Comparative Gravimetric Studies on Carbon Steel Corrosion in Selected Fruit Juices and Acidic Chloride Media (HCl) at Different pH
Abstract
:1. Introduction
1.1. Complexity of Food (Fruit Juice) Media and Its Implications on Corrosion and Corrosion Inhibition in Food Media
1.2. Chemical Composition of Test Media
1.2.1. Chemical Composition of Lemon Juice
1.2.2. Chemical Composition of Tomato Juice
1.2.3. Chemical Composition of Orange Juice
1.2.4. Chemical Composition of Pineapple Juice
2. Materials and Methods
2.1. Test Sample Preparation
2.2. Metallographic Examination
2.3. Test Solution Preparation
2.4. Weight Loss Experiments
2.5. Scanning Electron Microscopy
2.6. Atomic Absorption Spectroscopy
2.7. X-ray Diffraction
3. Results and Discussion
3.1. Compositional and Metallographic Analysis on Metal Samples
3.2. Parameters of the Test Solutions
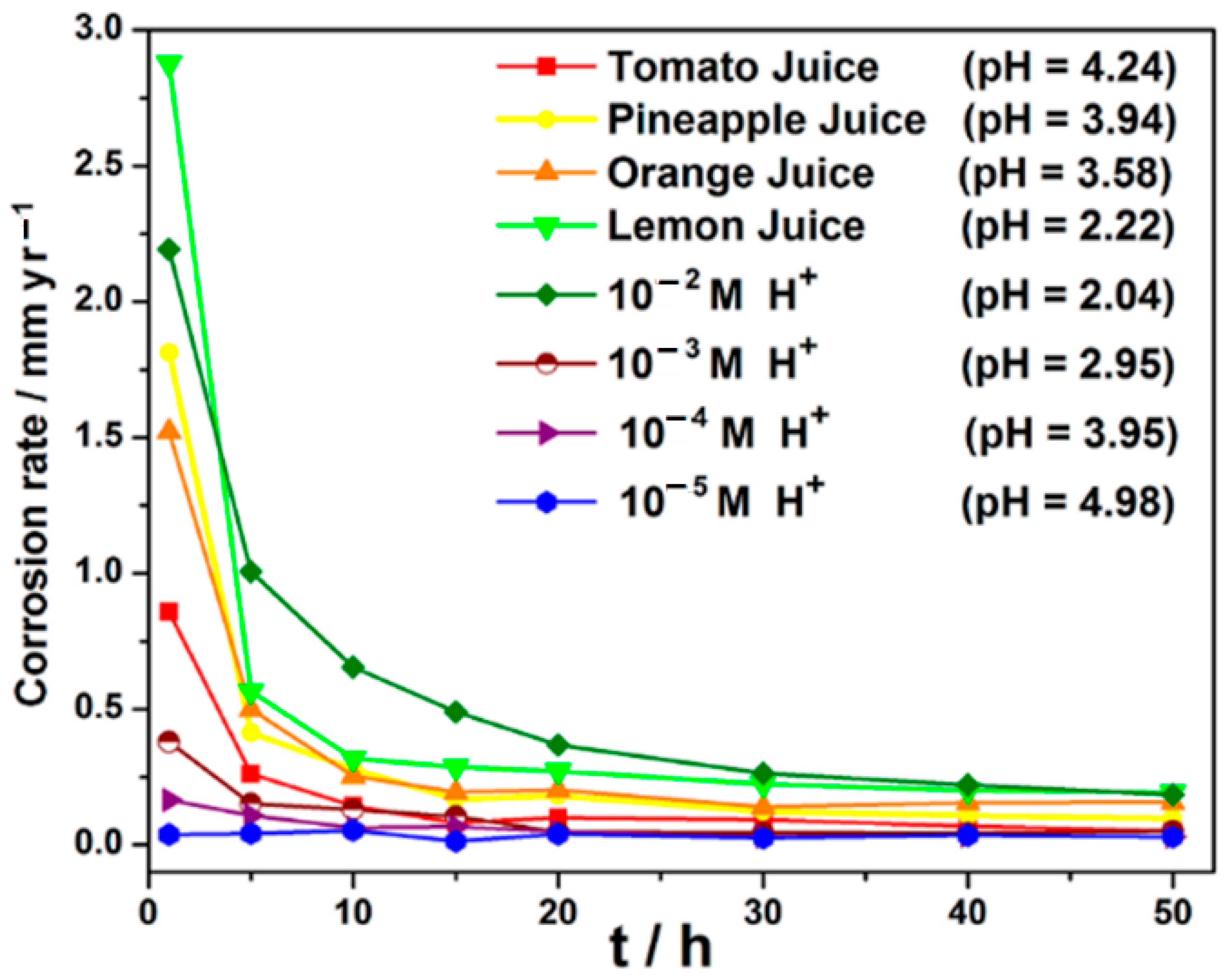
3.3. Results from Weight Loss Measurements
3.4. Results from Scanning Electron Microscopy
3.5. Results from X-ray Diffraction
3.6. Results from Atomic Absorption Spectrometry
3.7. Estimation of Apparent Elemental Metal Loss and Correlation of Weight Loss to Elemental Contamination of Fruit Juices
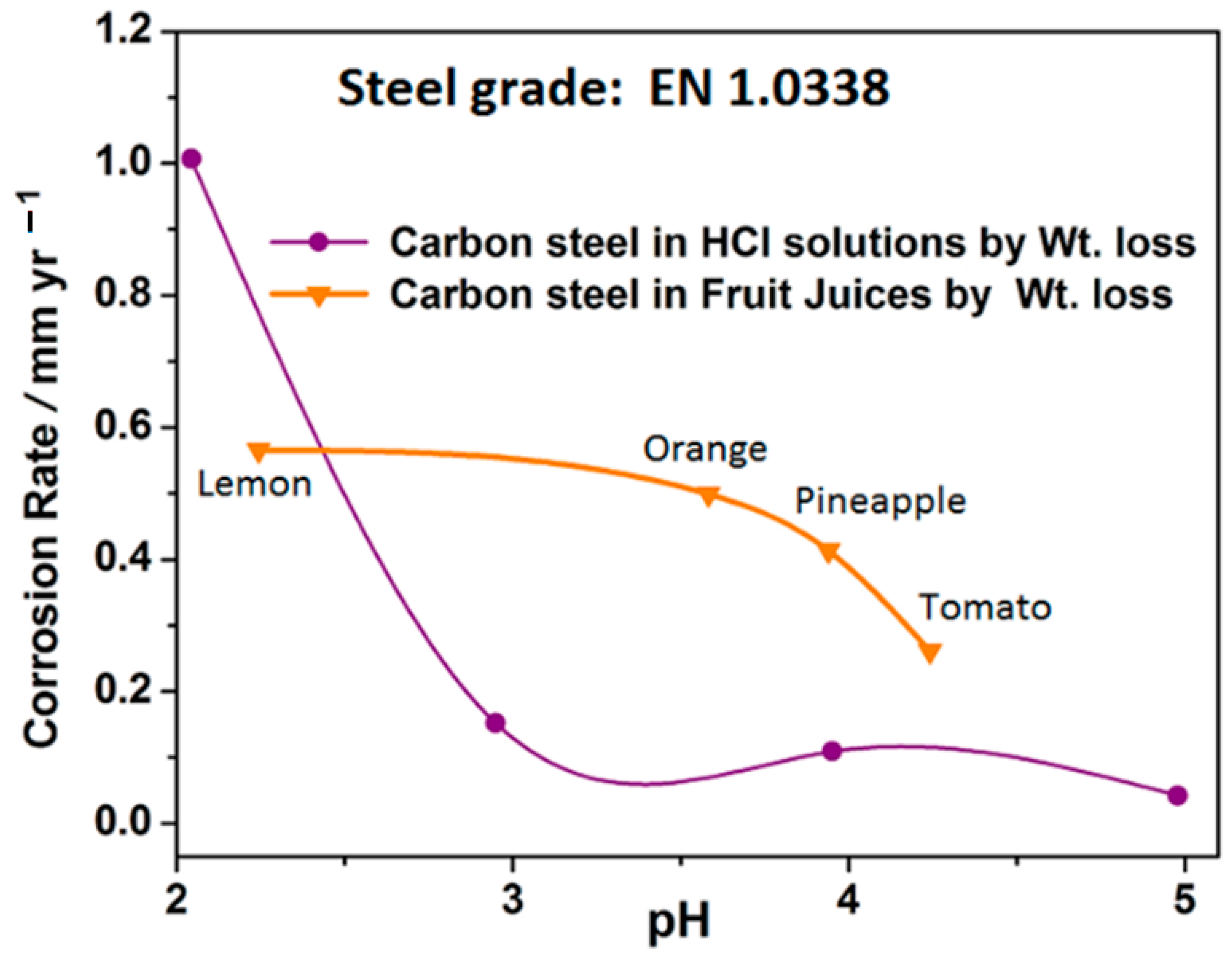
3.8. Correlation of Corrosion Rate of Carbon Steel in Fruit Juice of Varying pH with Acidic Chloride Media of Varying pH
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McSwane, D.R.; Rue, N.R.; Linton, R.; Willliams, A.G.; McSwane, D.; Rue, N. The Essentials of Food Safety and Sanitation, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2003. [Google Scholar]
- Reger, D.L.; Goode, S.R.; Ball, D.W. Chemistry: Principles and Practice; Thomson Brooks/Cole: Belmont, CA, USA, 2009. [Google Scholar]
- Vieira, E.R. Acidity in Foods. In Elementary Food Science; Food Science Text Series; Springer: Boston, MA, USA, 1996; pp. 383–384. [Google Scholar]
- McGlynn, W. Importance of Food pH in Commercial Canning Operations; FAPC-118; Oklahoma Cooperative Extension Service, Division of Agricultural Sciences and Natural Resources: Oklahoma City, OK, USA, 2003; Available online: https://shareok.org/bitstream/handle/11244/50205/oksd_fapc_118_2010-07.pdf?sequence=1 (accessed on 20 April 2018).
- Nummer, B. Food Acidity and Safety; FN/Food Safety/2008-01; Cooperative Extension, Utah State University: Logan, UT, USA, 2008; Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=2109&context=extension_curall (accessed on 21 April 2018).
- Ofoegbu, S.; Neife, S.; Okorie, B.; Ofoegbu, P. Corrosion Behaviour of Steels in Nigerian Food Processing Environments. J. Appl. Sci. Environ. Manag. 2011, 15, 135–139. [Google Scholar] [CrossRef]
- Pourbaix, M.; De Zoubov, N. Section 12.1: Iron. In Atlas of Electrochemical Equilibria in Aqueous Solutions; Pourbaix, M., Ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; pp. 307–321. [Google Scholar]
- Castellucci, N.T. Method of Providing Corrosion Resistance to Metal Surfaces. U.S. Patent 4120996, 17 October 1978. Available online: https://patentimages.storage.googleapis.com/0f/fa/02/1ad7dcd3bebb73/US4120996.pdf (accessed on 30 October 2017).
- Sekine, I.; Nakahata, Y.; Tanabe, H. The corrosion inhibition of mild steel by ascorbic and folic acids. Corros. Sci. 1988, 28, 987–1001. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Li, Y.; Wang, F.H. Effect of ascorbic acid on mild steel dissolution in sulphuric acid solution investigated by electrochemical polarization and surface probe techniques. J. Appl. Electrochem. 2007, 37, 1183–1190. [Google Scholar] [CrossRef]
- Talati, J.D.; Patel, A.S. Corrosion of copper by food acids containing colourants and sweetening agents—Part IV: Corrosion by lactic acid. Mater. Corros. 1988, 39, 27–33. [Google Scholar] [CrossRef]
- Talati, J.D.; Patel, A.S. Corrosion of copper by food acids containing colourants and sweetening agents—Part II: Corrosion by tartaric acid. Mater. Corros. 1986, 37, 504–510. [Google Scholar] [CrossRef]
- LaKind, J. Can coatings for foods and beverages: Issues and options. Int. J. Technol. Policy Manag. 2013, 13, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Vandercook, C.E.; Rolle, L.A.; Postlmayr, H.L.; Utterberg, R.A. Lemon Juice Composition. V. Effects of Some Fruit Storage and Processing Variables on the Characterization of Lemon Juice. J. Food Sci. 1966, 31, 58–62. [Google Scholar] [CrossRef]
- Severus, H. The use of aluminium—Especially as packaging material—In the food industry. In Aluminium in Food and the Environment; Royal Society of Chemistry: London, UK, 1989; pp. 88–101. [Google Scholar]
- Solmaz, R.; Kardaş, G.; Yazıcı, B.; Erbil, M. Citric acid as natural corrosion inhibitor for aluminium protection. Corros. Eng. Sci. Technol. 2008, 43, 186–191. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, C.; Spinelli, A. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5M HCl solution. Corros. Sci. 2014, 81, 75–84. [Google Scholar] [CrossRef]
- Chidiebere, M.A.; Oguzie, E.E.; Liu, L.; Li, Y.; Wang, F. Adsorption and corrosion inhibiting effect of riboflavin on Q235 mild steel corrosion in acidic environments. Mater. Chem. Phys. 2015, 156, 95–104. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Pavlovic, M.G.; Tomic, M.V. The inhibitive effect of vitamin-C on the corrosive performance of steel in HCl solutions. Int. J. Electrochem. Sci. 2013, 8, 1511–1519. [Google Scholar]
- Hoseizadeh, A.R.; Danaee, I.; Maddahy, M.H. Thermodynamic and Adsorption Behaviour of Vitamin B1 as a Corrosion Inhibitor for AISI 4130 Steel Alloy in HCl Solution. Z. Phys. Chem. 2013, 227, 403–418. [Google Scholar] [CrossRef]
- M’Hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- De Souza, F.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Soraya, N.; Rayenne, D.; Boulanouar, M.; Rabah, O. Structure-Corrosion Inhibition Performance Relationship: Application to Some Natural Free Acids and Antioxidants. Port. Electrochim. Acta 2018, 36, 23–34. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.; Khalil, N.; Mohamed, A. Inhibition by amino acids of the corrosion of steel in acid. Surf. Technol. 1985, 24, 383–389. [Google Scholar] [CrossRef]
- Hluchan, V.; Wheeler, B.L.; Hackerman, N. Amino acids as corrosion inhibitors in hydrochloric acid solutions. Mater. Corros. 1988, 39, 512–517. [Google Scholar] [CrossRef]
- Morad, M.S.S.; Hermas, A.A. Influence of some amino acids and vitamin C on the anodic dissolution of tin in sodium chloride solution. J. Chem. Technol. Biotechnol. 2001, 76, 401–410. [Google Scholar] [CrossRef]
- Morad, M.S. Corrosion inhibition of mild steel in sulfamic acid solution by S-containing amino acids. J. Appl. Electrochem. 2008, 38, 1509–1518. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F. Tryptophan as copper corrosion inhibitor in 0.5 M aerated sulfuric acid. Corros. Sci. 2002, 44, 1995–2011. [Google Scholar] [CrossRef]
- Oguzie, E.; Li, Y.; Wang, F. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.-P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.; Mohsen, Q.; Arida, H. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Eddy, N.O. Experimental and theoretical studies on some amino acids and their potential activity as inhibitors for the corrosion of mild steel, part 2. J. Adv. Res. 2011, 2, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Eddy, N.O. Part 3. Theoretical study on some amino acids and their potential activity as corrosion inhibitors for mild steel in HCl. Mol. Simul. 2010, 36, 354–363. [Google Scholar] [CrossRef]
- Mendonça, G.L.; Costa, S.N.; Freire, V.N.; Casciano, P.N.; Correia, A.; de Lima-Neto, P. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Zuo, R.; Örnek, D.; Wood, T.K. Aluminum- and mild steel-binding peptides from phage display. Appl. Microbiol. Biotechnol. 2005, 68, 505–509. [Google Scholar] [CrossRef]
- Little, B.J.; Sikes, C.S. Corrosion Inhibition by Thermal Polyaspartate. Surf. React. Pept. Polym. 1991, 444, 263–279. [Google Scholar] [CrossRef]
- Mueller, E.; Sikes, C.S.; Little, B.J. Peptide Interactions with Steel Surfaces: Inhibition of Corrosion and Calcium Carbonate Precipitation. Corrosion 1993, 49, 829–835. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; Maqueda, D.M.; De La Hoz, L.; Da Silva, V.S.N.; Pacheco, M.T.B.; Amigo, L.; Recio, I. Identification of iron-binding peptides from whey protein hydrolysates using iron (III)-immobilized metal ion affinity chromatographyand reversed phase-HPLC-tandem mass spectrometry. J. Dairy Sci. 2016, 99, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, B.O.; Oladunjoye, O.A. Corrosion of Tin Plate by Malic Acid Containing Colourants and Sweetening Agents. West India J. Eng. 2004, 27, 10–17. [Google Scholar]
- Vogel, A.I. A Textbook of Macro and Semimicro Qualitative Inorganic Analysis; Orient Longman Ltd.: New Delhi, India, 1977; p. 56. [Google Scholar]
- Al-Sogair, F.M.; Operschall, B.P.; Sigel, A.; Sigel, H.; Schnabl, J.; Sigel, R.K.O. Probing the Metal-Ion-Binding Strength of the Hydroxyl Group. Chem. Rev. 2011, 111, 4964–5003. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Psotová, J.; Lasovsky, J.; Vicar, J. Metal-chelating properties, electrochemical behavior, scavenging and cytopro-tective activities of six natural phenolics. Biomed. Pap. 2003, 147, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Symonowicz, M.; Kolanek, M. Flavonoids and their properties to form chelate complexes. Biotechnol. Food Sci. 2012, 76, 35–41. Available online: http://hdl.handle.net/11652/281 (accessed on 15 October 2017).
- Kurniawan, A.; Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Natural Clays/Clay Minerals and Modified Forms for Heavy Metals Removal. In Heavy Metals in Water: Presence Removal and Safety; Sharma, S.K., Ed.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 213–248. [Google Scholar]
- Blesa, M.; Weisz, A.; Morando, P.; Salfity, J.; Magaz, G.; Regazzoni, A. The interaction of metal oxide surfaces with complexing agents dissolved in water. Coord. Chem. Rev. 2000, 196, 31–63. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Katoh, M. Influence of chelating agent (citric acid) and F− on corrosion of Al. Corros. Sci. 1968, 8, 423–431. [Google Scholar] [CrossRef]
- Šeruga, M.; Hasenay, D. Electrochemical and surface properties of aluminium in citric acid solutions. J. Appl. Electrochem. 2001, 31, 961–967. [Google Scholar] [CrossRef]
- Müller, B. Citric acid as corrosion inhibitor for aluminium pigment. Corros. Sci. 2004, 46, 159–167. [Google Scholar] [CrossRef]
- Mustafa, C.M.; Haque, M.M. Corrosion Inhibition of Aluminium by Molybdate, Citric Acid, and Nitrite in Sim-ulated Cooling Water. Corros. Prev. Control 1997, 44, 49–55. [Google Scholar]
- Ashassi-Sorkhabi, H.; Asghari, E.; Mohammadi, M. Effects of Solution Hydrodynamics on Corrosion Inhibition of Steel by Citric Acid in Cooling Water. J. Mater. Eng. Perform. 2014, 23, 2992–3000. [Google Scholar] [CrossRef]
- Ma, L.Y.; Ding, Y.; Ma, L.Q.; Yao, C.R.; Shen, W.D. Study on the Citric Acid Passivation and the Pitting Corrosion Resistance of 316L Stainless Steel. Surf. Technol. 2007, 2, 015. [Google Scholar]
- Rose, A.; Regis, A.P.P.; Rajendran, S.; Rani, F.R.S.; Krishnaveni, A. Synergistic Effect of Fructose and Zn2+ in Con-trolling Corrosion of Carbon steel. Zast. Mater. 2010, 51, 221–226. [Google Scholar]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Yaro, A.S.; Khadom, A.; Ibraheem, H.F. Peach juice as an anti-corrosion inhibitor of mild steel. Anti-Corros. Methods Mater. 2011, 58, 116–124. [Google Scholar] [CrossRef]
- Olusegun, A.K.; Oforka, N.C.; Ebenso, E.E. The inhibition of mild steel corrosion in an acidic medium by the juice of Citrus paradisi (Grapefruit). J. Corros. Sci. Eng. 2004, 8, 1–10. [Google Scholar]
- Ogunleye, I.; Adeyemi, G. Effect of grape fruit juice on the corrosion behaviour of mild steel in acidic medium. Am. J. Sci. Ind. Res. 2011, 2, 611–615. [Google Scholar] [CrossRef]
- Gerengi, H. Anticorrosive Properties of Date Palm (Phoenix dactylifera L.) Fruit Juice on 7075 Type Aluminum Alloy in 3.5% NaCl Solution. Ind. Eng. Chem. Res. 2012, 51, 12835–12843. [Google Scholar] [CrossRef]
- Rajesh, K. Studying corrosion inhibitory effect of aloe vera juice on stainless steel used for orange juice storage. Int. J. Basic Appl. Chem. Sci. 2012, 2, 48–55. [Google Scholar]
- Ashassi-Sorkhabi, H.; Seifzadeh, D. The inhibition of steel corrosion in hydrochloric acid solution by juice of Prunus cerasus. Int. J. Electrochem. Sci. 2006, 1, 92–96. [Google Scholar]
- Wang, X.; Wang, Y.; Wang, Q.; Wan, Y.; Huang, X.; Jing, C. Viburnum Sargentii Koehne Fruit Extract as Corrosion Inhibitor For Mild Steel In Acidic Solution. Int. J. Electrochem. Sci. 2018, 13, 5228–5242. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 2019, 282, 366–384. [Google Scholar] [CrossRef]
- Souci, S.W.; Frachman, W.; Kraut, H. Food Composition and Nutrition Tables; Medpharm: Stuttgart, Germany; CRC Press: Bocca Raton, FL, USA, 1994. [Google Scholar]
- Saxholt, E.; Christensen, A.T.; Møller, A.; Hartkopp, H.B.; Hess Ygil, K.; Hels, O.H. Danish Food Composition Databank, Revision Department of Nutrition, National Food Institute, Technical University of Denmark. 2008. Available online: http://www.foodcomp.dk (accessed on 8 June 2017).
- United States Department of Agriculture (USDA). National Nutrient Databases for Standard Reference. Release 24. 2011. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/SR24/sr24_doc.pdf (accessed on 16 August 2021).
- Gould, W.A. Tomato Production, Processing and Technology, 3rd ed.; CTI Publications, Inc.: Baltimore, MD, USA, 1992. [Google Scholar]
- Kader, A.A.; Stevens, M.A.; Albright, M.; Morris, L.L. Amino acid composition and flavor of fresh market tomatoes as influenced by fruit ripeness when harvested. J. Am. Hortic. Sci. 1978, 103, 541–544. [Google Scholar]
- Karadeniz, F. Main organic acid distribution of authentic citrus juices in Turkey. Turk. J. Agric. For. 2004, 28, 267–271. [Google Scholar]
- Gorinstein, S.; Haruenkit, R.; Park, Y.-S.; Jung, S.-T.; Zachwieja, Z.; Jastrzebski, Z.; Katrich, E.; Trakhtenberg, S.; Martin-Belloso, O. Bioactive compounds and antioxidant potential in fresh and dried Jaffa® sweeties, a new kind of citrus fruit. J. Sci. Food Agric. 2004, 84, 1459–1463. [Google Scholar] [CrossRef]
- Rapisarda, P.; Tomaino, A.; Cascio, R.L.; Bonina, F.; De Pasquale, A.; Saija, A. Antioxidant Effectiveness as Influenced by Phenolic Content of Fresh Orange Juices. J. Agric. Food Chem. 1999, 47, 4718–4723. [Google Scholar] [CrossRef]
- Kelebek, H.; Canbas, A.; Selli, S. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chem. 2008, 107, 1710–1716. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Robards, K.; Antolovich, M. Methods for assessing the authenticity of orange juice: A review. Analyst 1995, 120, 1–28. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Aturki, Z.; Brandi, V.; Sinibaldi, M. Separation of Flavanone-7-O-glycoside Diastereomers and Analysis in Citrus Juices by Multidimensional Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5303–5308. [Google Scholar] [CrossRef] [PubMed]
- Akamine, E.K. Problems in shipping fresh Hawaiian tropical and subtropical fruits. In Proceedings of the I International Symposium on Tropical and Subtropical Fruits, Lima, Peru, 1 October 1976; Volume 57, pp. 151–162. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Kadam, S.S. (Eds.) Handbook of Fruit Science and Technology: Production, Composition, Storage, and Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 1995; p. 177. [Google Scholar]
- Cárnara, M.; Díez, C.; Torija, E. Chemical characterization of pineapple juices and nectars. Principal components analysis. Food Chem. 1995, 54, 93–100. [Google Scholar] [CrossRef]
- Bartolomé, A.P.; Rupérez, P.; Fúster, C. Pineapple fruit: Morphological characteristics, chemical composition and sensory analysis of red Spanish and Smooth Cayenne cultivars. Food Chem. 1995, 53, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Wrolstad, R. Phenolic Composition of Authentic Pineapple Juice. J. Food Sci. 2002, 67, 155–161. [Google Scholar] [CrossRef]
- De Ancos, B.; Sanchez-Moreno, C.; González-Aguilar, G.A. Pineapple composition and nutrition. In Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition; Lobo, M.G., Paull, R.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 221–239. [Google Scholar]
- American Society for Testing and Materials. ASTM Standard E3-2010: Standard Guide for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- American Society for Testing and Materials. ASTM Standard E4-2007: Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- American Society for Testing and Materials. ASTM Standard E112-2010: Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Hilbert, L.R. Monitoring corrosion rates and localised corrosion in low conductivity water. Corros. Sci. 2006, 48, 3907–3923. [Google Scholar] [CrossRef]
- Zakowski, K.; Narozny, M.; Szocinski, M.; Darowicki, K. Influence of water salinity on corrosion risk—The case of the southern Baltic Sea coast. Environ. Monit. Assess. 2014, 186, 4871–4879. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.; Brett, C. Characterisation of passive films formed on mild steels in bicarbonate solution by EIS. Electrochim. Acta 2002, 47, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.A.; Brett, C.M. Influence of alloying on the passive behaviour of steels in bicarbonate medium. Corros. Sci. 2002, 44, 1949–1965. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterisation of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Silva, V.M.; Williams, L.F. Pitting of plain carbon steels in acidic solution. Surf. Technol. 1977, 6, 131–137. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Corrosion behavior of mild steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2008, 3, 806–818. Available online: www.electrochemsci.org/papers/vol3/3070806.pdf (accessed on 25 July 2021).
- Kolawole, S.; Kolawole, F.; Enegela, O.; Adewoye, O.; Soboyejo, A.; Soboyejo, W. Pitting Corrosion of a Low Carbon Steel in Corrosive Environments: Experiments and Models. Adv. Mater. Res. 2015, 1132, 349–365. [Google Scholar] [CrossRef]
- Pessu, F.; Barker, R.; Neville, A. Pitting and uniform corrosion of X65 carbon steel in sour corrosion environments: The influence of CO2, H2S and temperature. Corrosion 2017, 73, 1168–1183. [Google Scholar] [CrossRef]
- Guo, P.; La Plante, E.C.; Wang, B.; Chen, X.; Balonis, M.; Bauchy, M.; Sant, G. Direct observation of pitting corrosion evolutions on carbon steel surfaces at the nano-to-micro-scales. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Refait, P. On the formation of β-FeOOH (akaganéite) in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]
- Büchler, M.; Schmuki, P.; Böhni, H. Iron Passivity in Borate Buffer: Formation of a Deposit Layer and Its Influence on the Semiconducting Properties. J. Electrochem. Soc. 1998, 145, 609–614. [Google Scholar] [CrossRef]
- Fursey, A. Oxide Films on Mild Steel. Nature 1965, 207, 747–748. [Google Scholar] [CrossRef]
- Dyson, D.J.; Keown, S.R.; Dyson, S.R.K.D.J. Detection of Magnetite as a Surface Impurity on Thin Foils of Ferrous Materials. Nature 1966, 209, 707–708. [Google Scholar] [CrossRef]
- Matsuyama, S.; Ishii, K.; Fujiwara, M.; Kikuchi, Y.; Nakhostin, M.; Kawamura, Y.; Tsuboi, S.; Yamanaka, K.; Watanabè, M.; Ohkura, S.; et al. Characterization of corrosion layer of carbon steel by micro-PIXE/RBS analysis. Int. J. PIXE 2009, 19, 61–66. [Google Scholar] [CrossRef]
- Suzuki, S. Surface Analysis of Oxides and Corrosion Products Formed on Surfaces of Iron-based Alloys. In Characterization of Corrosion Products on Steel Surfaces; Waseda, Y., Suzuki, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 131–158. [Google Scholar]
- Trévin, S. Flow accelerated corrosion (FAC) in nuclear power plant components. In Nuclear Corrosion Science and Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 186–229. [Google Scholar]
- Wilhelm, S.M.; Yun, K.S.; Ballenger, L.W.; Hackerman, N. Semiconductor Properties of Iron Oxide Electrodes. J. Electrochem. Soc. 1979, 126, 419–424. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Blaney, L. Magnetite (Fe3O4): Properties, synthesis, and applications. Lehigh Rev. 2007, 15, 33–81. Available online: https://preserve.lehigh.edu/cas-lehighreview-vol-15/5/ (accessed on 8 April 2018).
- Büchler, M.; Schmuki, P.; Böhni, H.; Stenberg, T.; Mäntylä, T. Comparison of the Semiconductive Properties of Sputter-Deposited Iron Oxides with the Passive Film on Iron. J. Electrochem. Soc. 1998, 145, 378–385. [Google Scholar] [CrossRef]
- Barakat, N.A.M. Synthesis and characterization of maghemite iron oxide (γ-Fe2O3) nanofibers: Novel semiconductor with magnetic feature. J. Mater. Sci. 2012, 47, 6237–6245. [Google Scholar] [CrossRef]
- Litter, M.I.; Blesa, M.A. Photodissolution of iron oxides. IV. A comparative study on the photodissolution of hematite, magnetite, and maghemite in EDTA media. Can. J. Chem. 1992, 70, 2502–2510. [Google Scholar] [CrossRef]
- Sato, N. Surface Oxides Affecting Metallic Corrosion. Corros. Rev. 2001, 19, 253–272. [Google Scholar] [CrossRef]
- Yuan, J.; Fujisawa, R.; Tsujikawa, S. Photopotentials of Copper Coated with TiO2 by Sol-Gel Method. Zairyo-to-kankyo 1994, 43, 433–440. [Google Scholar] [CrossRef]
- Yuan, J.; Tsujikawa, S. Characterization of Sol-Gel-Derived TiO2 Coatings and Their Photoeffects on Copper Substrates. J. Electrochem. Soc. 1995, 142, 3444–3450. [Google Scholar] [CrossRef]
- Fujisawa, R.; Tsujikawa, S. Photo-Protection of 304 Stainless Steel with TiO2 Coating. Mater. Sci. Forum 1995, 185, 1075. [Google Scholar] [CrossRef]
- Sato, N. The potentials of mixed electrodes of corrodible metal and metal oxide. Corros. Sci. 2000, 42, 1957–1973. [Google Scholar] [CrossRef]
- Sato, N. Electrochemistry at Metal and Semiconductor Electrodes; Elsevier: Amsterdam, The Netherlands, 1999; p. 87. [Google Scholar]
- Sidhu, P.S. Dissolution of Iron Oxides and Oxyhydroxides in Hydrochloric and Perchloric Acids. Clays Clay Miner. 1981, 29, 269–276. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, K.; Choi, W. Accelerated dissolution of iron oxides in ice. Atmos. Chem. Phys. 2012, 12, 11125–11133. [Google Scholar] [CrossRef] [Green Version]
- MacCarthy, J.; Nosrati, A.; Skinner, W.; Addai-Mensah, J. Effect of mineralogy and temperature on atmospheric acid leaching and rheological behaviour of model oxide and clay mineral dispersions. Powder Technol. 2015, 286, 420–430. [Google Scholar] [CrossRef]
- LaKind, J.; Stone, A. Reductive dissolution of goethite by phenolic reductants. Geochim. Cosmochim. Acta 1989, 53, 961–971. [Google Scholar] [CrossRef]
- Postma, D. The reactivity of iron oxides in sediments: A kinetic approach. Geochim. Cosmochim. Acta 1993, 57, 5027–5034. [Google Scholar] [CrossRef]
- LaQue, F.L.; Copson, H.R. (Eds.) Corrosion Resistance of Metals and Alloys; Reinhold Publishing Corporation: New York, NY, USA, 1963; Volume 158, p. 571. [Google Scholar]











| Carbon Steel Description | % C | % Si | % Mn | % P | % Ni | % Cr | % S | % Pb | % Fe |
|---|---|---|---|---|---|---|---|---|---|
| Sheet CR1 | 0.035 | 0.010 | 0.20 | 0.014 | 0.040 | 0.019 | 0.006 | 0.002 | 99.674 |
| Test Media | pH (Calc.) | pH (Measured) | Resistivity (Ω-cm) |
|---|---|---|---|
| Tomato | - | 4.242 | 3.04 × 102 |
| Orange | - | 3.582 | 3.10 × 102 |
| Lemon | - | 2.224 | 2.53 × 102 |
| Pineapple | - | 3.940 | 4.57 × 102 |
| 10−2 M HCl | 2 | 2.044 | 2.69 × 102 |
| 10−3 M HCl | 3 | 2.950 | 1.96 × 103 |
| 10−4 M HCl | 4 | 3.952 | 2.18 × 104 |
| 10−5 M HCl | 5 | 4.979 | 2.46 × 105 |
| Sample | Fe Content in Fresh Juice (ppm) | Fe Content after 50 h of Carbon Steel (8.96 cm2) Immersion (ppm) |
|---|---|---|
| Tomato Juice | 1.066 | 2766.5 |
| Pineapple Juice | 0.745 | 453.4 |
| Orange Juice | 0.881 | 1504.6 |
| Lemon Juice | 0.936 | 2096.2 |
| Test Media | Wt. Loss of Carbon Steel after 50 h Immersion in (mg) | Wt. Loss Attributable to Fe (mg) * | Fe Content from AAS after 50 h of Carbon Steel Immersion (ppm) | Molarity of Fe in Test Media from AAS (mol L−1) ** | Fe Content per Litre after 50 h of Carbon Steel Immersion (mg) | Fe Content in 100 mL of Solution (mg) | % of Wt. Loss Attributable to Fe Manifesting in Solution (%) |
|---|---|---|---|---|---|---|---|
| Tomato Juice | 0.7 | 0.697718 | 2766.5 | 0.0495 | 2.7643 | 0.276433 | 39.62 |
| Pineapple Juice | 1.13 | 1.1263162 | 453.4 | 0.0081 | 0.4523445 | 0.04523445 | 4.02 |
| Orange Juice | 1.8 | 1.794132 | 1504.6 | 0.0269 | 1.5022 | 0.150223 | 8.37 |
| Lemon Juice | 2.2 | 2.192828 | 2096.2 | 0.0375 | 2.0942 | 0.2094188 | 9.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofoegbu, S.U. Comparative Gravimetric Studies on Carbon Steel Corrosion in Selected Fruit Juices and Acidic Chloride Media (HCl) at Different pH. Materials 2021, 14, 4755. https://doi.org/10.3390/ma14164755
Ofoegbu SU. Comparative Gravimetric Studies on Carbon Steel Corrosion in Selected Fruit Juices and Acidic Chloride Media (HCl) at Different pH. Materials. 2021; 14(16):4755. https://doi.org/10.3390/ma14164755
Chicago/Turabian StyleOfoegbu, Stanley Udochukwu. 2021. "Comparative Gravimetric Studies on Carbon Steel Corrosion in Selected Fruit Juices and Acidic Chloride Media (HCl) at Different pH" Materials 14, no. 16: 4755. https://doi.org/10.3390/ma14164755
APA StyleOfoegbu, S. U. (2021). Comparative Gravimetric Studies on Carbon Steel Corrosion in Selected Fruit Juices and Acidic Chloride Media (HCl) at Different pH. Materials, 14(16), 4755. https://doi.org/10.3390/ma14164755





