Electrochemically Synthesized Poly(3-hexylthiophene) Nanowires as Photosensitive Neuronal Interfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
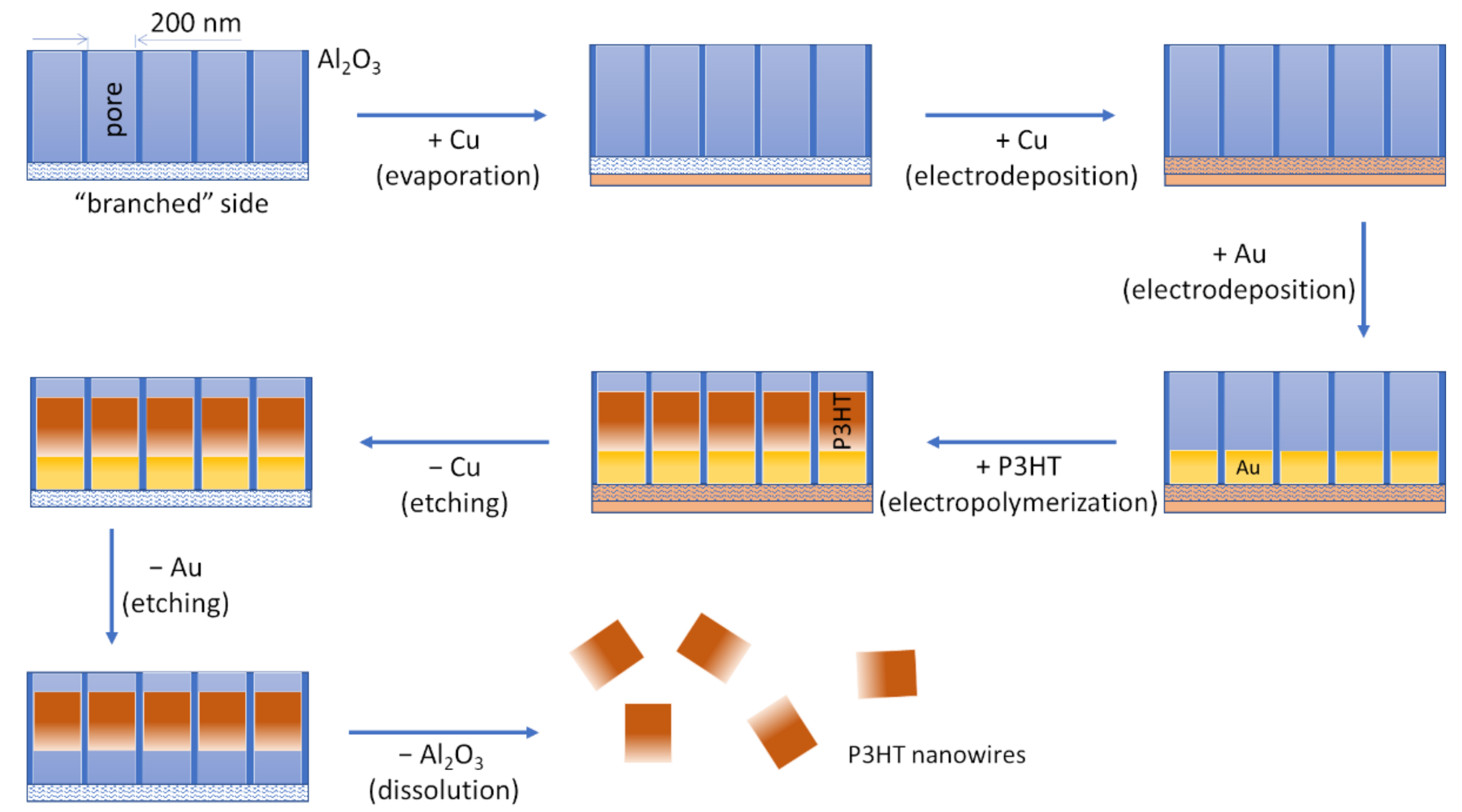
2.2. Template-Assisted Electrochemical Synthesis of P3HT Nanowiresaterials
2.3. Characterization of the P3HT Nanowires
2.4. Primary Neuronal Cultures
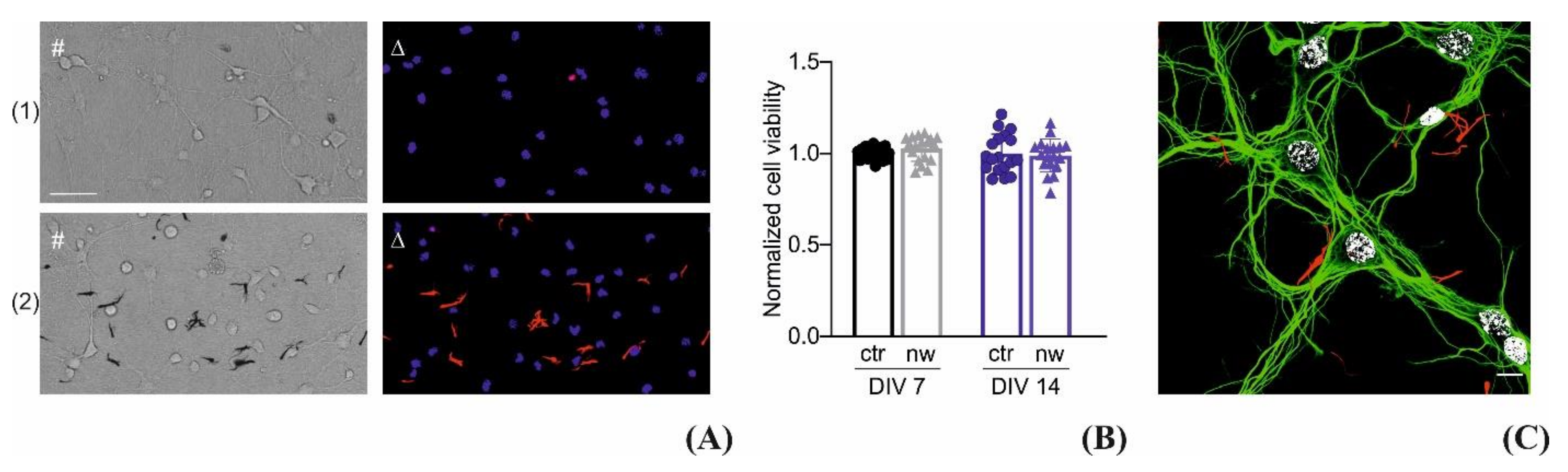
2.5. Cell Viability
2.6. Immunocytochemistry and Confocal Microscopy
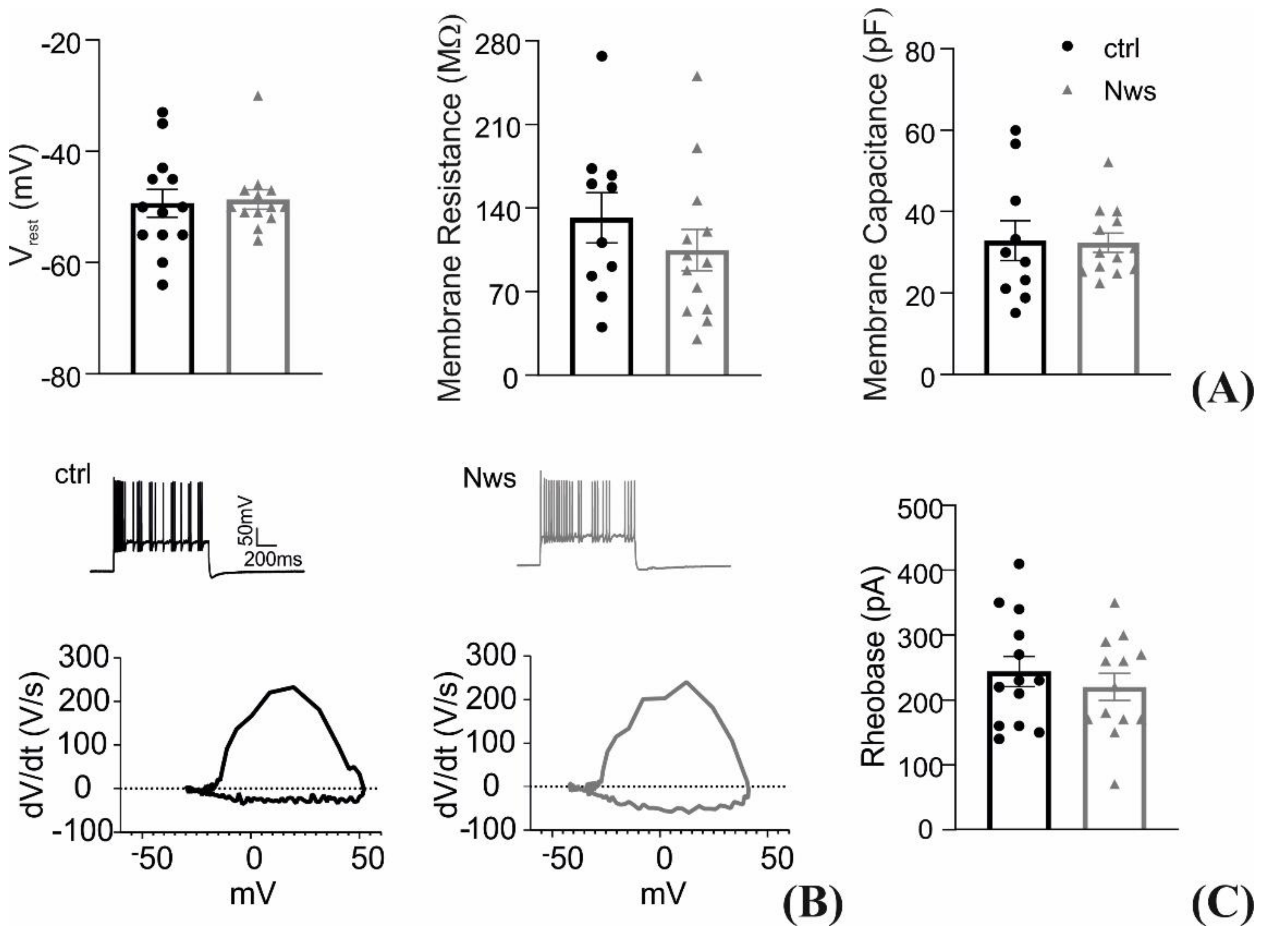
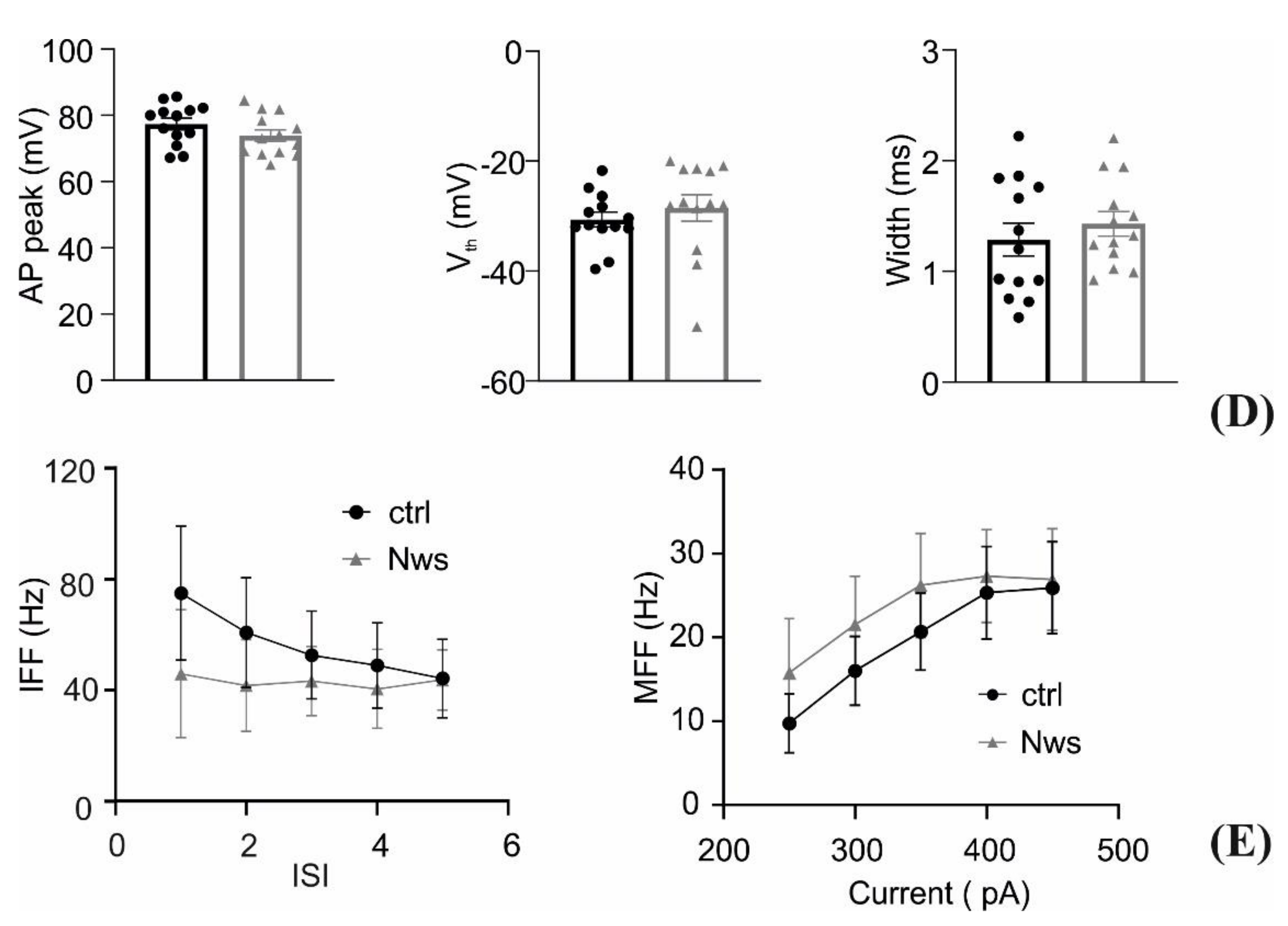
2.7. Patch-Clamp Recordings
3. Results and Discussion
3.1. Template-Assisted Electrochemical Synthesis of P3HT Nanowires
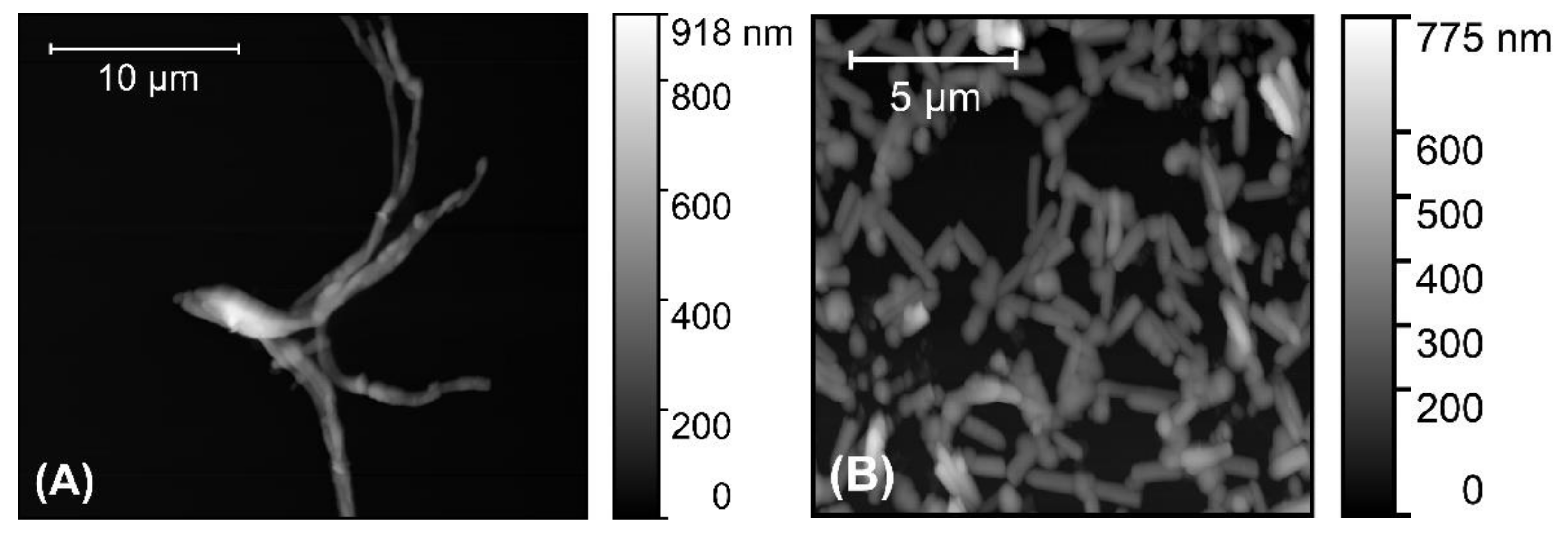
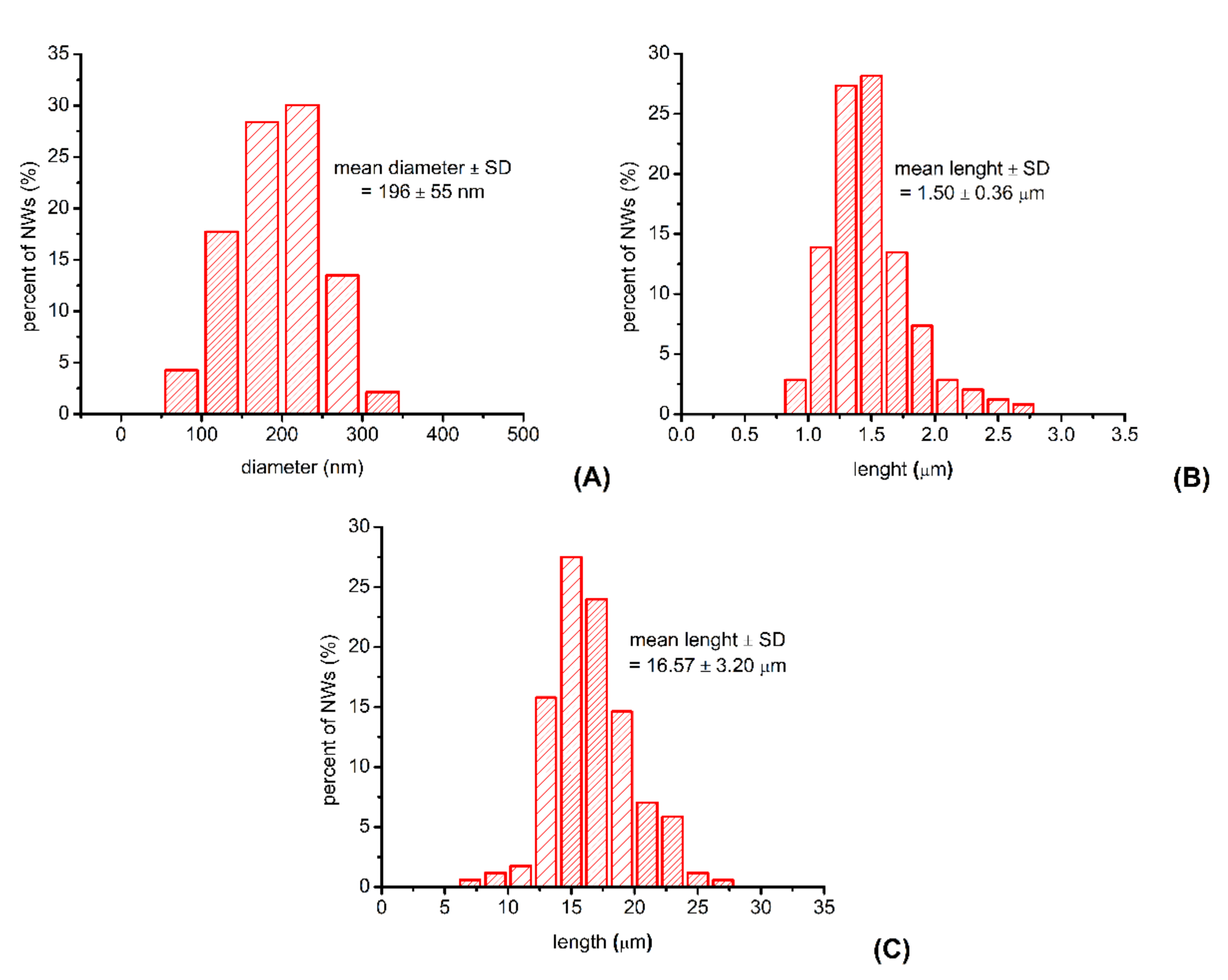
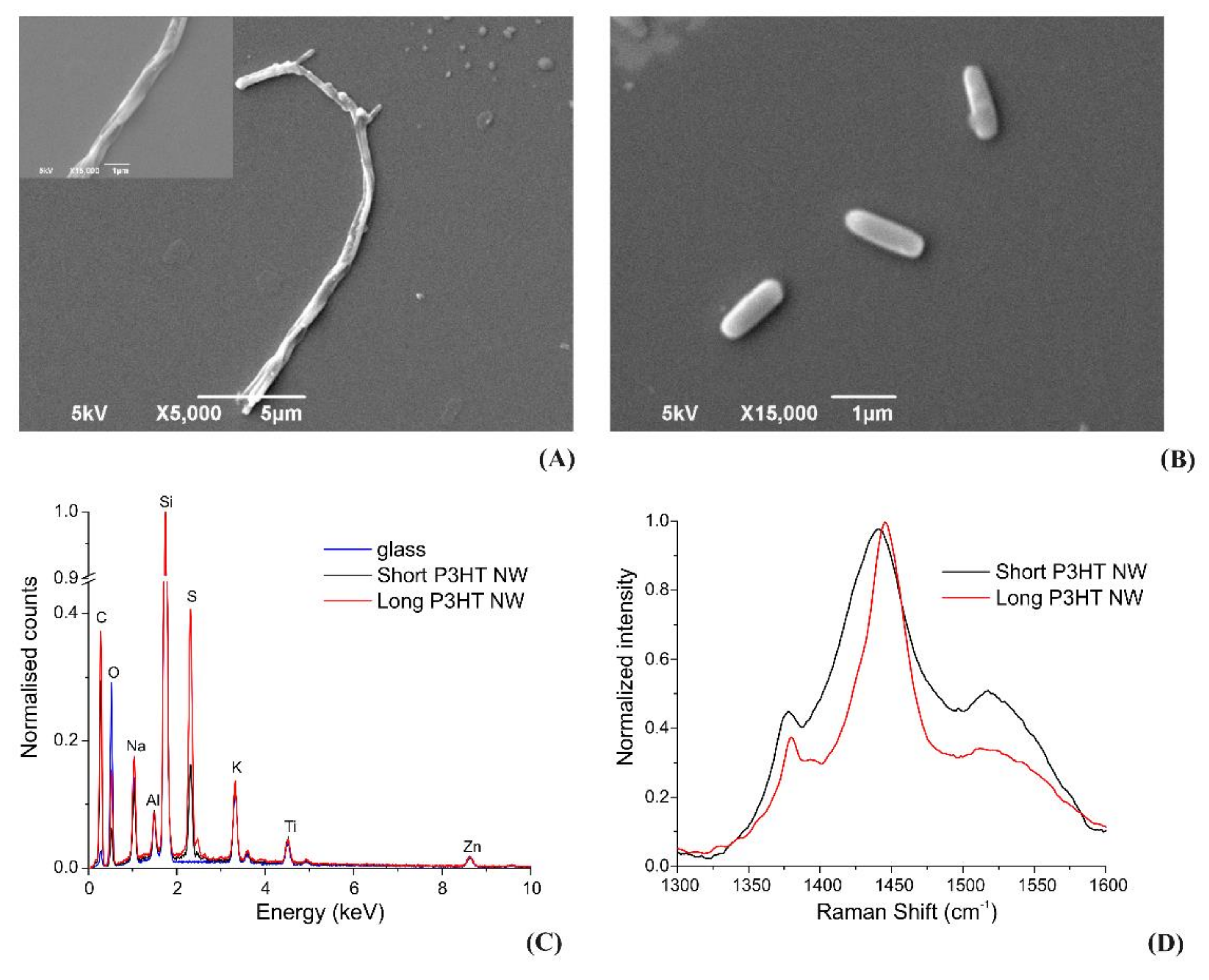
3.2. Characterization of the P3HT Nanowires
3.3. Interfacing the P3HT Nanowires with Neurons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sista, P.; Luscombe, C.K. Progress in the Synthesis of Poly (3-hexylthiophene). In P3HT Revisited—From Molecular Scale to Solar Cell Devices; Ludwigs, S., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–38. ISBN 978-3-662-45145-8. [Google Scholar]
- Pourjafari, D.; Vazquez, A.; Cavazos, J.; Gomez, I. Structural and Optoelectrical Comparison Between Chemical and Electrochemical Synthesized Poly (3-Hexylthiophene). Soft Mater. 2014, 12, 380–386. [Google Scholar] [CrossRef]
- Pourjafari, D.; Serrano, T.; Kharissov, B.; Peña, Y.; Gómez, I. Template Assisted Synthesis of Poly(3-Hexylthiophene) Nanorods and Nanotubes: Growth Mechanism and Corresponding Band Gap. Int. J. Mater. Res. 2015, 106, 414–420. [Google Scholar] [CrossRef]
- Endrődi, B.; Samu, G.F.; Azam, M.A.; Janáky, C.; Visy, C. Electrochemical Synthesis and Characterization of Poly(3-Hexylthiophene)/Single-Walled Carbon Nanotube Array Hybrid Materials. J. Solid State Electrochem. 2016, 20, 3179–3187. [Google Scholar] [CrossRef]
- Xiao, R.; Cho, S.I.; Liu, R.; Lee, S.B. Controlled Electrochemical Synthesis of Conductive Polymer Nanotube Structures. J. Am. Chem. Soc. 2007, 129, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Tateno, H.; Matsumura, Y.; Atobe, M. Synthesis and Molecular Weight Control of Poly(3-Hexylthiophene) Using Electrochemical Polymerization in a Flow Microreactor. React. Chem. Eng. 2017, 2, 642–645. [Google Scholar] [CrossRef]
- Kong, X.; Zong, K.; Lee, S.S. Nanoconfining Optoelectronic Materials for Enhanced Performance and Stability. Chem. Mater. 2019, 31, 4953–4970. [Google Scholar] [CrossRef]
- van Bavel, S.; Sourty, E.; de With, G.; Frolic, K.; Loos, J. Relation between Photoactive Layer Thickness, 3D Morphology, and Device Performance in P3HT/PCBM Bulk-Heterojunction Solar Cells. Macromolecules 2009, 42, 7396–7403. [Google Scholar] [CrossRef]
- van Bavel, S.; Bärenklau, M.; de With, G.; Hoppe, H.; Loos, J. P3HT/PCBM Bulk Heterojunction Solar Cells: Impact of Blend Composition and 3D Morphology on Device Performance. Adv. Funct. Mater. 2010, 20, 1458–1463. [Google Scholar] [CrossRef]
- Labastide, J.A.; Baghgar, M.; Dujovne, I.; Venkatraman, B.H.; Ramsdell, D.C.; Venkataraman, D.; Barnes, M.D. Time- and Polarization-Resolved Photoluminescence of Individual Semicrystalline Polythiophene (P3HT) Nanoparticles. J. Phys. Chem. Lett. 2011, 2, 2089–2093. [Google Scholar] [CrossRef]
- Hu, J.; Clark, K.W.; Hayakawa, R.; Li, A.-P.; Wakayama, Y. Enhanced Electrical Conductivity in Poly(3-Hexylthiophene)/Fluorinated Tetracyanoquinodimethane Nanowires Grown with a Porous Alumina Template. Langmuir 2013, 29, 7266–7270. [Google Scholar] [CrossRef]
- Millstone, J.E.; Kavulak, D.F.J.; Woo, C.H.; Holcombe, T.W.; Westling, E.J.; Briseno, A.L.; Toney, M.F.; Fréchet, J.M.J. Synthesis, Properties, and Electronic Applications of Size-Controlled Poly(3-Hexylthiophene) Nanoparticles. Langmuir 2010, 26, 13056–13061. [Google Scholar] [CrossRef]
- Samitsu, S.; Shimomura, T.; Heike, S.; Hashizume, T.; Ito, K. Effective Production of Poly(3-Alkylthiophene) Nanofibers by Means of Whisker Method Using Anisole Solvent: Structural, Optical, and Electrical Properties. Macromolecules 2008, 41, 8000–8010. [Google Scholar] [CrossRef]
- Wirix, M.J.M.; Bomans, P.H.H.; Friedrich, H.; Sommerdijk, N.A.J.M.; de With, G. Three-Dimensional Structure of P3HT Assemblies in Organic Solvents Revealed by Cryo-TEM. Nano Lett. 2014, 14, 2033–2038. [Google Scholar] [CrossRef]
- Martín, J.; Nogales, A.; Martín-González, M. The Smectic–Isotropic Transition of P3HT Determines the Formation of Nanowires or Nanotubes into Porous Templates. Macromolecules 2013, 46, 1477–1483. [Google Scholar] [CrossRef]
- Rojo, M.M.; Martín, J.; Grauby, S.; Borca-Tasciuc, T.; Dilhaire, S.; Martin-Gonzalez, M. Decrease in Thermal Conductivity in Polymeric P3HT Nanowires by Size-Reduction Induced by Crystal Orientation: New Approaches towards Thermal Transport Engineering of Organic Materials. Nanoscale 2014, 6, 7858–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.; Campoy-Quiles, M.; Nogales, A.; Garriga, M.; Alonso, M.I.; Goñi, A.R.; Martín-González, M. Poly(3-Hexylthiophene) Nanowires in Porous Alumina: Internal Structure under Confinement. Soft Matter 2014, 10, 3335. [Google Scholar] [CrossRef] [PubMed]
- Bedolla-Valdez, Z.I.; Xiao, R.; Cendra, C.; Fergerson, A.S.; Chen, Z.; Gonel, G.; Salleo, A.; Yu, D.; Moulé, A.J. Reversible Doping and Photo Patterning of Polymer Nanowires. Adv. Electron. Mater. 2020, 6, 2000469. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Gu, Z. A Comprehensive Review of Template-Synthesized Multi-Component Nanowires: From Interfacial Design to Sensing and Actuation Applications. Sens. Actuators Rep. 2021, 3, 100029. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S.; et al. A Fully Organic Retinal Prosthesis Restores Vision in a Rat Model of Degenerative Blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Manfredi, G.; Mete, M.; Colombo, E.; Bramini, M.; Di Marco, S.; Shmal, D.; Mantero, G.; Dipalo, M.; Rocchi, A.; et al. Subretinally Injected Semiconducting Polymer Nanoparticles Rescue Vision in a Rat Model of Retinal Dystrophy. Nat. Nanotechnol. 2020, 15, 698–708. [Google Scholar] [CrossRef]
- Aria, M.M.; Srivastava, S.B.; Sekerdag, E.; Dikbas, U.M.; Sadeghi, S.; Pering, S.R.; Cameron, P.J.; Gursoy-Ozdemir, Y.; Kavakli, I.H.; Nizamoglu, S. Perovskite-Based Optoelectronic Biointerfaces for Non-Bias-Assisted Photostimulation of Cells. Adv. Mater. Interfaces 2019, 6, 1900758. [Google Scholar] [CrossRef] [Green Version]
- DiFrancesco, M.L.; Colombo, E.; Papaleo, E.D.; Maya-Vetencourt, J.F.; Manfredi, G.; Lanzani, G.; Benfenati, F. A Hybrid P3HT-Graphene Interface for Efficient Photostimulation of Neurons. Carbon 2020, 162, 308–317. [Google Scholar] [CrossRef]
- Liu, Y.; Feig, V.R.; Bao, Z. Conjugated Polymer for Implantable Electronics toward Clinical Application. Adv. Healthc. Mater. 2021, 2001916. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.-I.; Pavel, I.-A.; David, S.; Gáspár, S. Modification with Hemeproteins Increases the Diffusive Movement of Nanorods in Dilute Hydrogen Peroxide Solutions. Chem. Commun. 2013, 49, 8803–8805. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Bunea, A.-I.; David, S.; Gáspár, S. Nanorods with Biocatalytically Induced Self-Electrophoresis. ChemCatChem 2014, 6, 866–872. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Pavel, I.-A.; David, S.; Gáspár, S. Sensing Based on the Motion of Enzyme-Modified Nanorods. Biosens. Bioelectron. 2015, 67, 42–48. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, D.; Zhang, F.-H.; Gan, Y. AFM Tip-Sample Convolution Effects for Cylinder Protrusions. Appl. Surf. Sci. 2017, 422, 482–491. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Serrà, A.; Vallés, E. Advanced Electrochemical Synthesis of Multicomponent Metallic Nanorods and Nanowires: Fundamentals and Applications. Appl. Mater. Today 2018, 12, 207–234. [Google Scholar] [CrossRef]
- Osawa, S.; Ito, M.; Iwase, S.; Yajima, H.; Endo, R.; Tanaka, K. Effects of Molecular Weight on the Electrical Properties of Electrochemically Synthesized Poly(3-Hexylthiophene). Polymer 1992, 33, 914–919. [Google Scholar] [CrossRef]
- Sulka, G.; Zaraska, L.; Stępniowski, W. Anodic porous alumina as a template for nanofabrication. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2011; Volume 11, pp. 261–349. [Google Scholar]
- Rahimi, K.; Botiz, I.; Agumba, J.O.; Motamen, S.; Stingelin, N.; Reiter, G. Light Absorption of Poly(3-Hexylthiophene) Single Crystals. RSC Adv. 2014, 4, 11121–11123. [Google Scholar] [CrossRef] [Green Version]
- Zucchetti, E.; Zangoli, M.; Bargigia, I.; Bossio, C.; Maria, F.D.; Barbarella, G.; D’Andrea, C.; Lanzani, G.; Antognazza, M.R. Poly(3-Hexylthiophene) Nanoparticles for Biophotonics: Study of the Mutual Interaction with Living Cells. J. Mater. Chem. B 2017, 5, 565–574. [Google Scholar] [CrossRef]
- Bareket, L.; Waiskopf, N.; Rand, D.; Lubin, G.; David-Pur, M.; Ben-Dov, J.; Roy, S.; Eleftheriou, C.; Sernagor, E.; Cheshnovsky, O.; et al. Semiconductor Nanorod–Carbon Nanotube Biomimetic Films for Wire-Free Photostimulation of Blind Retinas. Nano Lett. 2014, 14, 6685–6692. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Yiliguma; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire Arrays Restore Vision in Blind Mice. Nat. Commun. 2018, 9, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelidova, D.; Morikawa, R.K.; Cowan, C.S.; Raics, Z.; Goldblum, D.; Scholl, H.P.N.; Szikra, T.; Szabo, A.; Hillier, D.; Roska, B. Restoring Light Sensitivity Using Tunable Near-Infrared Sensors. Science 2020, 368, 1108–1113. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gáspár, S.; Ravasenga, T.; Munteanu, R.-E.; David, S.; Benfenati, F.; Colombo, E. Electrochemically Synthesized Poly(3-hexylthiophene) Nanowires as Photosensitive Neuronal Interfaces. Materials 2021, 14, 4761. https://doi.org/10.3390/ma14164761
Gáspár S, Ravasenga T, Munteanu R-E, David S, Benfenati F, Colombo E. Electrochemically Synthesized Poly(3-hexylthiophene) Nanowires as Photosensitive Neuronal Interfaces. Materials. 2021; 14(16):4761. https://doi.org/10.3390/ma14164761
Chicago/Turabian StyleGáspár, Szilveszter, Tiziana Ravasenga, Raluca-Elena Munteanu, Sorin David, Fabio Benfenati, and Elisabetta Colombo. 2021. "Electrochemically Synthesized Poly(3-hexylthiophene) Nanowires as Photosensitive Neuronal Interfaces" Materials 14, no. 16: 4761. https://doi.org/10.3390/ma14164761





