| VC | 0 | 0 | none | |
| PDMS% (V/V) | 6.25 | 0 | 0 | none |
| 12.5 | 0 | 0 | none | |
| 25 | 0 | 0 | none | |
| 50 | 0 | 0 | none | |
| 100 | 0 | 1 | none/slight | |
| PDMS/Chi-77KS/HA% (V/V) | 6.25 | 0 | 0 | none |
| 12.5 | 0 | 0 | none | |
| 25 | 0 | 0 | none | |
| 50 | 0 | 0 | none | |
| 100 | 0 | 1 | none/slight | |
| PDMS/Chi-77KS/AMOX/HA% (V/V) | 6.25 | 0 | 0 | none |
| 12.5 | 0 | 0 | none | |
| 25 | 0 | 0 | none | |
| 50 | 0–1 | 1 | none-slight/slight | |
| 100 | 0–1 | 2 | none-slight/mild | |
| PC | 3 | 4 | moderate/severe | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajdnik, U.; Luxbacher, T.; Vesel, A.; Štern, A.; Žegura, B.; Trček, J.; Fras Zemljič, L. Polysaccharide-Based Bilayer Coatings for Biofilm-Inhibiting Surfaces of Medical Devices. Materials 2021, 14, 4720. https://doi.org/10.3390/ma14164720
Ajdnik U, Luxbacher T, Vesel A, Štern A, Žegura B, Trček J, Fras Zemljič L. Polysaccharide-Based Bilayer Coatings for Biofilm-Inhibiting Surfaces of Medical Devices. Materials. 2021; 14(16):4720. https://doi.org/10.3390/ma14164720
Chicago/Turabian StyleAjdnik, Urban, Thomas Luxbacher, Alenka Vesel, Alja Štern, Bojana Žegura, Janja Trček, and Lidija Fras Zemljič. 2021. "Polysaccharide-Based Bilayer Coatings for Biofilm-Inhibiting Surfaces of Medical Devices" Materials 14, no. 16: 4720. https://doi.org/10.3390/ma14164720







