Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemical Measurements
2.2. Contact Angle Measurements
2.3. Electrode Materials
3. Results and Discussion
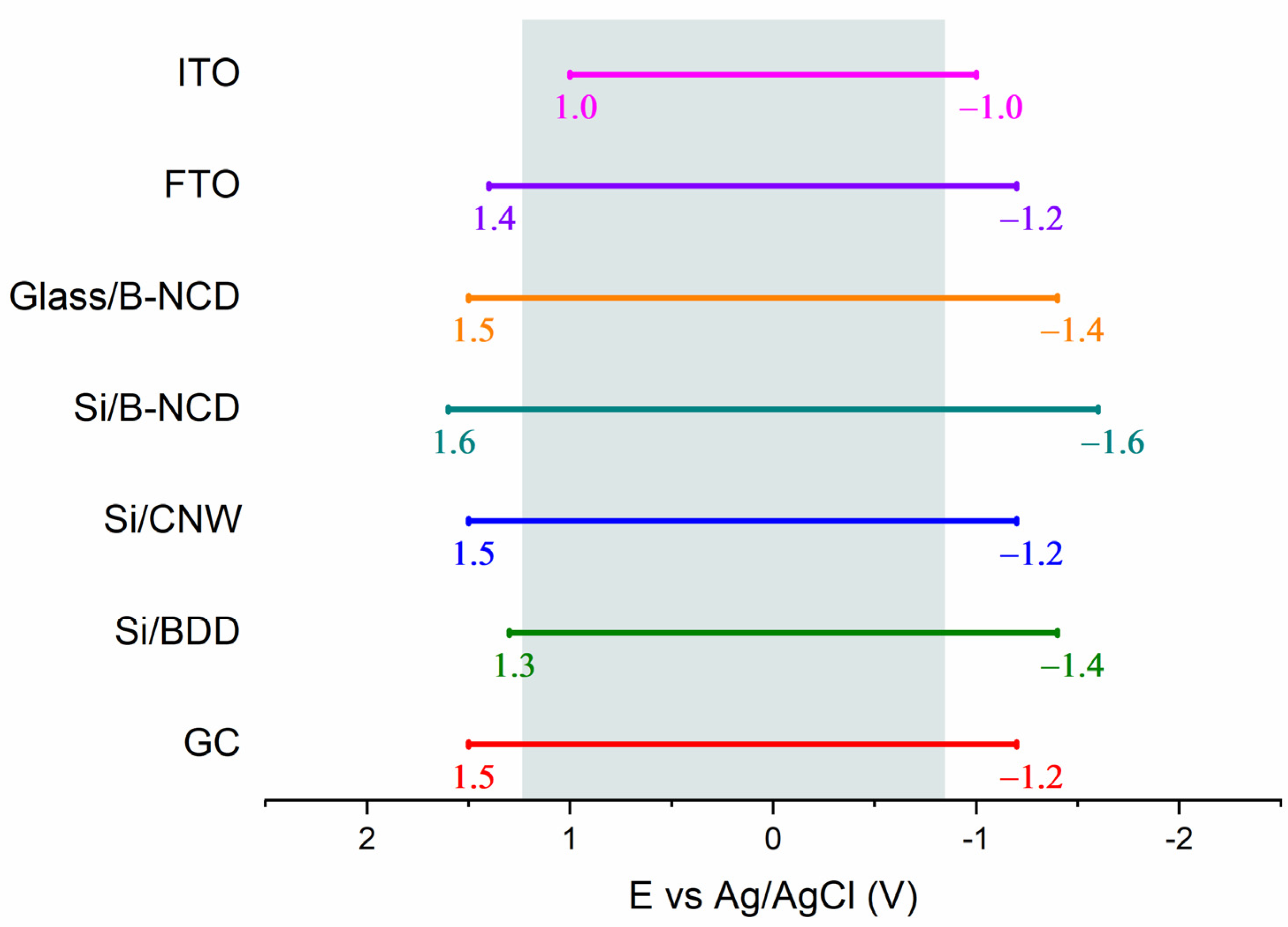
3.1. Electrochemical Properties
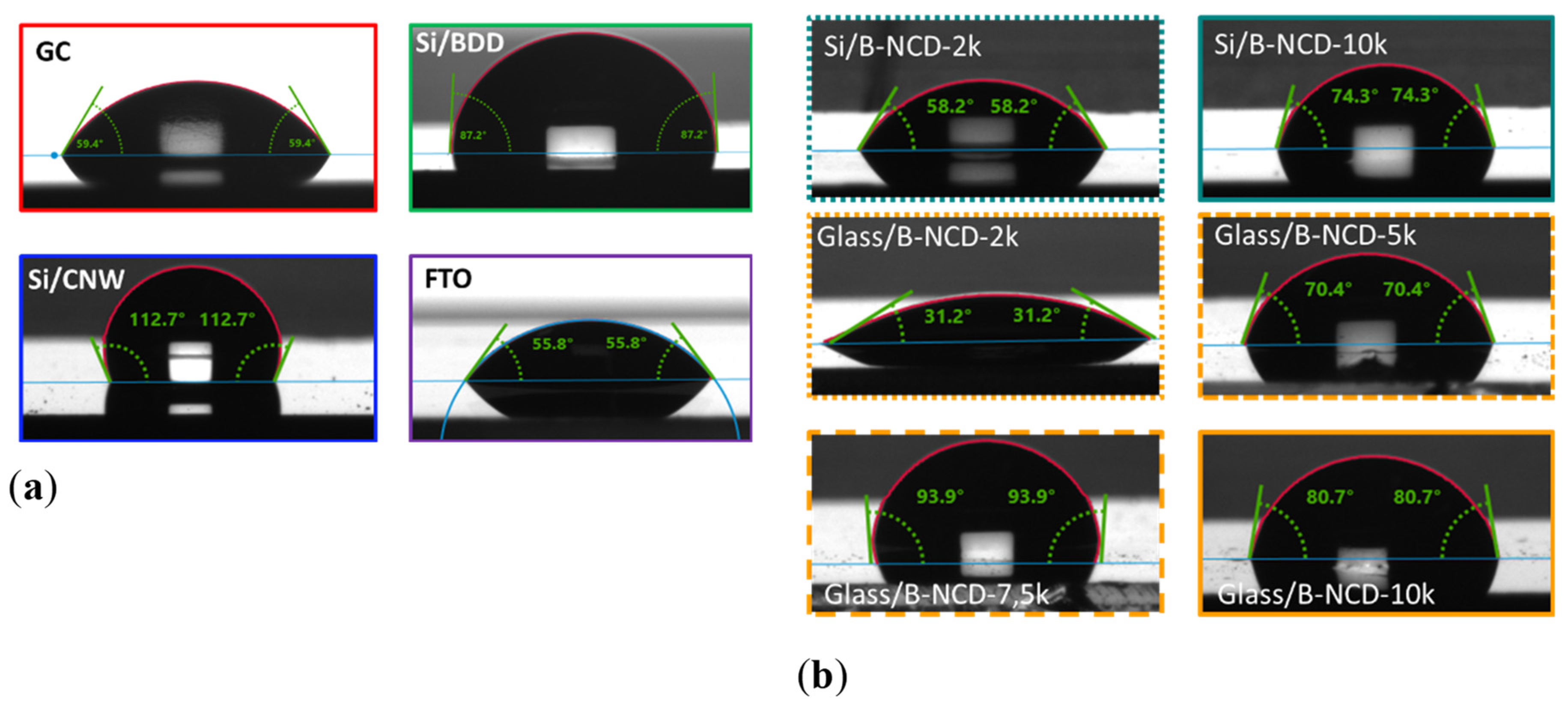
3.2. Wettability
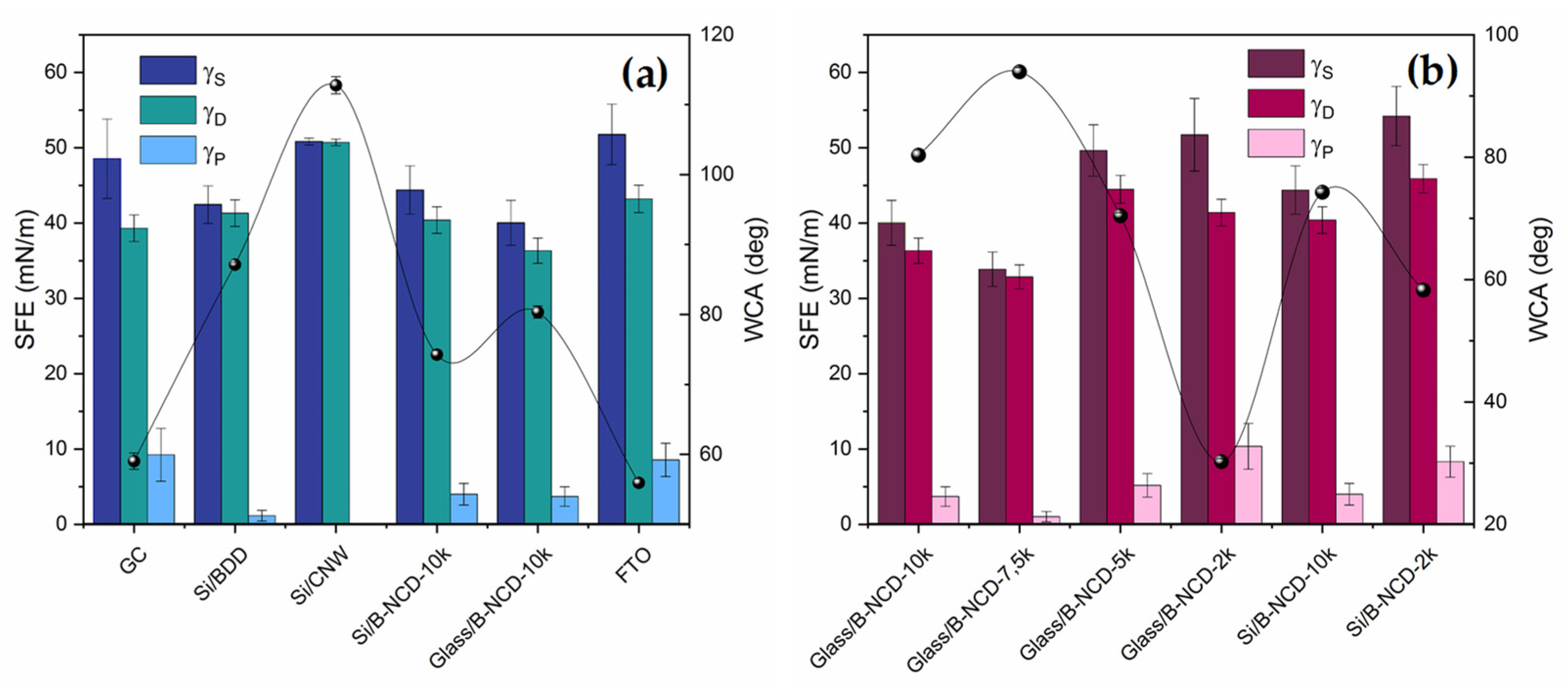
3.3. Surface Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Linden, W.; Dieker, J. Glassy carbon as electrode material in electro- analytical chemistry. Anal. Chim. Acta 1980, 119, 1–24. [Google Scholar] [CrossRef]
- Pesin, L.A. Review Structure and properties of glass-like carbon. J. Mater. Sci. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H. A Sensitive Chlorpromazine Voltammetric Sensor Based on Graphene Oxide Modified Glassy Carbon Electrode. Anal. Bioanal. Chem. Res. 2019, 6, 171–182. [Google Scholar] [CrossRef]
- Masikini, M.; Ghica, M.E.; Baker, P.; Iwuoha, E.; Brett, C.M.A. Electrochemical Sensor Based on Multi-walled Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrode for Detection of Estradiol in Environmental Samples. Electroanalysis 2019, 31, 1925–1933. [Google Scholar] [CrossRef]
- Sohouli, E.; Keihan, A.H.; Shahdost-Fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A glassy carbon electrode modified with carbon nanoonions for electrochemical determination of fentanyl. Mater. Sci. Eng. C 2020, 110, 110684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.G.; Silva, L.; Corat, E.J.; Trava-Airoldi, V. Kinetics study of diamond electrodes at different levels of boron doping as quasi-reversible systems. Diam. Relat. Mater. 2002, 11, 1523–1531. [Google Scholar] [CrossRef]
- Fujishima, A.; Einaga, Y.; Rao, T.N.; Tryk, D.A. Diamond Electrochemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Yang, N. Diamond Electrochemical Devices. In Novel Aspects of Diamond: From Growth to Applications; (Topics in Applied Physics); Yang, N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 223–256. ISBN 978-3-030-12469-4. [Google Scholar]
- Compton, R.G.; Foord, J.S.; Marken, F. Electroanalysis at Diamond-Like and Doped-Diamond Electrodes. Electroanalysis 2003, 15, 1349–1363. [Google Scholar] [CrossRef]
- Qureshi, A.; Kang, W.P.; Davidson, J.L.; Gurbuz, Y. Review on carbon-derived, solid-state, micro and nano sensors for electrochemical sensing applications. Diam. Relat. Mater. 2009, 18, 1401–1420. [Google Scholar] [CrossRef] [Green Version]
- Niedzialkowski, P.; Ossowski, T.; Zięba, P.; Cirocka, A.; Rochowski, P.; Pogorzelski, S.; Ryl, J.; Sobaszek, M.; Bogdanowicz, R. Poly-l-lysine-modified boron-doped diamond electrodes for the amperometric detection of nucleic acid bases. J. Electroanal. Chem. 2015, 756, 84–93. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Tian, R.H.; Zhi, J.F. Amperometric biosensor based on tyrosinase immobilized on a boron-doped diamond electrode. Biosens. Bioelectron. 2007, 22, 822–828. [Google Scholar] [CrossRef]
- Geng, R.; Zhao, G.; Liu, M.; Li, M. A sandwich structured SiO2/cytochrome c/SiO2 on a boron-doped diamond film electrode as an electrochemical nitrite biosensor. Biomaterials 2008, 29, 2794–2801. [Google Scholar] [CrossRef]
- Costa, D.J.; Santos, J.C.; Sanches-Brandão, F.A.; Ribeiro, W.F.; Banda, G.; Araujo, M. Boron-doped diamond electrode acting as a voltammetric sensor for the detection of methomyl pesticide. J. Electroanal. Chem. 2017, 789, 100–107. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.; Galushin, A.A.; Karyakin, A.A. Prussian Blue modified boron-doped diamond interfaces for advanced H2O2 electrochemical sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Different strategies for functionalization of diamond surfaces. J. Solid State Electrochem. 2007, 12, 1205–1218. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Sawczak, M.; Niedzialkowski, P.; Zieba, P.; Finke, B.; Ryl, J.; Ossowski, T. Direct amination of boron-doped diamond by plasma polymerized allylamine film. Phys. Status Solidi (a) 2014, 211, 2319–2327. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Metal Nanoparticle Modified Boron Doped Diamond Electrodes for Use in Electroanalysis. Electroanalysis 2010, 22, 1947–1956. [Google Scholar] [CrossRef]
- Pecková, K.; Musilová, J.; Barek, J. Boron-Doped Diamond Film Electrodes—New Tool for Voltammetric Determination of Organic Substances. Crit. Rev. Anal. Chem. 2009, 39, 148–172. [Google Scholar] [CrossRef]
- Niedziałkowski, P.; Cebula, Z.; Malinowska, N.; Białobrzeska, W.; Sobaszek, M.; Ficek, M.; Bogdanowicz, R.; Anand, J.S.; Ossowski, T. Comparison of the paracetamol electrochemical determination using boron-doped diamond electrode and boron-doped carbon nanowalls. Biosens. Bioelectron. 2019, 126, 308–314. [Google Scholar] [CrossRef]
- Martin, K.K.; Ouattara, L. Electroanalytical Investigation on Paracetamol on Boron-Doped Diamond Electrode by Voltammetry. Am. J. Anal. Chem. 2019, 10, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Kowalcze, M.; Jakubowska, M. Voltammetric determination of nicotine in electronic cigarette liquids using a boron-doped diamond electrode (BDDE). Diam. Relat. Mater. 2020, 103, 107710. [Google Scholar] [CrossRef]
- Stotter, J.; Zak, J.; Behler, Z.; Show, Y.; Swain, G. Optical and Electrochemical Properties of Optically Transparent, Boron-Doped Diamond Thin Films Deposited on Quartz. Anal. Chem. 2002, 74, 5924–5930. [Google Scholar] [CrossRef]
- Sobaszek, M.; Siuzdak, K.; Skowroński, Ł.; Bogdanowicz, R.; Pluciński, J. Optically transparent boron-doped nanocrystalline diamond films for spectroelectrochemical measurements on different substrates. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012024. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, A.; Souza, F.; Matsushima, J.; Baldan, M.R.; Ferreira, N.G. Detection of phenol at boron-doped nanocrystalline diamond electrodes. J. Electroanal. Chem. 2011, 658, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Dincer, C.; Ktaich, R.; Laubender, E.; Hees, J.J.; Kieninger, J.; Nebel, C.E.; Heinze, J.; Urban, G.A. Nanocrystalline boron-doped diamond nanoelectrode arrays for ultrasensitive dopamine detection. Electrochim. Acta 2015, 185, 101–106. [Google Scholar] [CrossRef]
- Zak, J.K.; Butler, J.E.; Swain, G.M. Diamond Optically Transparent Electrodes: Demonstration of Concept with Ferri/Ferrocyanide and Methyl Viologen. Anal. Chem. 2001, 73, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Auciello, O.; Butler, J.; Cai, W.; Carlisle, J.A.; Gerbi, J.E.; Gruen, D.M.; Knickerbocker, T.; Lasseter, T.L.; Russell, J.N., Jr.; et al. DNA-modified nanocrystalline diamond thin-films as stable, biologically active substrates. Nat. Mater. 2002, 1, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Akin, D.; Gupta, A.; Sherman, D.; Shi, B.; Auciello, O.; Bashir, R. Ultrananocrystalline diamond film as an optimal cell interface for biomedical applications. Biomed. Microdevices 2007, 9, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobaszek, M.; Skowroński, Ł.; Bogdanowicz, R.; Siuzdak, K.; Cirocka, A.; Zięba, P.; Gnyba, M.; Naparty, M.; Gołuński, Ł.; Płotka, P. Optical and electrical properties of ultrathin transparent nanocrystalline boron-doped diamond electrodes. Opt. Mater. 2015, 42, 24–34. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon Nanowalls Grown by Microwave Plasma Enhanced Chemical Vapor Deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Siuzdak, K.; Ficek, M.; Sobaszek, M.; Ryl, J.; Gnyba, M.; Niedzialkowski, P.; Malinowska, N.; Karczewski, J.; Bogdanowicz, R. Boron-Enhanced Growth of Micron-Scale Carbon-Based Nanowalls: A Route toward High Rates of Electrochemical Biosensing. ACS Appl. Mater. Interfaces 2017, 9, 12982–12992. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanimura, M.; Nakai, H.; Yoshimura, A.; Yoshimura, H.; Kojima, K.; Tachibana, M. Nanographite domains in carbon nanowalls. J. Appl. Phys. 2007, 101, 94306. [Google Scholar] [CrossRef]
- Kondo, S.; Kawai, S.; Takeuchi, W.; Yamakawa, K.; Den, S.; Kano, H.; Hiramatsu, M.; Hori, M. Initial growth process of carbon nanowalls synthesized by radical injection plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2009, 106, 094302. [Google Scholar] [CrossRef]
- Teii, K.; Shimada, S.; Nakashima, M.; Chuang, A.T.H. Synthesis and electrical characterization of n-type carbon nanowalls. J. Appl. Phys. 2009, 106, 84303. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.; Kang, H.; Kim, J.H.; Choi, W. Adhesion-Increased Carbon Nanowalls for the Electrodes of Energy Storage Systems. Energies 2019, 12, 4759. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, L.; Makris, T.; Giorgi, R.; Lisi, N.; Salernitano, E. Electrochemical properties of carbon nanowalls synthesized by HF-CVD. Sens. Actuators B Chem. 2007, 126, 144–152. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Yu, H.; Chen, S. Fabrication of a TiO2/carbon nanowall heterojunction and its photocatalytic ability. Carbon 2008, 46, 1126–1132. [Google Scholar] [CrossRef]
- Krivchenko, V.A.; Itkis, D.; Evlashin, S.; Semenenko, D.A.; Goodilin, E.A.; Rakhimov, A.T.; Stepanov, A.S.; Suetin, N.V.; Pilevsky, A.A.; Voronin, P.V. Carbon nanowalls decorated with silicon for lithium-ion batteries. Carbon 2012, 50, 1438–1442. [Google Scholar] [CrossRef]
- Li, B.; Li, S.; Liu, J.; Wang, B.; Yang, S. Vertically Aligned Sulfur–Graphene Nanowalls on Substrates for Ultrafast Lithium–Sulfur Batteries. Nano Lett. 2015, 15, 3073–3079. [Google Scholar] [CrossRef]
- Shin, S.C.; Yoshimura, A.; Matsuo, T.; Mori, M.; Tanimura, M.; Ishihara, A.; Ota, K.-I.; Tachibana, M. Carbon nanowalls as platinum support for fuel cells. J. Appl. Phys. 2011, 110, 104308. [Google Scholar] [CrossRef]
- Yang, J.; Wei, D.; Tang, L.; Song, X.; Luo, W.; Chu, J.; Gao, T.; Shi, H.; Du, C. Wearable temperature sensor based on graphene nanowalls. RSC Adv. 2015, 5, 25609–25615. [Google Scholar] [CrossRef]
- Slobodian, P.; Cvelbar, U.; Riha, P.; Olejnik, R.; Matyas, J.; Filipič, G.; Watanabe, H.; Tajima, S.; Kondo, H.; Sekine, M.; et al. High sensitivity of a carbon nanowall-based sensor for detection of organic vapours. RSC Adv. 2015, 5, 90515–90520. [Google Scholar] [CrossRef]
- Choi, H.; Kwon, S.H.; Kang, H.; Kim, J.H.; Choi, W. Zinc-oxide-deposited Carbon Nanowalls for Acetone Sensing. Thin Solid Films 2020, 700, 137887. [Google Scholar] [CrossRef]
- Andersson, A.; Johansson, N.; Bröms, P.; Yu, N.; Lupo, D.; Salaneck, W.R. Fluorine Tin Oxide as an Alternative to Indium Tin Oxide in Polymer LEDs. Adv. Mater. 1998, 10, 859–863. [Google Scholar] [CrossRef]
- Patni, N.; Sharma, P.; Pillai, S. Newer approach of using alternatives to (Indium doped) metal electrodes, dyes and electrolytes in dye sensitized solar cell. Mater. Res. Express 2018, 5, 045509. [Google Scholar] [CrossRef]
- Lee, K.-T.; Liu, D.-M.; Liang, Y.-Y.; Matsushita, N.; Ikoma, T.; Lu, S.-Y. Porous fluorine-doped tin oxide as a promising substrate for electrochemical biosensors-demonstration in hydrogen peroxide sensing. J. Mater. Chem. B 2014, 2, 7779–7784. [Google Scholar] [CrossRef]
- Banyamin, Z.Y.; Kelly, P.J.; West, G.; Boardman, J. Electrical and Optical Properties of Fluorine Doped Tin Oxide Thin Films Prepared by Magnetron Sputtering. Coatings 2014, 4, 732–746. [Google Scholar] [CrossRef]
- Bierwagen, O. Indium oxide—A transparent, wide-band gap semiconductor for (opto)electronic applications. Semicond. Sci. Technol. 2015, 30, 024001. [Google Scholar] [CrossRef]
- Ouerfelli, J.; Djobo, S.O.; Bernède, J.; Cattin, L.; Morsli, M.; Berredjem, Y. Organic light emitting diodes using fluorine doped tin oxide thin films, deposited by chemical spray pyrolysis, as anode. Mater. Chem. Phys. 2008, 112, 198–201. [Google Scholar] [CrossRef]
- Jasiecki, S.; Czupryniak, J.; Ossowski, T.; Schroeder, G. FTO Coated Glass Electrode Functionalization with Transition Metal Cations Receptors via Electrostatic Self-Assembly. Int. J. Electrochem. Sci. 2013, 8, 12543–12556. [Google Scholar]
- Ahuja, T.; Rajesh; Kumar, D.; Tanwar, V.K.; Sharma, V.; Singh, N.; Biradar, A.M. An amperometric uric acid biosensor based on Bis[sulfosuccinimidyl] suberate crosslinker/3-aminopropyltriethoxysilane surface modified ITO glass electrode. Thin Solid Films 2010, 519, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.O.; Hong, S.-Y.; Kim, M.; Park, S.-M.; Park, J.W. Modification of indium–tin oxide (ITO) glass with aziridine provides a surface of high amine density. J. Colloid Interface Sci. 2004, 277, 499–504. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Martínez, M.; Blázquez, O.; Hernández, S.; Garrido, B.; de Miguel, M.L. Organosilane-functionalization of nanostructured indium tin oxide films. Interface Focus 2016, 6, 20160056. [Google Scholar] [CrossRef] [Green Version]
- Muthurasu, A.; Ganesh, V. Electrochemical characterization of Self-assembled Monolayers (SAMs) of silanes on indium tin oxide (ITO) electrodes – Tuning electron transfer behaviour across electrode–electrolyte interface. J. Colloid Interface Sci. 2012, 374, 241–249. [Google Scholar] [CrossRef]
- Göbel, G.; Talke, A.; Lisdat, F. FTO—An Electrode Material for the Stable Electrochemical Determination of Dopamine. Electroanalysis 2018, 30, 225–229. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Gietzelt, T.; Grundke, K.; Jacobasch, A.H.-J.; Neumann, A.W. Contact Angle Measurements and Contact Angle Interpretation. 1. Contact Angle Measurements by Axisymmetric Drop Shape Analysis and a Goniometer Sessile Drop Technique. Langmuir 1997, 13, 2880–2894. [Google Scholar] [CrossRef]
- Kwok, D.; Neumann, A. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurements and interpretation: Wetting behavior and solid surface tensions for poly(alkyl methacrylate) polymers. J. Adhes. Sci. Technol. 2000, 14, 719–743. [Google Scholar] [CrossRef]
- Cirocka, A.; Zarzeczańska, D.; Wcisło, A.; Ryl, J.; Bogdanowicz, R.; Finke, B.; Ossowski, T. Tuning of the electrochemical properties of transparent fluorine-doped tin oxide electrodes by microwave pulsed-plasma polymerized allylamine. Electrochim. Acta 2019, 313, 432–440. [Google Scholar] [CrossRef]
- Niedziałkowski, P.; Bojko, M.; Ryl, J.; Wcisło, A.; Spodzieja, M.; Magiera-Mularz, K.; Guzik, K.; Dubin, G.; Holak, T.A.; Ossowski, T.; et al. Ultrasensitive electrochemical determination of the cancer biomarker protein sPD-L1 based on a BMS-8-modified gold electrode. Bioelectrochemistry 2021, 139, 107742. [Google Scholar] [CrossRef]
- Szczepańska, E.; Synak, A.; Bojarski, P.; Niedziałkowski, P.; Wcisło, A.; Ossowski, T.; Grobelna, B. Dansyl-Labelled Ag@SiO2 Core-Shell Nanostructures—Synthesis, Characterization, and Metal-Enhanced Fluorescence. Materials 2020, 13, 5168. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, T.; Wcisło, A.; Majstrzyk, W.; Niedziałkowski, P.; Ossowski, T.; Więckiewicz, W.; Gotszalk, T. Adhesion as a component of retention force of overdenture prostheses-study on selected Au based dental materials used for telescopic crowns using atomic force microscopy and contact angle techniques. J. Mech. Behav. Biomed. Mater. 2021, 121, 104648. [Google Scholar] [CrossRef]
- Jańczuk, B.; Białlopiotrowicz, T. Surface free-energy components of liquids and low energy solids and contact angles. J. Colloid Interface Sci. 1989, 127, 189–204. [Google Scholar] [CrossRef]
- Hołysz, L.; Szcześ, A. Determination of surface free energy components of organic liquids by the thin layer wicking method. Ann. Univ. Mariae Curie-Sklodowska Sect. AA—Chem. 2017, 71, 11. [Google Scholar] [CrossRef]
- Swebocki, T.; Niedziałkowski, P.; Cirocka, A.; Szczepańska, E.; Ossowski, T.; Wcisło, A. In pursuit of key features for constructing electrochemical biosensors—Electrochemical and acid-base characteristic of self-assembled monolayers on gold. Supramol. Chem. 2020, 32, 256–266. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Bogdanowicz, R.; Zięba, P.; Wysocka, J.; Ryl, J.; Sobaszek, M.; Ossowski, T. Melamine-modified Boron-doped Diamond towards Enhanced Detection of Adenine, Guanine and Caffeine. Electroanalysis 2015, 28, 211–221. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Sawczak, M.; Niedzialkowski, P.; Zieba, P.; Finke, B.; Ryl, J.; Karczewski, J.; Ossowski, T. Novel Functionalization of Boron-Doped Diamond by Microwave Pulsed-Plasma Polymerized Allylamine Film. J. Phys. Chem. C 2014, 118, 8014–8025. [Google Scholar] [CrossRef]
- Wang, S.; Swope, V.M.; Butler, J.E.; Feygelson, T.; Swain, G.M. The structural and electrochemical properties of boron-doped nanocrystalline diamond thin-film electrodes grown from Ar-rich and H2-rich source gases. Diam. Relat. Mater. 2009, 18, 669–677. [Google Scholar] [CrossRef]
- Hupert, M.; Muck, A.; Wang, J.; Stotter, J.; Cvackova, Z.; Haymond, S.; Show, Y.; Swain, G.M. Conductive diamond thin-films in electrochemistry. Diam. Relat. Mater. 2003, 12, 1940–1949. [Google Scholar] [CrossRef]
- Matveeva, E. Electrochemistry of the Indium-Tin Oxide Electrode in 1 M NaOH Electrolyte. J. Electrochem. Soc. 2005, 152, H138–H145. [Google Scholar] [CrossRef]
- Benck, J.D.; Pinaud, B.A.; Gorlin, Y.; Jaramillo, T.F. Substrate Selection for Fundamental Studies of Electrocatalysts and Photoelectrodes: Inert Potential Windows in Acidic, Neutral, and Basic Electrolyte. PLoS ONE 2014, 9, e107942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallon, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]




| Electrode | Redox System | Ea (V) | Ek (V) | ΔE (V) | ja (µA/cm2) | jk (µA/cm2) |
|---|---|---|---|---|---|---|
| GC | (Fe(CN)6)3−/4− | 0.283 | 0.112 | 0.171 | 585.5 | −692.9 |
| H2Q/Q | 0.450 | 0.063 | 0.387 | 1617.4 | −993.7 | |
| Si/BDD | (Fe(CN)6)3−/4− | 0.376 | −0.092 | 0.468 | 318.7 | −319.3 |
| H2Q/Q | 0.990 | −0.387 | 1.377 | 730.2 | −450.7 | |
| Si/CNW | (Fe(CN)6)3−/4− | 0.229 | 0.137 | 0.092 | 794.0 | −877.1 |
| H2Q/Q | 0.403 | 0.079 | 0.324 | 1465.0 | −1143.9 | |
| FTO | (Fe(CN)6)3−/4− | 0.214 | 0.049 | 0.165 | 1460.2 | −1139.6 |
| H2Q/Q | 1.151 | −0.260 | 1.411 | 798.9 | −451.9 |
| Electrode | (B)/(C) | Ea (V) | Ek (V) | ΔE (V) | ja (µA/cm2) | jk (µA/cm2) |
|---|---|---|---|---|---|---|
| Si/B-NCD | 10k | 0.328 | 0.022 | 0.306 | 562.0 | −588.9 |
| 5k | 0.436 | −0.077 | 0.513 | 473.1 | −533.4 | |
| 2k | 0.634 | −0.257 | 0.891 | 395.8 | −493.3 | |
| Glass/B-NCD | 10k | 0.188 | 0.062 | 0.126 | 844.9 | −972.8 |
| 7.5k | 0.197 | 0.053 | 0.144 | 779.8 | −889.1 | |
| 5k | 0.206 | 0.044 | 0.162 | 875.0 | −991.3 | |
| 2k | 0.436 | −0.221 | 0.657 | 515.9 | −612.8 |
| Measured Value | Electrode | GC | Si/BDD | Si/CNW | FTO |
|---|---|---|---|---|---|
| Contact angle Ɵ (°) | Water | 59.03 (±1.17) | 87.13 (±0.30) | 112.79 (±1.23) | 55.93 (±0.59) |
| Surface Free Energy (mN/m) | γS | 48.54 (±5.26) | 42.46 (±2.49) | 50.82 (±0.46) | 51.77 (±4.02) |
| γD | 39.32 (±1.76) | 41.32 (±1.77) | 50.72 (±0.44) | 43.22 (±1.82) | |
| γP | 9.22 (±3.50) | 1.14 (±0.72) | 0.11 (±0.02) | 8.55 (±2.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirocka, A.; Zarzeczańska, D.; Wcisło, A. Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO). Materials 2021, 14, 4743. https://doi.org/10.3390/ma14164743
Cirocka A, Zarzeczańska D, Wcisło A. Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO). Materials. 2021; 14(16):4743. https://doi.org/10.3390/ma14164743
Chicago/Turabian StyleCirocka, Anna, Dorota Zarzeczańska, and Anna Wcisło. 2021. "Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO)" Materials 14, no. 16: 4743. https://doi.org/10.3390/ma14164743
APA StyleCirocka, A., Zarzeczańska, D., & Wcisło, A. (2021). Good Choice of Electrode Material as the Key to Creating Electrochemical Sensors—Characteristics of Carbon Materials and Transparent Conductive Oxides (TCO). Materials, 14(16), 4743. https://doi.org/10.3390/ma14164743