Experimental Study on Thermal Runaway Process of 18650 Lithium-Ion Battery under Different Discharge Currents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Battery Samples
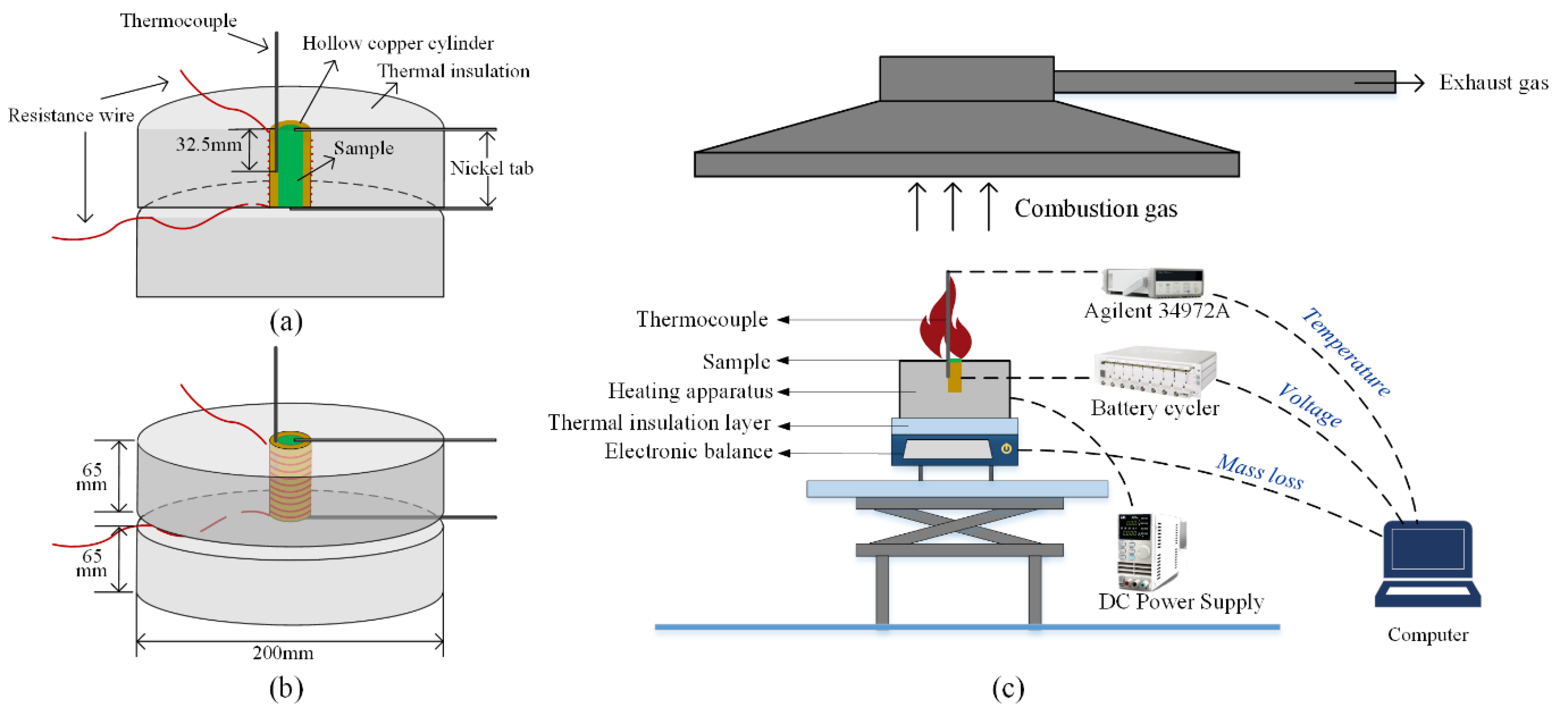
2.2. Apparatus
2.3. Test Conditions
3. Results
3.1. Thermal Response of the LIB in an Open Circuit
3.2. Discharge Tests on the LIBs with the Same C0
3.3. Discharge Tests on the LIBs with the Same CTR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- Bhide, S.; Shim, T. Novel Predictive Electric Li-Ion Battery Model Incorporating Thermal and Rate Factor Effects. IEEE Trans. Veh. Technol. 2011, 60, 819–829. [Google Scholar] [CrossRef]
- Tran, M.K.; Fowler, M. A Review of Lithium-Ion Battery Fault Diagnostic Algorithms: Current Progress and Future Challenges. Algorithms 2020, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.K.; Fowler, M. Sensor Fault Detection and Isolation for Degrading Lithium-Ion Batteries in Electric Vehicles Using Parameter Estimation with Recursive Least Squares. Batteries 2020, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Mao, B.; Huang, P.; Chen, H.; Wang, Q.; Sun, J. Self-heating reaction and thermal runaway criticality of the lithium ion battery. Int. J. Heat Mass Transf. 2020, 149, 119178. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process. Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Wu, T.Q.; Chen, H.D.; Wang, Q.S.; Sun, J.H. Comparison analysis on the thermal runaway of lithium-ion battery under two heating modes. J. Hazard. Mater. 2018, 344, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Jhu, C.Y.; Wang, Y.W.; Shu, C.M.; Chang, J.C.; Wu, H.C. Thermal explosion hazards on 18650 lithium ion batteries with a VSP2 adiabatic calorimeter. J. Hazard. Mater. 2011, 192, 99–107. [Google Scholar] [CrossRef]
- Jhu, C.Y.; Wang, Y.W.; Wen, C.Y.; Shu, C.M. Thermal runaway potential of LiCoO2 and Li(Ni1/3Co1/3Mn1/3)O2 batteries determined with adiabatic calorimetry methodology. Appl. Energy 2012, 100, 127–131. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Huang, C.-Y. Thermal explosion energy evaluated by thermokinetic analysis for series- and parallel-circuit NMC lithium battery modules. Process. Saf. Environ. Prot. 2020, 142, 295–307. [Google Scholar] [CrossRef]
- Roder, P.; Baba, N.; Wiemhofer, H.D. A detailed thermal study of a Li[Ni0.33C0.33Mn0.33]O-2/LiMn2O4-based lithium ion cell by accelerating rate and differential scanning calorimetry. J. Power Sources 2014, 248, 978–987. [Google Scholar] [CrossRef]
- Ribiere, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Wang, Q.S.; Huang, P.F.; Ping, P.; Du, Y.L.; Li, K.; Sun, J.H. Combustion behavior of lithium iron phosphate battery induced by external heat radiation. J. Loss Prev. Process. Ind. 2017, 49, 961–969. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Jiang, L.; Zhao, C.; Sun, J.; Wang, Q. Refined study on lithium ion battery combustion in open space and a combustion chamber. Process. Saf. Environ. Prot. 2020, 139, 133–146. [Google Scholar] [CrossRef]
- Manikandan, B.; Yap, C.; Balaya, P. Towards Understanding Heat Generation Characteristics of Li-Ion Batteries by Calorimetry, Impedance, and Potentiometry Studies. J. Electrochem. Soc. 2017, 164, A2794–A2800. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Shi, S.; Tang, J.C.; Wu, H.W.; Yu, J.Z. Experimental and analytical study on heat generation characteristics of a lithium-ion power battery. Int. J. Heat Mass Transf. 2018, 122, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Lyu, P.Z.; Huo, Y.T.; Qu, Z.G.; Rao, Z.H. Investigation on the thermal behavior of Ni-rich NMC lithium ion battery for energy storage. Appl. Therm. Eng. 2020, 166, 114746. [Google Scholar] [CrossRef]
- Bai, Y.; Li, L.M.; Li, Y.; Chen, G.H.; Zhao, H.C.; Wang, Z.H.; Wu, C.; Ma, H.Y.; Wang, X.Q.; Cui, H.Y.; et al. Reversible and irreversible heat generation of NCA/Si-C pouch cell during electrochemical energy-storage process. J. Energy Chem. 2019, 29, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, C.; Guo, X.; Cheng, S.; Gao, Y.; Wang, R.; Sun, Y.; Yan, P. Thermal characteristics of ultrahigh power density lithium-ion battery. J. Power Sources 2021, 506, 230205. [Google Scholar] [CrossRef]
- Seong Kim, U.; Yi, J.; Shin, C.B.; Han, T.; Park, S. Modeling the Dependence of the Discharge Behavior of a Lithium-Ion Battery on the Environmental Temperature. J. Electrochem. Soc. 2011, 158, A611. [Google Scholar] [CrossRef]
- Onda, K.; Kameyama, H.; Hanamoto, T.; Ito, K. Experimental study on heat generation behavior of small lithium-ion secondary batteries. J. Electrochem. Soc. 2003, 150, A285–A291. [Google Scholar] [CrossRef]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J. Power Sources 2006, 158, 535–542. [Google Scholar] [CrossRef]
- “7.18” Hangzhou Electric Car Caught Fire Culprit: Lithium Battery Became “Invisible Bomb”. Available online: https://www.thepaper.cn/newsDetail_forward_13698240 (accessed on 23 June 2021).
- Vape Pen Explodes, Rips Away Chunk of Man’s Face. Available online: https://web.calce.umd.edu/batteries/news.html (accessed on 8 April 2019).
- National Transportation Safety Board. Preliminary report—Battery fire in electric-powered passenger car. In National Transportation Safety Board; National Transportation Safety Board: West Hollywood, CA, USA, 2018. Available online: https://www.ntsb.gov/investigations/accidentreports/pages/hwy18fh014-preliminary.aspx (accessed on 20 October 2018).
- Sun, P.Y.; Bisschop, R.; Niu, H.H.; Huang, X.Y. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Chen, S.; Guo, L. Hazardous characteristics of charge and discharge of lithium-ion batteries under adiabatic environment and hot environment. Int. J. Heat Mass Transf. 2019, 141, 419–431. [Google Scholar] [CrossRef]
- Guo, L.S.; Wang, Z.R.; Wang, J.H.; Luo, Q.K.; Liu, J.J. Effects of the environmental temperature and heat dissipation condition on the thermal runaway of lithium ion batteries during the charge-discharge process. J. Loss Prev. Process. Ind. 2017, 49, 953–960. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Gong, J.; Liu, K.; Wang, H.; Guo, L. Experimental Study of Thermal Runaway Process of 18650 Lithium-Ion Battery. Materials 2017, 10, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Stoliarov, S.I.; Denlinger, M.; Masias, A.; Snyder, K. Comprehensive calorimetry of the thermally-induced failure of a lithium ion battery. J. Power Sources 2015, 280, 516–525. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Stoliarov, S.I.; Denlinger, M.; Masias, A.; Snyder, K. Heat release during thermally-induced failure of a lithium ion battery: Impact of cathode composition. Fire Saf. J. 2016, 85, 10–22. [Google Scholar] [CrossRef]
- Zhong, G.; Mao, B.; Wang, C.; Jiang, L.; Xu, K.; Sun, J.; Wang, Q. Thermal runaway and fire behavior investigation of lithium ion batteries using modified cone calorimeter. J. Therm. Anal. Calorim. 2018, 135, 2879–2889. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Loren, A.; Mellander, B.E. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar] [CrossRef]
- Wang, Q.S.; Ping, P.; Zhao, X.J.; Chu, G.Q.; Sun, J.H.; Chen, C.H. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard. Mater. 2019, 381, 120916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, H.; Zhong, G.; Wang, Y.; Wang, Q. Experimental study on thermal runaway risk of 18650 lithium ion battery under side-heating condition. J. Loss Prev. Process. Ind. 2019, 61, 122–129. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Wang, Q.S.; Sun, J.H.; Yao, X.L.; Chen, C.H. Thermal behavior of lithiated graphite with electrolyte in lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A329–A333. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Feng, X.N.; Ouyang, M.G.; Liu, X.; Lu, L.G.; Xia, Y.; He, X.M. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.S.; Hsu, H.J.; Feng, X.N.; Xu, G.L.; Zhuang, M.H.; Gao, H.; Lu, L.G.; Han, X.B.; Chu, Z.Y.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef] [Green Version]
- Utsunomiya, T.; Hatozaki, O.; Yoshimoto, N.; Egashira, M.; Morita, M. Self-discharge behavior and its temperature dependence of carbon electrodes in lithium-ion batteries. J. Power Sources 2011, 196, 8598–8603. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- He, X.Z.; Restuccia, F.; Zhang, Y.; Hu, Z.W.; Huang, X.Y.; Fang, J.; Rein, G. Experimental Study of Self-heating Ignition of Lithium-Ion Batteries During Storage: Effect of the Number of Cells. Fire Technol. 2020, 56, 2649–2669. [Google Scholar] [CrossRef]
- Venugopal, G. Characterization of thermal cut-off mechanisms in prismatic lithium-ion batteries. J. Power Sources 2001, 101, 231–237. [Google Scholar] [CrossRef]
- Feng, X.N.; Fang, M.; He, X.M.; Ouyang, M.G.; Lu, L.G.; Wang, H.; Zhang, M.X. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. NIST-JANAF thermochemical tables, fourth edition. J. Phys.Chem. Ref. Data Monogr. 1998, 9, 1e1951. [Google Scholar]
- Zhou, Z.; Zhou, X.; Peng, Y.; Li, L.; Cao, J.; Yang, L.; Cao, B. Quantitative study on the thermal failure features of lithium iron phosphate batteries under varied heating powers. Appl. Therm. Eng. 2021, 185, 116346. [Google Scholar] [CrossRef]












| Date | Location | Incident |
|---|---|---|
| July 2021 | Hangzhou, China | An electro mobile caught fire while in motion [24]. |
| April 2019 | Cordova, Spain | The battery inside an e-cigarette exploded when using [25]. |
| June 2018 | California, USA | A Tesla car released smoke while being driven [26]. |
| January 2016 | Mudeungsan, Korea | A Renault-Samsung electric vehicle caught fire while driving [27]. |
| Group No | Test No | Discharge Current (A) | C0 (mAh) |
|---|---|---|---|
| i | 1 | — | 1650 |
| ii | 2 | 1 | 1650 |
| 3 | 2 | ||
| 4 | 3 | ||
| 5 | 4 | ||
| 6 | 6 | ||
| iii | 7 | 1 | 1775 |
| 8 | 2 | 1900 | |
| 9 | 3 | 2000 | |
| 10 | 4 | 2050 | |
| 11 | 6 | 2125 |
| Test No | Discharge Current (A) | C0 (mAh) | Energy Consumption 1 (mAh) | CTR (mAh) |
|---|---|---|---|---|
| 2 | 1 | 1650 | 127.68 ± 0.92 | 1522.32 ± 0.92 |
| 3 | 2 | 1650 | 224.66 ± 2.66 | 1425.34 ± 2.66 |
| 4 | 3 | 1650 | 292.98 ± 1.98 | 1357.02 ± 1.98 |
| 5 | 4 | 1650 | 357.24 ± 5.96 | 1292.76 ± 5.96 |
| 6 | 6 | 1650 | 422.10 ± 6.65 | 1227.90 ± 6.65 |
| Test No | Discharge Current /A | Onset of Safety Venting | Onset of Thermal Runaway | Tmax/°C | (dT/dt)max/°C·s−1 | ∆m/g | ||
|---|---|---|---|---|---|---|---|---|
| tSV/s | TSV/°C | tTR/s | TTR/°C | |||||
| 1 | — | 585 ± 12 | 168.75 ± 2.26 | 932 ± 5 | 262.11 ± 0.35 | 621.61 ± 1.74 | 36.68 ± 1.17 | 9.30 ± 0.24 |
| 2 | 1 | 554 ± 8 | 170.55 ± 5.01 | 917 ± 15 | 272.86 ± 3.01 | 620.49 ± 13.69 | 29.67 ± 1.77 | 8.85 ± 0.10 |
| 3 | 2 | 546 ± 2 | 169.50 ± 3.81 | 905 ± 11 | 274.19 ± 4.03 | 618.98 ± 4.63 | 23.87 ± 1.92 | 8.43 ± 0.21 |
| 4 | 3 | 541 ± 5 | 174.57 ± 5.13 | 900 ± 12 | 282.77 ± 3.2 | 611.43 ± 8.31 | 19.73 ± 2.48 | 8.00 ± 0.17 |
| 5 | 4 | 540 ± 10 | 177.19 ± 2.53 | 897 ± 3 | 282.92 ± 2.25 | 600.87 ± 1.33 | 17.24 ± 4.00 | 7.65 ± 0.19 |
| 6 | 6 | 538 ± 7 | 180.50 ± 1.96 | 872 ± 1 | 286.47 ± 7.41 | 585.26 ± 3.86 | 13.68 ± 0.40 | 7.39 ± 0.23 |
| Test No | Discharge Current (A) | C0 (mAh) | Energy Consumption (mAh) | CTR (mAh) |
|---|---|---|---|---|
| 7 | 1 | 1775 | 126.89 ± 2.69 | 1648.11 ± 2.69 |
| 8 | 2 | 1900 | 240.68 ± 7.01 | 1659.32 ± 7.01 |
| 9 | 3 | 2000 | 339.07 ± 5.39 | 1660.93 ± 5.39 |
| 10 | 4 | 2050 | 392.42 ± 3.56 | 1657.58 ± 3.56 |
| 11 | 6 | 2150 | 457.57 ± 4.68 | 1662.43 ± 4.68 |
| Test No | Discharge Current /A | Onset of Safety Venting | Onset of Thermal Runaway | Tmax/°C | (dT/dt)max/°C·s−1 | ∆m/g | ||
|---|---|---|---|---|---|---|---|---|
| tSV/s | TSV/°C | tTR/s | TTR/°C | |||||
| 1 | — | 585 ± 12 | 168.75 ± 2.26 | 932 ± 5 | 262.11 ± 0.35 | 621.61 ± 1.74 | 36.68 ± 1.17 | 9.30 ± 0.24 |
| 7 | 1 | 542 ± 7 | 165.28 ± 6.34 | 920 ± 8 | 264.01 ± 1.69 | 621.16 ± 2.90 | 36.73 ± 2.28 | 9.52 ± 0.09 |
| 8 | 2 | 541 ± 9 | 165.28 ± 6.34 | 916 ± 18 | 264.11 ± 1.57 | 618.37 ± 4.73 | 35.27 ± 0.30 | 9.57 ± 0.29 |
| 9 | 3 | 525 ± 16 | 166.89 ± 2.45 | 906 ± 18 | 264.30 ± 1.85 | 628.79 ± 14.18 | 33.70 ± 0.49 | 9.26 ± 0.15 |
| 10 | 4 | 505 ± 5 | 164.85 ± 3.34 | 881 ± 17 | 264.68 ± 2.05 | 619.14 ± 2.36 | 36.18 ± 2.64 | 9.30 ± 0.06 |
| 11 | 6 | 499 ± 11 | 166.97 ± 1.65 | 863 ± 24 | 263.09 ± 2.66 | 627.94 ± 18.78 | 33.98 ± 1.72 | 9.58 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Ju, X.; Zhou, X.; Peng, Y.; Zhou, Z.; Cao, B.; Yang, L. Experimental Study on Thermal Runaway Process of 18650 Lithium-Ion Battery under Different Discharge Currents. Materials 2021, 14, 4740. https://doi.org/10.3390/ma14164740
Li L, Ju X, Zhou X, Peng Y, Zhou Z, Cao B, Yang L. Experimental Study on Thermal Runaway Process of 18650 Lithium-Ion Battery under Different Discharge Currents. Materials. 2021; 14(16):4740. https://doi.org/10.3390/ma14164740
Chicago/Turabian StyleLi, Lun, Xiaoyu Ju, Xiaodong Zhou, Yang Peng, Zhizuan Zhou, Bei Cao, and Lizhong Yang. 2021. "Experimental Study on Thermal Runaway Process of 18650 Lithium-Ion Battery under Different Discharge Currents" Materials 14, no. 16: 4740. https://doi.org/10.3390/ma14164740





