Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode
Abstract
:1. Introduction
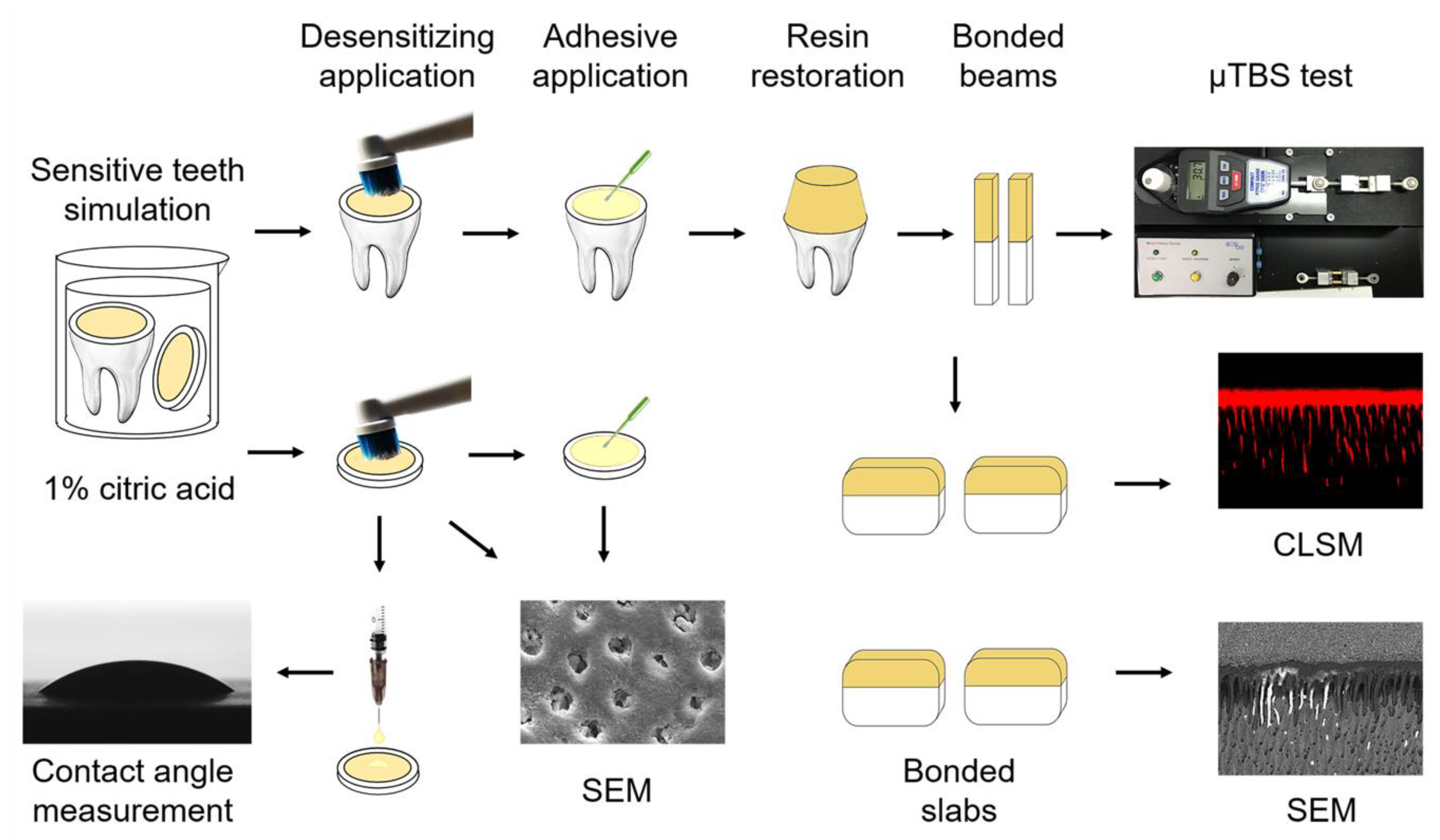
2. Materials and Methods
2.1. Sensitive Specimen Preparation
2.2. Surface Desensitization
2.3. Bonding Procedure
2.4. Micro-Tensile Bond Testing (μTBS)
2.5. Dentin Tubular Sealing Observation
2.6. Contact Angle
2.7. Confocal Laser Scanning Microscopy Analysis
2.8. Nanoleakage Evaluation
2.9. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength
3.2. Dentin Tubular Sealing Observation
3.3. Contact Angle
3.4. Confocal Laser Scanning Microscopy Analysis
3.5. Nanoleakage Evaluation
4. Discussion
5. Conclusions
- nHAp-based desensitizing agents compromise the bond strength and adhesive resin penetration of universal adhesives with self-etch mode.
- Compared with self-etch mode, the etch-and-rinse technique produces comparable bond strength on nHAp-based desensitized dentin, but increases nanoleakage of the adhesive interfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine—Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.Y.; Tian, F.C.; Niu, L.N.; Ochala, K.; Chen, C.; Fu, B.P.; Wang, X.Y.; Pashley, D.H.; Tay, F.R. Defying ageing: An expectation for dentine bonding with universal adhesives? J. Dent. 2016, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Michaud, P.L.; Brown, M. Effect of universal adhesive etching modes on bond strength to dual-polymerizing composite resins. J. Prosthet. Dent. 2018, 119, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Dačić, S.; Miljković, M.; Mitić, A.; Radenković, G.; Anđelković-Apostolović, M.; Jovanović, M. Influence of etching mode and composite resin type on bond strength to dentin using universal adhesive system. Microsc. Res. Tech. 2020, 84, 1211–1219. [Google Scholar] [CrossRef]
- Marto, C.M.; Baptista Paula, A.; Nunes, T.; Pimenta, M.; Abrantes, A.M.; Pires, A.S.; Laranjo, M.; Coelho, A.; Donato, H.; Botelho, M.F.; et al. Evaluation of the efficacy of dentin hypersensitivity treatments—A systematic review and follow-up analysis. J. Oral. Rehabil. 2019, 46, 952–990. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bakri, M.M.; Yahya, F.; Ando, H.; Unno, S.; Kitagawa, J. The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int. J. Mol. Sci. 2019, 20, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.L.; Zheng, G.; Lin, H.; Yang, M.; Zhang, Y.D.; Han, J.M. Network meta-analysis on the effect of desensitizing toothpastes on dentine hypersensitivity. J. Dent. 2019, 88, 103170. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, D.H.; Yoo, K.H.; Yoon, S.Y.; Kim, Y.; Bae, M.K.; Chung, J.; Ko, C.C.; Kwon, Y.H.; Kim, Y.I. Dentin sealing and antibacterial effects of silver-doped bioactive glass/mesoporous silica nanocomposite: An in vitro study. Clin. Oral. Investig. 2019, 23, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Bekes, K. Clinical presentation and physiological mechanisms of dentine hypersensitivity. In Dentine Hypersensitivity: Developing a Person-Centred Approach to Oral; Robinson, P.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 21–32. [Google Scholar] [CrossRef]
- Mantzourani, M.; Sharma, D. Dentine sensitivity: Past, present and future. J. Dent. 2013, 41 (Suppl. 4), S3–S17. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Yu, J.; Yang, H.; Song, F.; Huang, C. Application of electrophoretic deposition to occlude dentinal tubules in vitro. J. Dent. 2018, 71, 43–48. [Google Scholar] [CrossRef]
- Li, C.; Lu, D.; Deng, J.; Zhang, X.; Yang, P. Amyloid-Like Rapid Surface Modification for Antifouling and In-Depth Remineralization of Dentine Tubules to Treat Dental Hypersensitivity. Adv. Mater. 2019, 31, e1903973. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Tenenbaum, H.C.; Wilder, R.S.; Quock, R.; Hewlett, E.R.; Ren, Y.F. Pathogenesis, diagnosis and management of dentin hypersensitivity: An evidence-based overview for dental practitioners. BMC Oral. Health 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral. Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative Evaluation of Effect of Nano-hydroxyapatite and 8% Arginine Containing Toothpastes in Managing Dentin Hypersensitivity: Double Blind Randomized Clinical Trial. Acta Med. (Hradec Kralove) 2017, 60, 114–119. [Google Scholar] [CrossRef]
- de Melo Alencar, C.; de Paula, B.L.F.; Guanipa Ortiz, M.I.; Baraúna Magno, M.; Martins Silva, C.; Cople Maia, L. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: A systematic review and meta-analysis. J. Dent. 2019, 82, 11–21. [Google Scholar] [CrossRef]
- Kurt, S.; Kırtıloğlu, T.; Yılmaz, N.A.; Ertaş, E.; Oruçoğlu, H. Evaluation of the effects of Er:YAG laser, Nd:YAG laser, and two different desensitizers on dentin permeability: In vitro study. Lasers Med. Sci. 2018, 33, 1883–1890. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, J.; Malmstrom, H.S.; Ren, Y.F. Protective effects of resin sealant and flowable composite coatings against erosive and abrasive wear of dental hard tissues. J. Dent. 2016, 49, 68–74. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, J.; Zhang, S.; Malmstrom, H.S.; Ren, Y.F. Effectiveness of resin-based materials against erosive and abrasive enamel wear. Clin. Oral. Investig. 2017, 21, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hua, F.; Xu, P.; Huang, C.; Yang, H. Effects of Desensitizers on Adhesive-Dentin Bond Strength: A Systematic Review and Meta-analysis. J. Adhes. Dent. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Pei, D.; Meng, Y.; Li, Y.; Liu, J.; Lu, Y. Influence of nano-hydroxyapatite containing desensitizing toothpastes on the sealing ability of dentinal tubules and bonding performance of self-etch adhesives. J. Mech. Behav. Biomed. Mater. 2019, 91, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Makkar, S.; Goyal, M.; Kaushal, A.; Hegde, V. Effect of desensitizing treatments on bond strength of resin composites to dentin—An in vitro study. J. Conserv. Dent. 2014, 17, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Arisu, H.D.; Dalkihç, E.; Üçtaşli, M.B. Effect of desensitizing agents on the microtensile bond strength of a two-step self-etch adhesive to dentin. Oper. Dent. 2011, 36, 153–161. [Google Scholar] [CrossRef]
- Escalante-Otárola, W.G.; Castro-Núñez, G.M.; Jordão-Basso, K.C.F.; Guimarães, B.M.; Palma-Dibb, R.G.; Kuga, M.C. Evaluation of dentin desensitization protocols on the dentinal surface and their effects on the dentin bond interface. J. Dent. 2018, 75, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, Z.; Yan, H.; Huang, C. Effects of calcium-containing desensitizers on the bonding stability of an etch-and-rinse adhesive against long-term water storage and pH cycling. Dent. Mater. J. 2018, 37, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Cortiano, F.M.; Rached, R.N.; Mazur, R.F.; Vieira, S.; Freire, A.; de Souza, E.M. Effect of desensitizing agents on the microtensile bond strength of two-step etch-and-rinse adhesives to dentin. Eur. J. Oral. Sci. 2016, 124, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Wang, Y.L.; Lin, P.Y.; Chen, Y.Y.; Chien, C.Y.; Lin, H.P.; Lin, C.P. A mesoporous biomaterial for biomimetic crystallization in dentinal tubules without impairing the bonding of a self-etch resin to dentin. J. Formos. Med. Assoc. 2016, 115, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, H.; Li, K.; Lei, J.; Zhou, L.; Huang, C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: An in vitro study. J. Dent. 2016, 50, 21–29. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Geng Vivanco, R.; Tonani-Torrieri, R.; Souza, A.B.S.; Marquele-Oliveira, F.; Pires-de-Souza, F.C.P. Effect of natural primer associated to bioactive glass-ceramic on adhesive/dentin interface. J. Dent. 2021, 106, 103585. [Google Scholar] [CrossRef]
- Arhun, N.; Halacoglu, D.M.; Ozduman, Z.C.; Tuncer, D. Efficacy of multi-mode adhesive systems on dentin wettability and microtensile bond strength of resin composite. J. Adhes. Sci. Technol. 2018, 32, 2405–2418. [Google Scholar] [CrossRef]
- Liu, J.; Lü, P.; Sun, Y.; Wang, Y. Wettability of dentin after Yb:KYW thin-disk femtosecond ablation. Lasers Med. Sci. 2015, 30, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Hiller, K.A.; Buchalla, W.; Grillmeier, I.; Neubauer, C.; Schmalz, G. In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci. Rep. 2018, 8, 4888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Liu, S.; Lv, Y.; Liu, W.; Ma, W.; Xu, P. Effect of a dentifrice containing different particle sizes of hydroxyapatite on dentin tubule occlusion and aqueous Cr (VI) sorption. Int. J. Nanomed. 2019, 14, 5243–5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglar, S.; Erdem, U.; Dogan, M.; Turkoz, M. Dentinal tubule occluding capability of nano-hydroxyapatite; The in-vitro evaluation. Microsc. Res. Tech. 2018, 81, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Wang, X.; Egusa, H.; Sun, J. High-Performance Dental Adhesives Containing an Ether-Based Monomer. J. Dent. Res. 2020, 99, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Takamizawa, T.; Ishii, R.; Tsujimoto, A.; Hirokane, E.; Barkmeier, W.W.; Latta, M.A.; Miyazaki, M. Influence of Application Time on Dentin Bond Performance in Different Etching Modes of Universal Adhesives. Oper. Dent. 2020, 45, 183–195. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Wilwerding, T.M.; Latta, M.A.; Miyazaki, M. Interfacial Characteristics and Bond Durability of Universal Adhesive to Various Substrates. Oper. Dent. 2017, 42, E59–E70. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.M.; Alderete, L.; Farge, P. Dentinal tubules driven wetting of dentin: Cassie-Baxter modelling. Eur. Phys. J. E Soft Matter 2009, 30, 187–195. [Google Scholar] [CrossRef]
- Stasic, J.N.; Selaković, N.; Puač, N.; Miletić, M.; Malović, G.; Petrović, Z.L.; Veljovic, D.N.; Miletic, V. Effects of non-thermal atmospheric plasma treatment on dentin wetting and surface free energy for application of universal adhesives. Clin. Oral. Investig. 2019, 23, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Choi, Y.J.; Bae, M.K.; Kim, Y.I.; Park, J.K.; Son, S.A. Effects of microsurface structure of bioactive nanoparticles on dentinal tubules as a dentin desensitizer. PLoS ONE 2020, 15, e0237726. [Google Scholar] [CrossRef]
- Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Berry, T.P.; Watanabe, H.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Influence of different etching modes on bond strength and fatigue strength to dentin using universal adhesive systems. Dent. Mater. 2016, 32, e9–e21. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, M.G.; Woo, S.U.; Lee, C.O.; Yi, J.K.; Kim, D.S. Comparative study of the dentin bond strength of a new universal adhesive. Dent. Mater. J. 2016, 35, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.A.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.H.; Loguercio, A.D. In vitro longevity of bonding properties of universal adhesives to dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef]
- Yan, H.; Wang, S.; Han, L.; Peng, W.; Yi, L.; Guo, R.; Liu, S.; Yang, H.; Huang, C. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 2018, 78, 83–90. [Google Scholar] [CrossRef] [PubMed]








| Adhesive | pH | Basic Compositions | Etch-and-Rinse Strategy | Self-Etch Strategy |
|---|---|---|---|---|
| All-Bond Universal (Bisco, USA) | 3.2 | 10-MDP, HEMA, Bis-GMA, ethanol, water, photoinitiators | 1. Apply etchant for 15 s; 2. Rinse thoroughly with water spray and remove excess water by air-drying or blotting with cotton pellets; leaving the surface moist; 3. Apply adhesive as the self-etch mode. | 1. Apply two separate coats of adhesive and scrub for 10–15 s per coat; 2. Evaporate excess solvent by thoroughly air-drying for at least 10 s until there is no visible movement of the adhesive; 3. The surface should have a uniform glossy appearance; otherwise, apply an additional coat of adhesive and repeat Step 2; 4. Light cure for 10 s. |
| Single Bond Universal (3M ESPE, USA) | 2.7 | 10-MDP, HEMA, Bis-GMA, DCDMA, MPTMS, VitrebondTM copolymer, silane, ethanol, water, photoinitiators | 1. Apply etchant for 15 s; 2. Rinse thoroughly with water spray and dry and remove excess water by water- and oil-free air or blotting with cotton pellets; leaving moist; 3. Apply adhesive as the self-etch mode. | 1. Apply the adhesive and rub for 20 s; 2. Evaporate solvent by gently air-drying for approximately 5 s; 3. Light cure for 10 s. |
| Clearfil Universal Bond Quick (Kuraray Noritake Dental, Japan) | 2.3 | 10-MDP, 2-HEMA, Bis-GMA, hydrophilic amide methacrylate, MPTMS, NaF, colloidal silica, silane coupling agent, photoinitiators | 1. Apply etchant, leave it in place for 15 s, then rinse and dry; 2. Apply adhesive as the self-etch mode. | 1. Apply the adhesive with a rubbing motion without waiting time; 2. Dry the surface sufficiently by blowing mild air for more than 5 s until the adhesive does not move; 3. Light-cure for 10 s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Huang, F.; Wang, S.; Li, M.; Lu, Y.; Pei, D.; Li, A. Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode. Materials 2021, 14, 4746. https://doi.org/10.3390/ma14164746
Meng Y, Huang F, Wang S, Li M, Lu Y, Pei D, Li A. Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode. Materials. 2021; 14(16):4746. https://doi.org/10.3390/ma14164746
Chicago/Turabian StyleMeng, Yuchen, Fan Huang, Silin Wang, Meiwen Li, Yi Lu, Dandan Pei, and Ang Li. 2021. "Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode" Materials 14, no. 16: 4746. https://doi.org/10.3390/ma14164746
APA StyleMeng, Y., Huang, F., Wang, S., Li, M., Lu, Y., Pei, D., & Li, A. (2021). Bonding Performance of Universal Adhesives Applied to Nano-Hydroxyapatite Desensitized Dentin Using Etch-and-Rinse or Self-Etch Mode. Materials, 14(16), 4746. https://doi.org/10.3390/ma14164746





