Characterization and Hydration Mechanism of Ammonia Soda Residue and Portland Cement Composite Cementitious Material
Abstract
:1. Introduction
2. Raw Materials and Methods
2.1. Raw Materials
2.2. Mix Proportions and Specimen Preparation
2.3. Testing Methods
3. Results
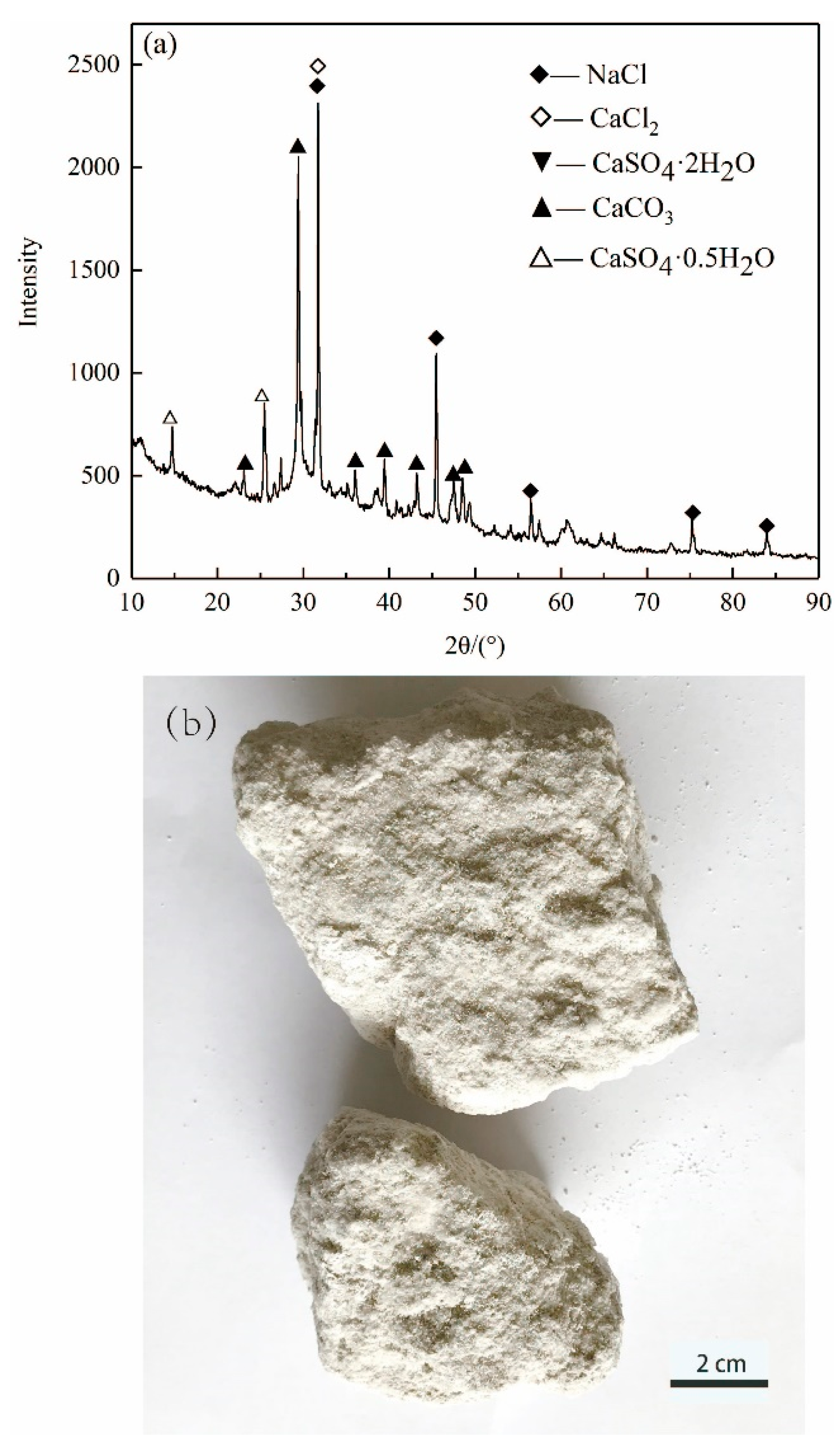
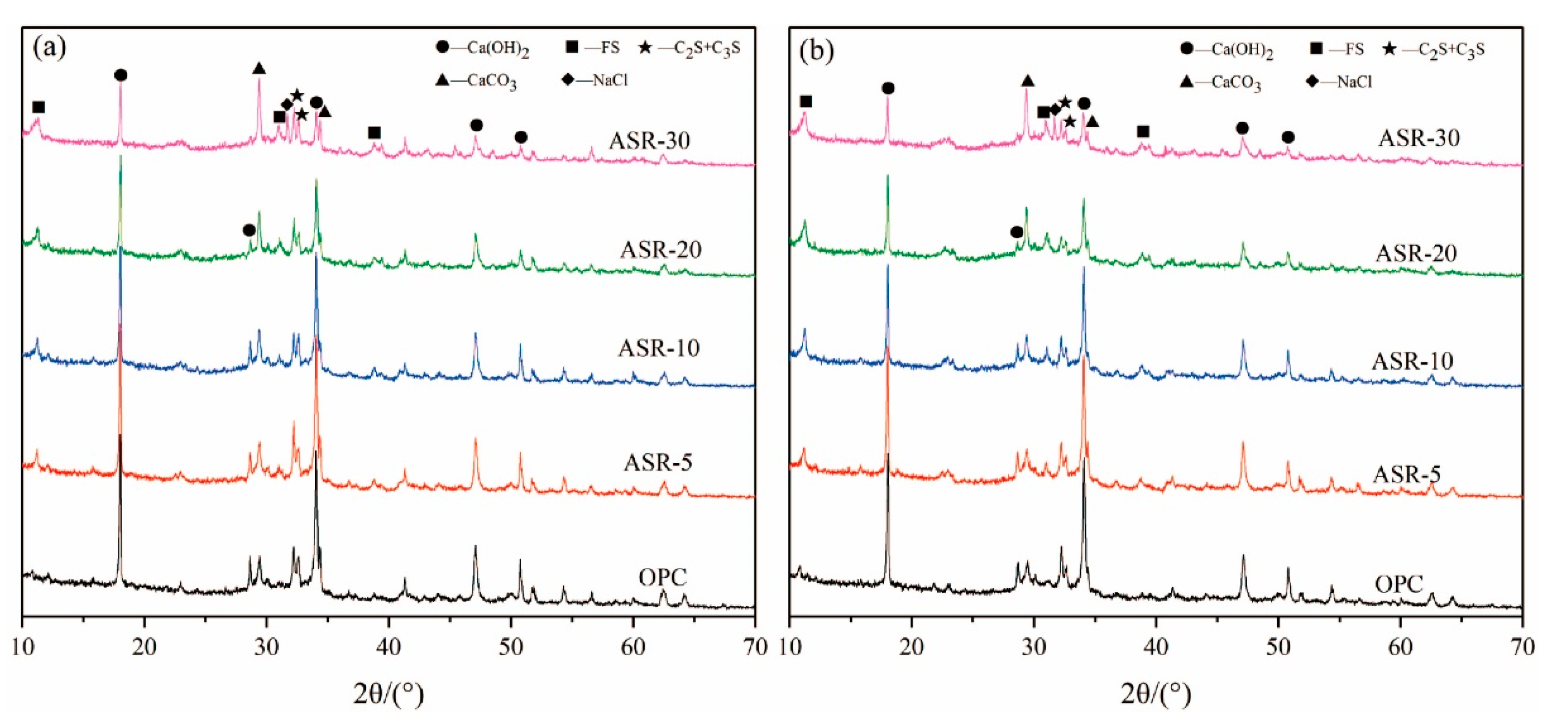
3.1. XRD Analysis
3.2. SEM Analysis
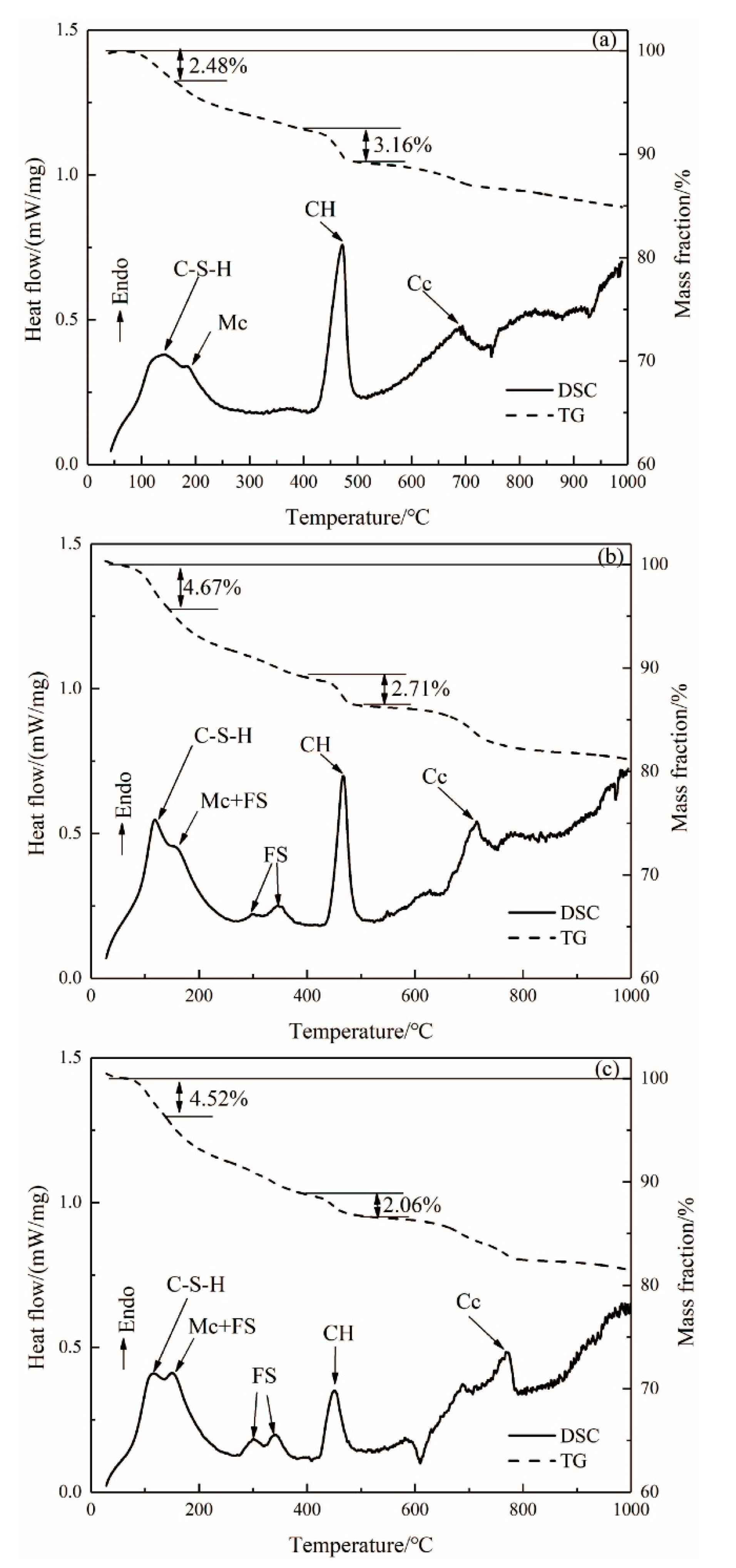
3.3. TG-DSC Analysis
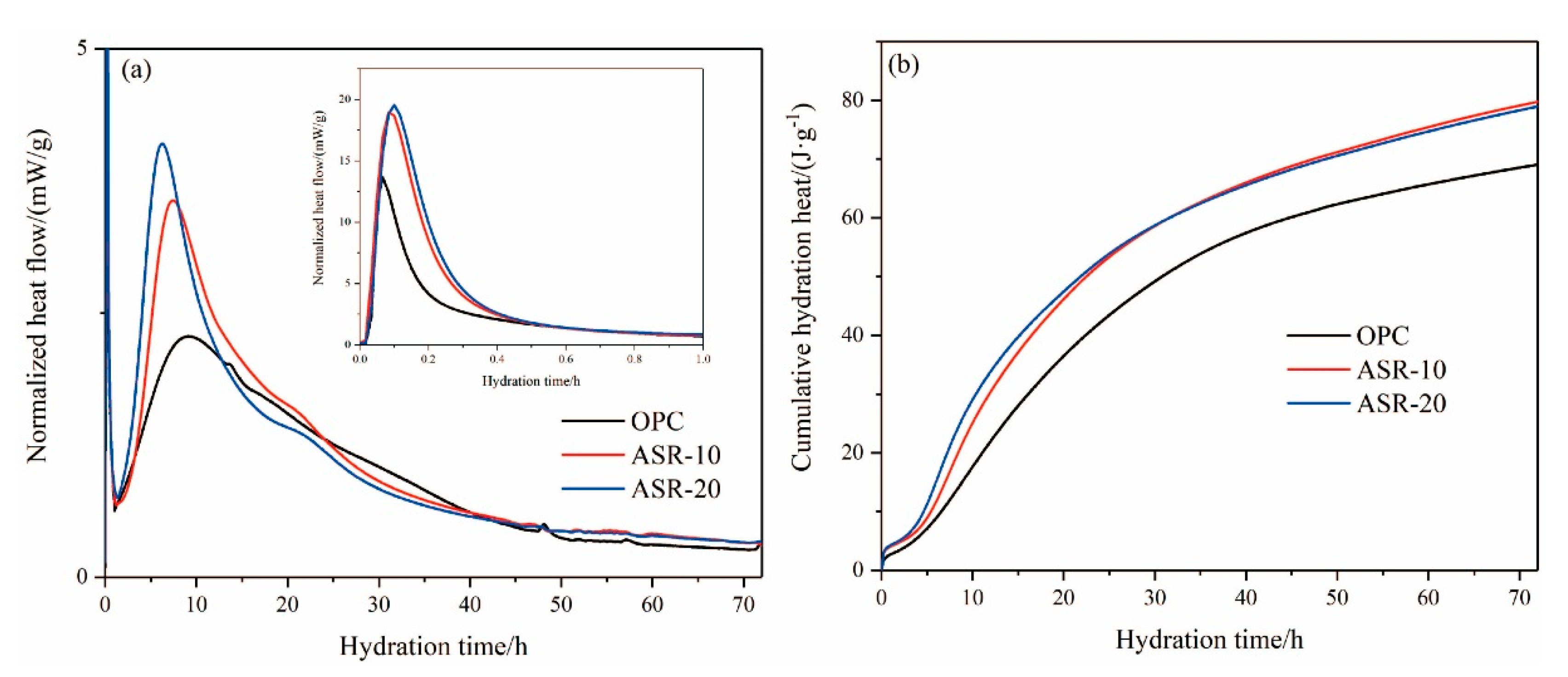
3.4. Hydration Heat
3.5. Pore Structure
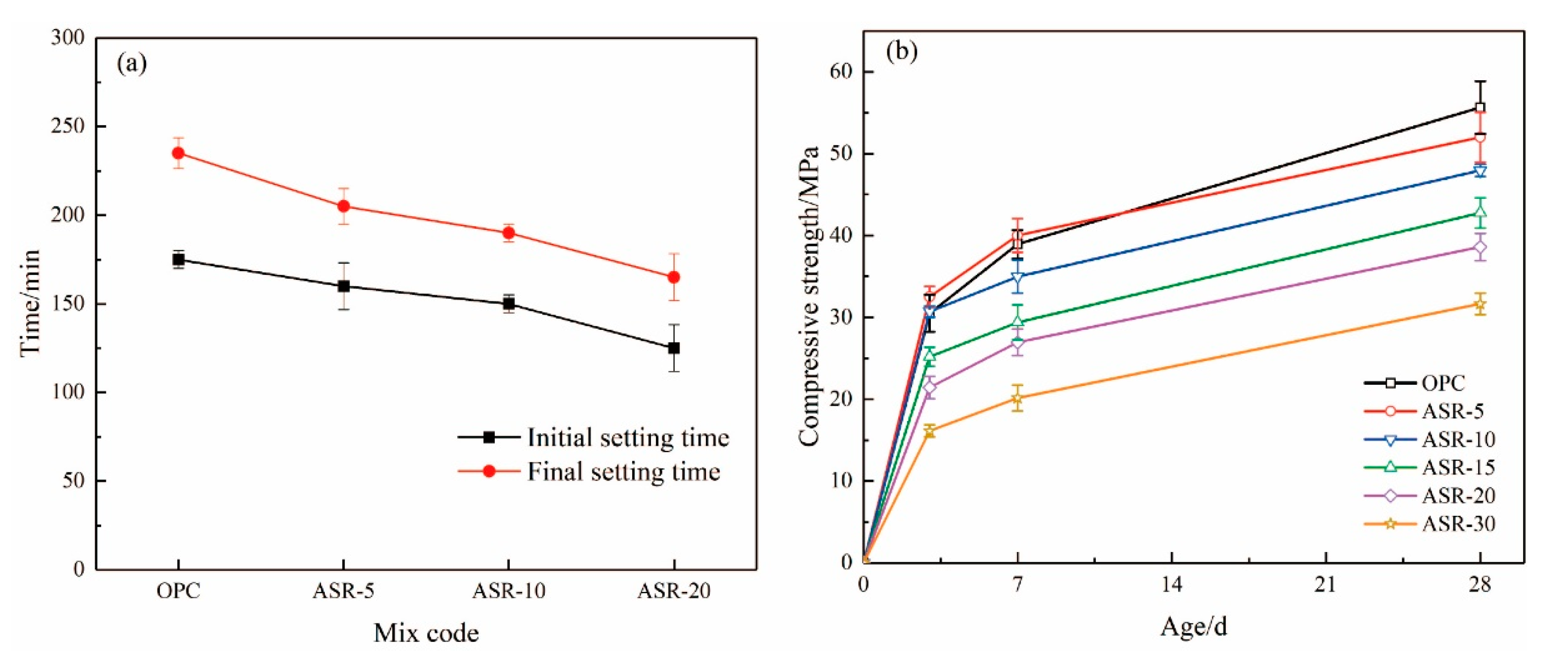
3.6. Setting Time and Compressive Strength
4. Further Discussion
4.1. Hydration Mechanism
4.2. Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Xu, D.; Zhao, X. Effect of soda residue addition and its chemical composition on physical properties and hydration products of soda residue-activated slag cementitious materials. Materials 2020, 13, 1789. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.B.; Yong-Qiang, P.U.; Yan, W.J.; Guo, W.Y.; Wang, H.C. Microstructure and chloride ion dissolution characteristics of soda residue. J. South China Univ. Technol. Nat. Sci. Ed. 2017, 45, 82–89. [Google Scholar]
- Wang, X.-B.; Yan, X.; Li, X.-Y. Environmental risk for application of ammonia-soda white mud in soils in China. J. Integr. Agric. 2020, 19, 601–611. [Google Scholar] [CrossRef]
- Lin, S.; Luo, H.J. Preparation of soil nutrient amendment using white mud produced in ammonia-soda process and its environmental assessment. T. Nonferr. Metal. Soc. 2009, 19, 1383–1388. [Google Scholar]
- Li, Y.; Song, X.; Chen, G.; Sun, Z.; Xu, Y.; Yu, J. Preparation of calcium carbonate and hydrogen chloride from distiller waste based on reactive extraction–crystallization process. Chem. Eng. J. 2015, 278, 55–61. [Google Scholar] [CrossRef]
- Kasikowski, T.; Buczkowski, R.; Igliński, B.; Peszyńska-Białczyk, K.; Lemanowska, E. Utilization of distiller waste from am-monia-soda processing. J. Clean. Prod. 2004, 12, 759–769. [Google Scholar] [CrossRef]
- He, J.; Shi, X.-K.; Li, Z.-X.; Zhang, L.; Feng, X.-Y.; Zhou, L.-R. Strength properties of dredged soil at high water content treated with soda residue, carbide slag, and ground granulated blast furnace slag. Constr. Build. Mater. 2020, 242, 118126. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Long, X.; Kang, B.; Zhang, J. Strength and microstructure characteristics of cement-soda residue solidi-fied/stabilized zinc contaminated soil subjected to freezing–thawing cycles. Cold Reg. Sci. Technol. 2020, 172, 102992. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yao, G.; Zhu, X.; Hu, S.; Qiu, J.; Chen, P.; Lyu, X. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Bai, Y.; Zhao, G.; Sang, Z.; Zhao, Q. Development and characterization of a new multi-strength level binder system using soda residue-carbide slag as composite activator. Constr. Build. Mater. 2021, 291, 123367. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Li, K. Ammonia-soda residue and metallurgical slags from iron and steel industries as cementitious materials for clinker-free concretes. J. Clean. Prod. 2021, 307, 127262. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Menke, R.; Price, R. The CO2 abatement cost curve for the thailand cement industry. J. Clean. Prod. 2010, 18, 1509–1518. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Raj, A.S.; Srinivasan, S.; Babu, K.G. Quantification of hydrated cement products of blended cements in low and medium strength concrete using TG and DTA technique. Thermochim. Acta 2003, 407, 49–60. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Qiao, C.; Ni, W.; Wang, Q.; Weiss, J. Chloride diffusion and wicking in concrete exposed to NaCl and MgCl2 solutions. J. Mater. Civ. Eng. 2018, 30, 04018015. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; Lothenbach, B.; Weerdt, K.D.; Garzón, S.F.; Enemark-Rasmussen, K.; Skibsted, J. Friedel’s salt profiles from thermogravimetric analysis and thermodynamic modelling of Portland cement-based mortars exposed to sodium chloride solution. Cem. Concr. Comp. 2017, 78, 73–83. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, M.; Jun, Y. Influence of classified steel slag with particle sizes smaller than 20 μm on the properties of cement and concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar]
- Das, B.; Kondraivendhan, B. Implication of pore size distribution parameters on compressive strength, permeability and hydraulic diffusivity of concrete. Constr. Build. Mater. 2012, 28, 382–386. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P. Concrete: Microstructure, Properties, and Materials; McGraw Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Zeng, Q.; Li, K.; Teddy, F.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.; Nonat, A.; Scherer, G.; Schweitzer, J.S.; Scrivener, K.; Thomas, J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of friedel’s salt formation in cements rich in tri-calcium alu-minate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Wang, X.; Thiery, M.; Saillio, M.; Barberon, F. Prediction of chloride binding isotherms of cementitious materials by analytical model or numerical inverse analysis. Cem. Concr. Res. 2012, 42, 1207–1224. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Jones, C.; Ramanathan, S.; Suraneni, P.; Hale, W.M. Calcium oxychloride: A critical review of the literature surrounding the formation, deterioration, testing procedures, and recommended mitigation techniques. Cem. Concr. Compos. 2020, 113, 103663. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Weiss, J. Flexural strength reduction of cement pastes exposed to CaCl2 solutions. Cem. Concr. Compos. 2018, 86, 297–305. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Tosun, K.; Felekoğlu, B.; Baradan, B.; Altun, İ.A. Effects of limestone replacement ratio on the sulfate resistance of Portland limestone cement mortars exposed to extraordinary high sulfate concentrations. Constr. Build. Mater. 2009, 23, 2534–2544. [Google Scholar] [CrossRef]
- Li, C.; Liang, Y.; Jiang, L.; Zhang, C.; Wang, Q. Characteristics of ammonia-soda residue and its reuse in magnesium oxychloride cement pastes. Constr. Build. Mater. 2021, 300, 123981. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Jiang, Y. Preparation of clinker-free concrete by using soda residue composite cementitious material. J. Harbin Inst. Technol. 2020, 52, 151–160. [Google Scholar]
- Lin, Y.; Xu, D.; Zhao, X. Properties and hydration mechanism of soda residue-activated ground granulated blast furnace slag cementitious materials. Materials 2021, 14, 2883. [Google Scholar] [CrossRef]



| Composition | Cement | ASR |
|---|---|---|
| CaO | 62.38 | 52.88 |
| SiO2 | 22.11 | 10.19 |
| MgO | 2.28 | 8.35 |
| SO3 | 2.62 | 8.87 |
| Na2O | 1.73 | 1.84 |
| Al2O3 | 4.43 | 3.25 |
| Fe2O3 | 3.13 | 1.23 |
| K2O | 0.26 | 0.38 |
| TiO2 | 0.35 | 0.19 |
| MnO | 0.3 | 0.06 |
| Cl | 0.012 | 11.95 |
| Mix Code | Cement | ASR | Water |
|---|---|---|---|
| OPC | 100 | 0 | 50 |
| ASR-5 | 95 | 5 | 50 |
| ASR-10 | 90 | 10 | 50 |
| ASR-20 | 80 | 20 | 50 |
| ASR-30 | 70 | 30 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Fu, P.; Ni, W.; Wang, Q.; Li, K. Characterization and Hydration Mechanism of Ammonia Soda Residue and Portland Cement Composite Cementitious Material. Materials 2021, 14, 4794. https://doi.org/10.3390/ma14174794
Xu D, Fu P, Ni W, Wang Q, Li K. Characterization and Hydration Mechanism of Ammonia Soda Residue and Portland Cement Composite Cementitious Material. Materials. 2021; 14(17):4794. https://doi.org/10.3390/ma14174794
Chicago/Turabian StyleXu, Dong, Pingfeng Fu, Wen Ni, Qunhui Wang, and Keqing Li. 2021. "Characterization and Hydration Mechanism of Ammonia Soda Residue and Portland Cement Composite Cementitious Material" Materials 14, no. 17: 4794. https://doi.org/10.3390/ma14174794






