Characteristics of Porous Aluminium Materials Produced by Pressing Sodium Chloride into Their Melts
Abstract
:1. Introduction
2. Materials and Methods
Calculation the Amount of Salt and Alloy for Production of Aluminium Alloy Porous Samples
3. Results
3.1. The Properties of Porous Materials
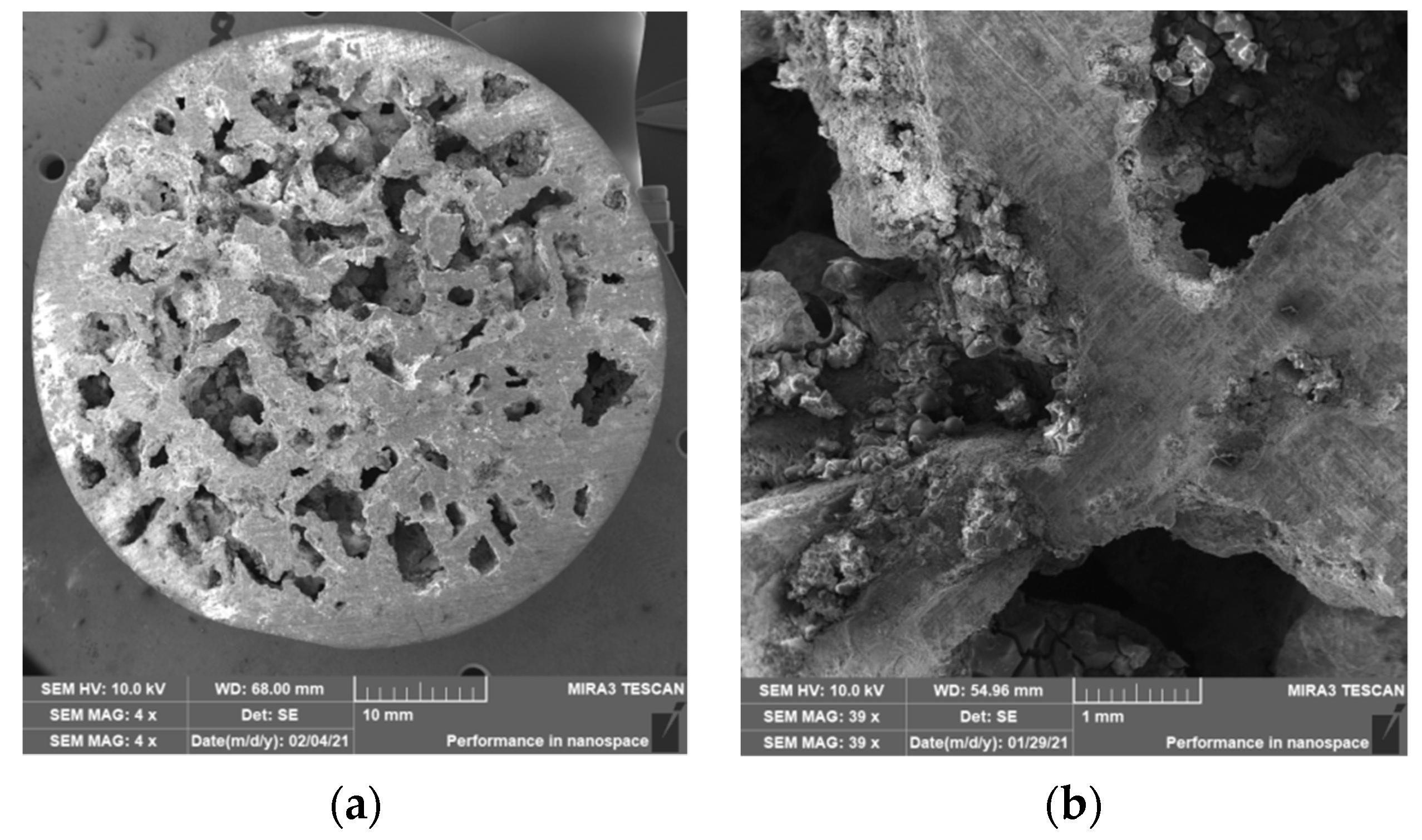
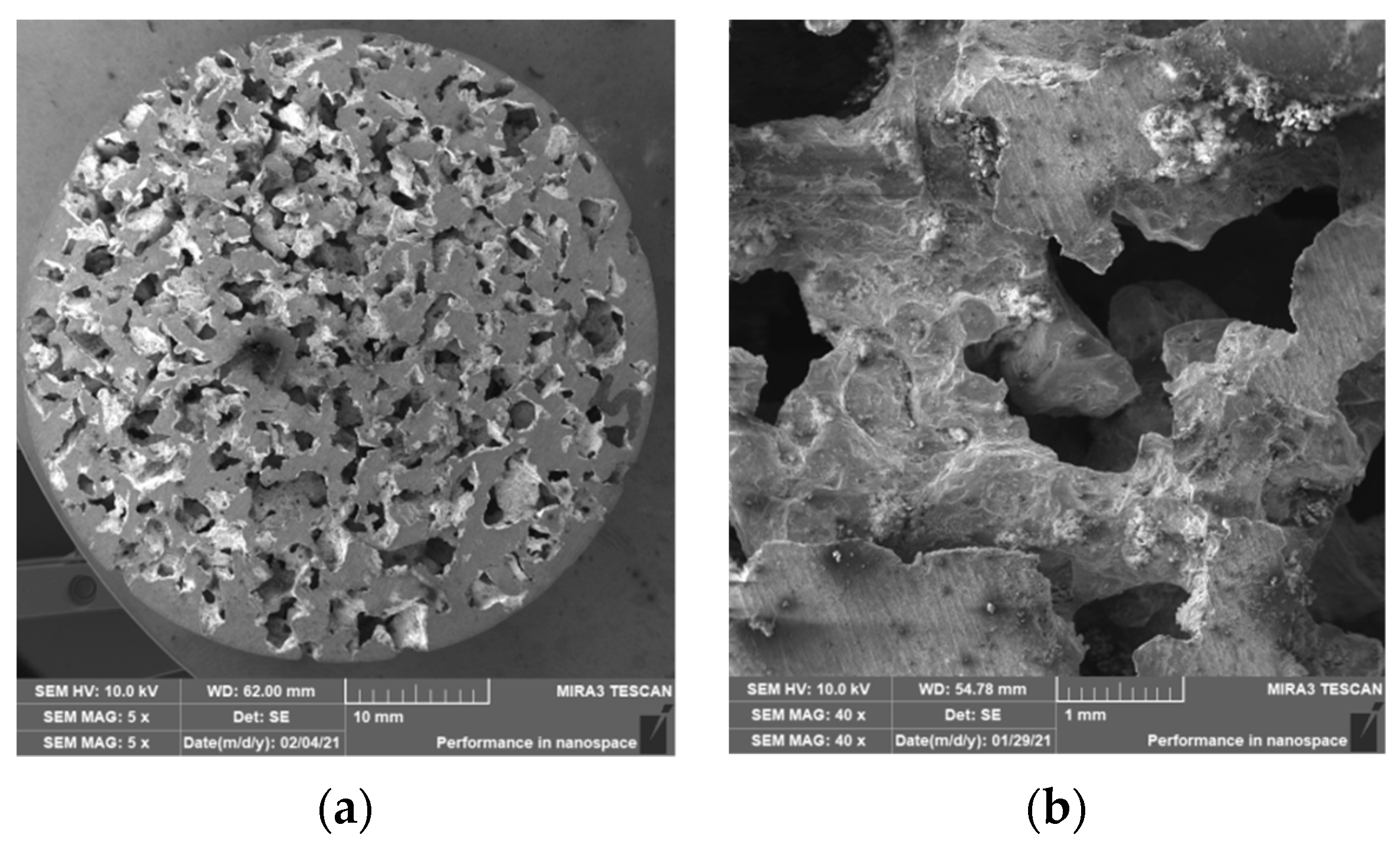
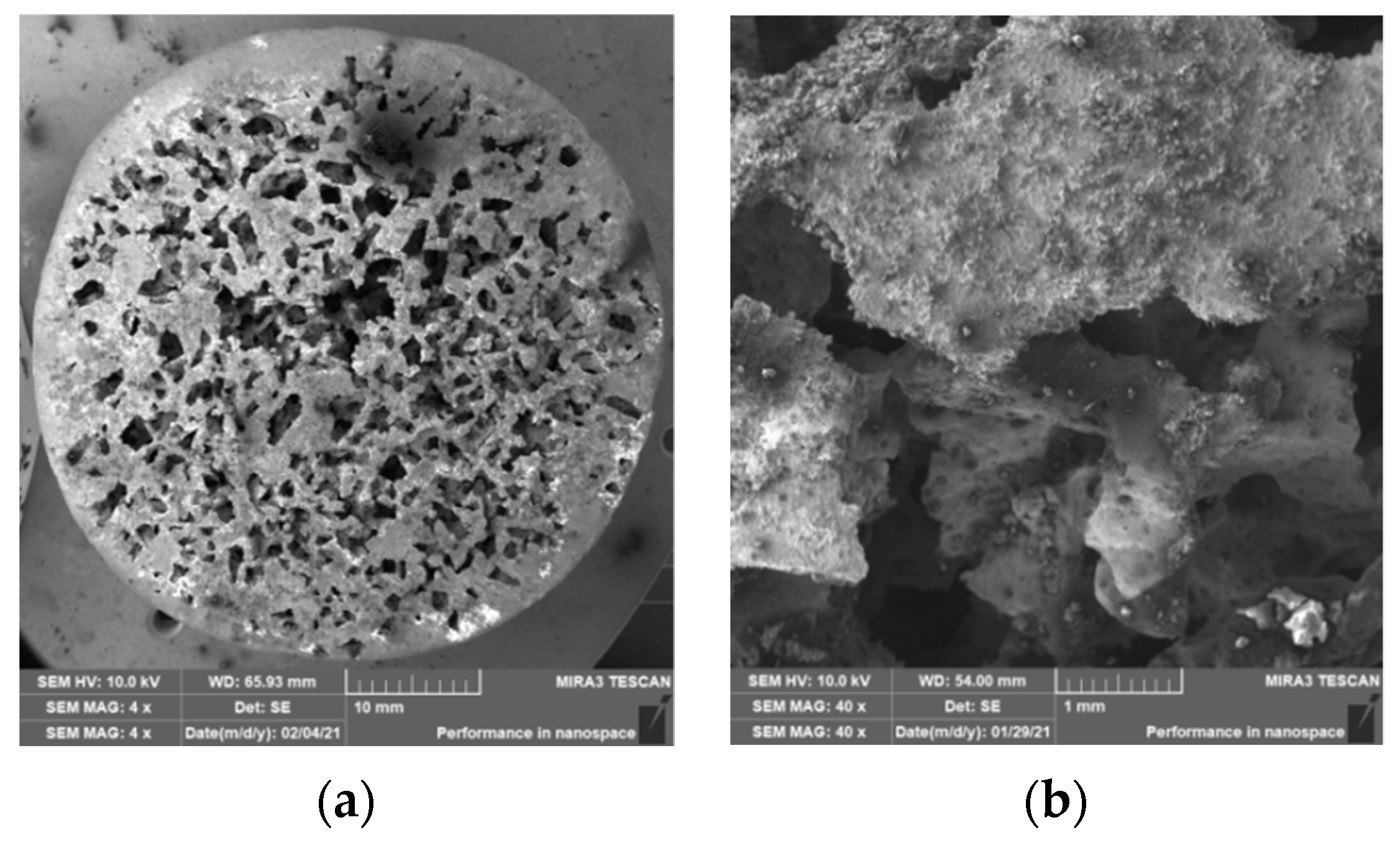
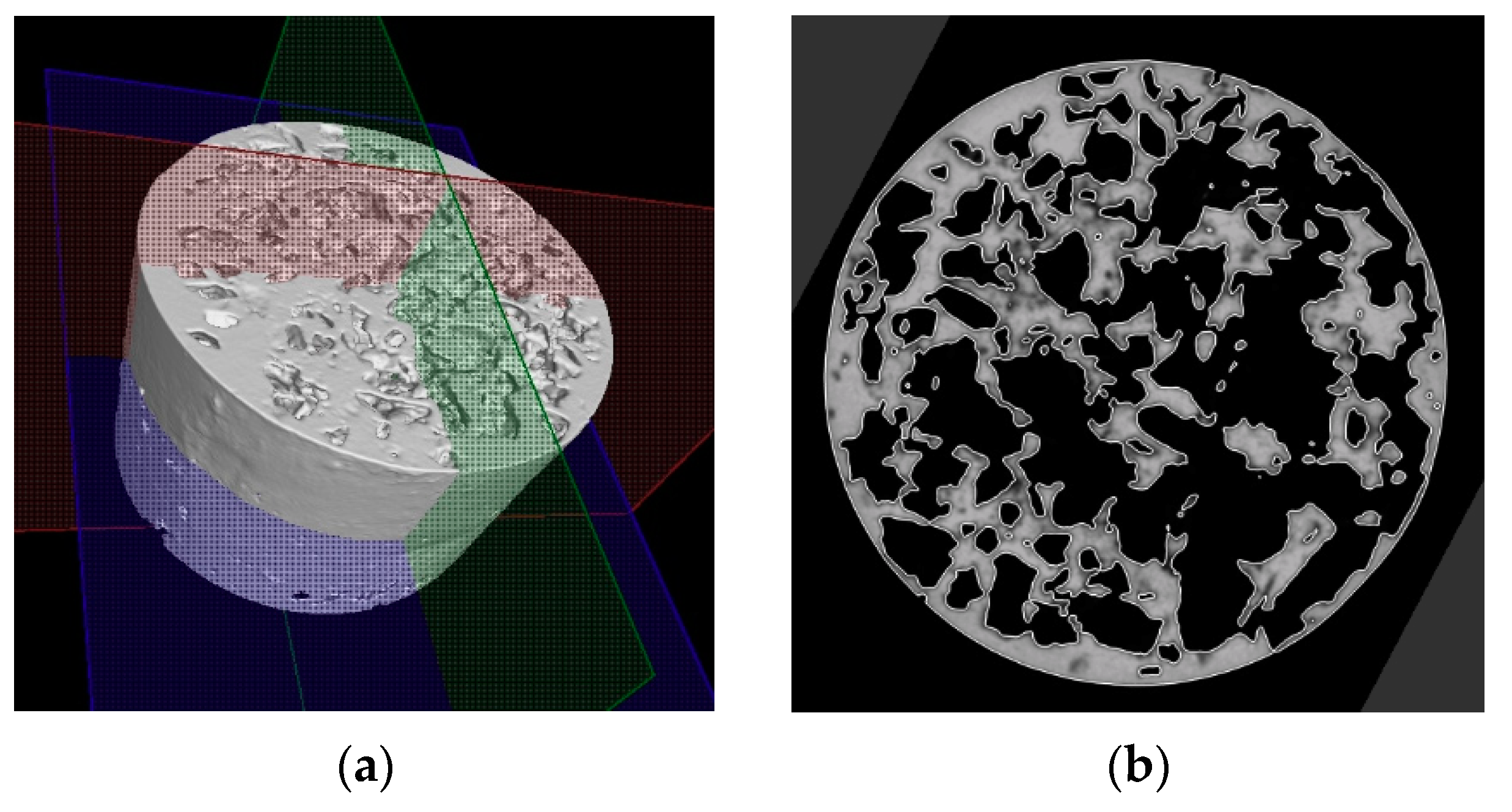
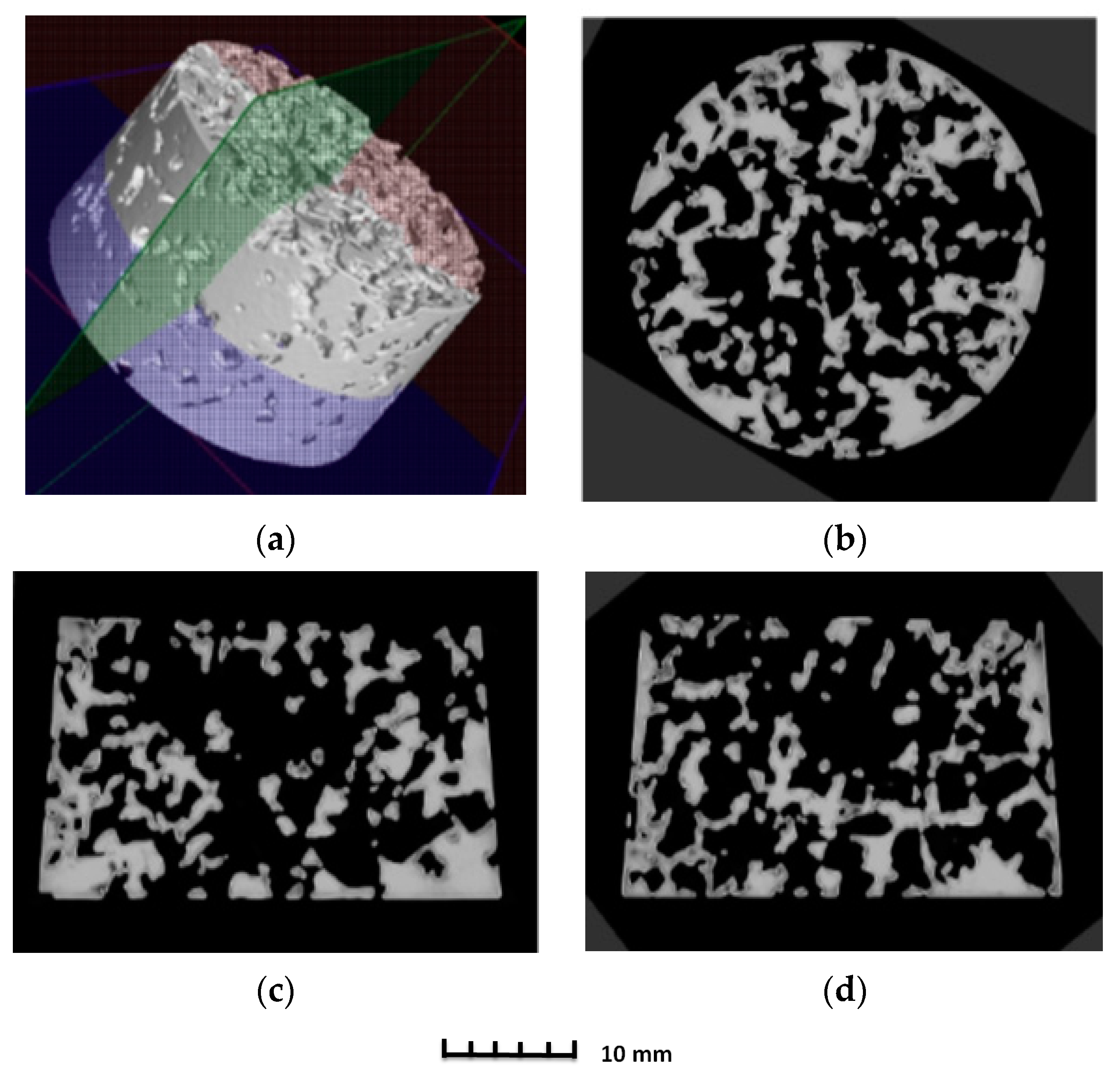
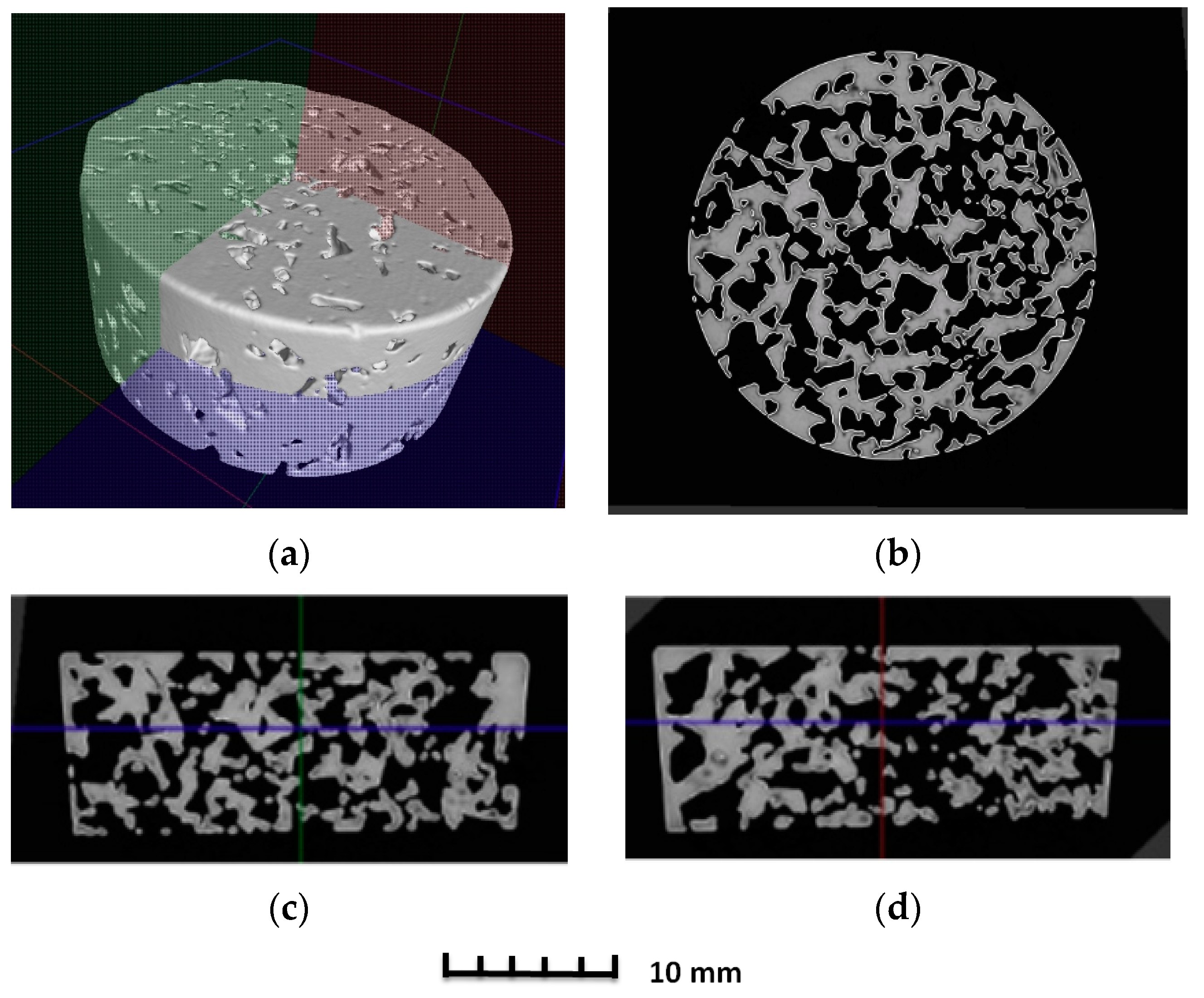
3.2. Structure of Porous Materials
4. Discussion
5. Conclusions
- (a)
- For the implementation of the experiments, a hydraulic forming press is required to develop a minimum pressure in the range of 100 MPa to 150 MPa. At the same time, it is necessary to prepare a foundry mould with a cavity that has a simple shape.
- (b)
- Moreover, considerable attention must be paid to the metallurgical preparation of the melt, as well as its treatment and temperature measurement, before its casting into the mould cavity. It is also necessary to preheat the mould to 550 °C and to preheat the sodium chloride to 150 °C. In addition, treatment of the mould cavity with a suitable protective coating is required.
- (c)
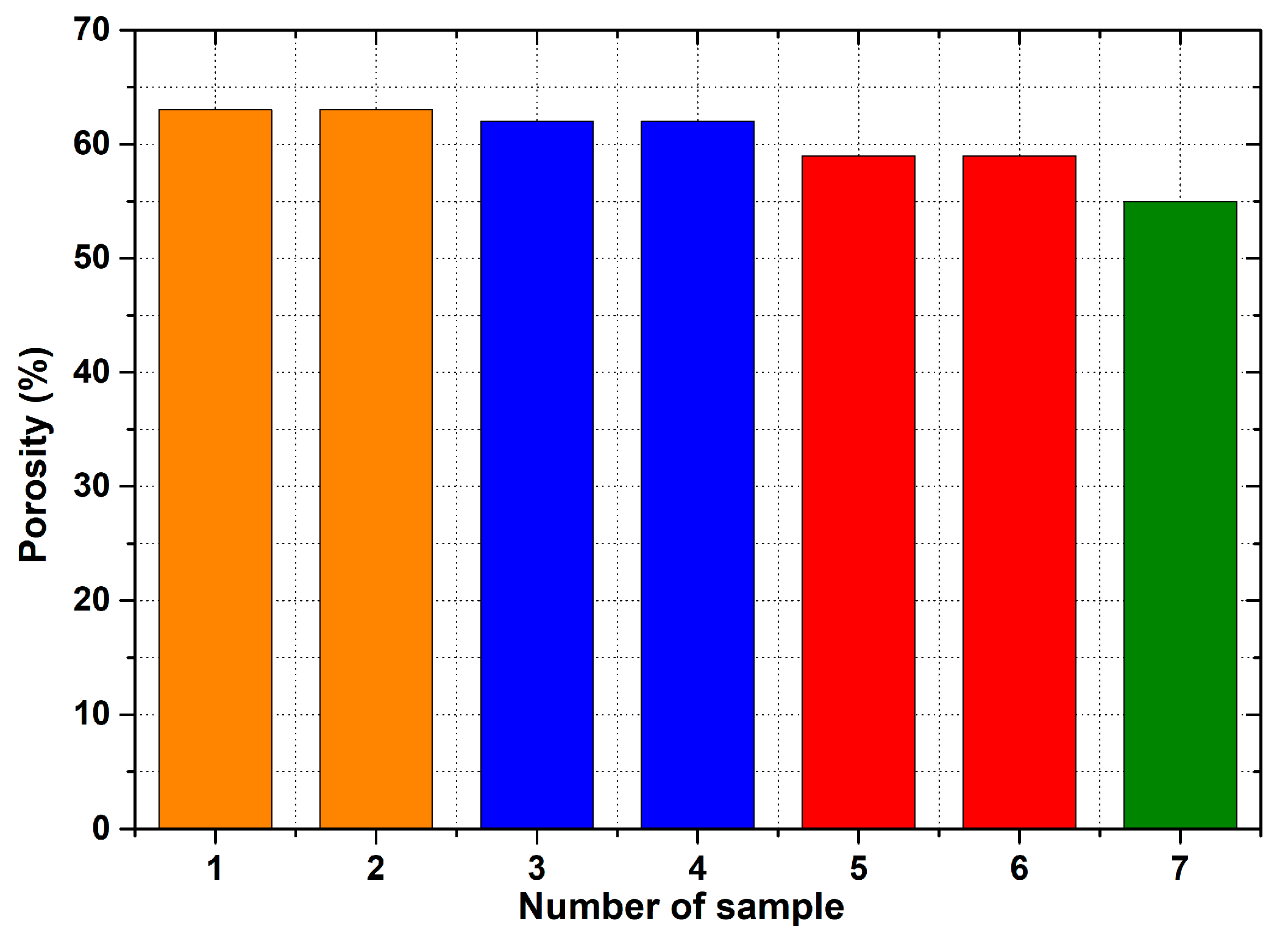
- For the production of porous materials, a 50% volume of sodium chloride and a 50% volume of aluminium alloy AlSi12 were used. Four different sizes of sodium chloride particles were used: from 8 to 10 mm, from 5 to 7 mm, from 3 to 5 mm and from 1 to 3 mm, respectively. Through the calculation of the material density, it can be found that larger salt particle sizes led to the lower density of produced sample. This fact is consistent with the well-known general theory of porous materials. The density of the produced porous samples varied within the range of 975 to 1186 kg·m−3. Note that the same material samples, without any porosity, had a density of 2650 kg·m−3. In sum, the porous material samples had a density that was from 2.0 to 2.7 times smaller than raw material without porosity, and the calculated porosity of the produced porous samples varied from 55% to 62% (the larger the sodium chloride particles, the greater the porosity).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ashby, M.F. The Mechanical Properties of Cellular Solids. Metall. Trans. A 1983, 14, 1755–1769. [Google Scholar] [CrossRef]
- Banhart, J. Foam Metal: The Resipe. Eur. News 1999, 30, 17–22. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Banhart, J. Light-Metal Foams, History of Innovation and Technological Challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Luna, E.M.E.; Barari, F.; Woolley, R.; Goodall, R. Casting Protocols for the Production of Open Cell Aluminium Foams by the Replication Technique and the Effect on Porosity. J. Vis. Exp. 2014, 94, e52568. [Google Scholar] [CrossRef] [Green Version]
- Surace, L.; De Filippis, L.A.C.; Ludovico, A.D.; Boghetich, G. Influence of Processing an Aluminium Foam Produced by Space Holder Technique. Mater. Des. 2009, 30, 1878–1885. [Google Scholar] [CrossRef]
- Luna, E.M.E. Investigation of Porous Metals as Improved Efficing Regeneration. Ph.D. Thesis, The University of Sheffield–Faculty of Engineering, Sheffield, UK, March 2016. [Google Scholar]
- Conde, Y.; Despois, J.F.; Goodall, R.; Marmottant, A.; Salvo, L.; San Marchi, C.; Mortensen, A. Replication Processing of Highly Porous Materials. Adv. Eng. Mater. 2006, 8, 795–803. [Google Scholar] [CrossRef]
- Goodall, R. Porous metals: Foams and sponges. In Advances in Powder Metallurgy: Properties, Processing and Applications, 1st ed.; Chang, I.T., Zhao, Y.Y., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 273–307. [Google Scholar]
- Ashby, M.F.; Evans, A.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: Burlington, MA, USA, 2000; p. 251. [Google Scholar]
- Gaillard, C.; Despois, J.F.; Mortensen, A. Processing of NaCl Powders of Controlled Size and Shape for the Microstructural Tailoring of Aluminium Foams. Mater. Sci. Eng. 2004, 374, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Czyzewski, A. Nature Inspires New Methods of Making Porous Materials. Available online: http://www.theengineer.co.uk/civil/news/nature-inspires-new-methods-of-making-porousmaterial/1009543.article (accessed on 1 August 2011).
- Rouquerol, J.; Anvir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Liu, P.; Hu, B.; Yu, A.; Liang, K.; Gu, S. Development in Applications of Porous Metals. Trans. Nonferr. Met. Soc. China 2001, 11, 629–638. [Google Scholar] [CrossRef]
- Moreno, F.G. Commercial Application of metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Aida, S.F.; Hijrah, M.N.; Amirah, A.H.; Zuhailawati, H.; Anasyida, A.S. Effect of NaCl as a Space Holder in Producing Open Cell A356 Aluminium Foam by Gravity die Casting Process. Procedia Chem. 2016, 19, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Suffin, N.S.A. Microstructure and Mechanical Behaviour of Aluminium Foam Produced by Sintering Dissolution Process Using NaCl Space Holder. J. Eng. Sci. 2011, 7, 37–49. [Google Scholar]
- Báez-Pimientoa, S.; Hernández–Rojasb, M.E.; Palomar-Pardavéc, M.E. Processing and Characterization of Open–Cell Aluminum Foams Obtained Through Infiltration Processes. Procedia Mater. Sci. 2015, 9, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Bafti, H.; Habibolahzadeh, A. Production of Aluminum Foam by Spherical Carbamide Space Holder Technique-Processing Parameters. Mater. Des. 2010, 31, 4122–4129. [Google Scholar] [CrossRef]
- Hassein-Bey, A.H.; Belhadj, A.; Gavrus, A. Elaboration and Mechanical-Electrochemical Characterisation of Open Cell Antimonial-Lead Foams Made by the “Excess Salt Replication Method” for Eventual Applications in Lead-Acid Batteries Manufacturing. Kem. Ind. 2020, 69, 387–398. [Google Scholar] [CrossRef]
- Lipinski, T.; Karpitz, D. Eeffect of Modification Time on Microstructure and Tensile Strength AlSi9Mg Alloy with Sr, Ti and B Additions Using in Agricultural Machinery. Eng. Rural. Dev. 2020, 19, 1476–1481. [Google Scholar] [CrossRef]
- Lipinski, T. Modification of Al-Si of Alloys with the use of Homogenous Modifiers. Arch. Metall. Mater. 2008, 53, 193–197. [Google Scholar]
- Hassani, A.; Habibolahzadeh, A.; Bafti, H. Production of Graded Aluminium Foams via Powder Space Holder Technique. Mater. Des. 2012, 40, 510–515. [Google Scholar] [CrossRef]
- Nikitha, D.S.; Karthik, S.B.; Kishore, N. Development and Evaluation of Density and Porosity of Aluminium Foams. Int. J. Res. Eng. Technol. 2015, 4, 115–122. [Google Scholar]
- Papantoniou, I.G.; Markopoulos, A.P.; Pantelis, D.I.; Manolakos, D.E.; Pantelis, D.I.; Manolakos, D.E. Application of Aluminium Flakes in Fabrication of Open–cell Aluminium foams by Space Holder Methos. Materials 2018, 11, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, N.; Vesenjak, M.; Duarte, I.; Tanaka, S.; Hokamoto, K.; Krstulovic-Opara, L.; Guo, B.; Chen, P.; Ren, Z. Compressive Behaviour or Cloused-Cell Aluminium Foam at Different Strain Rates. Materials 2019, 12, 4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nová, I.; Fraňa, K.; Sobotka, J.; Solfronk, P.; Koreček, D.; Nováková, I. Production of Porous Aluminium Using Sodium Chloride. Manuf. Technol. 2019, 19, 817–822. [Google Scholar] [CrossRef]
- Schindler, A.; Schöneich, M. Presentation of the Results of the Analysis of Sodium Chloride-Investigation of Alkali Salts with the ST 449 F5 Jupiter®; Application Note; Technical Materials, NETZSCH Gerätebau GmbH: Selb, Germany, 2010; Available online: www.netzsch.com (accessed on 19 August 2021).
- Nová, I.; Fraňa, K.; Solfronk, P.; Koreček, D. Monitoring the Influence of Sodium Chloride Particle Size on the Physical, Mechanical Properties and Structure of Samples of Porous Aluminium Materials. Manuf. Technol. 2021, 21, 109–116. [Google Scholar] [CrossRef]
- Costanza, G.; Tata, M.E. Mechanical behaviour of PCMT and SDP Al Foams: A comparison. Procedia Struct. Integr. 2020, 25, 55–62. [Google Scholar] [CrossRef]
- Exxentis AG–EIN.eu. Switzerland, Porous Aluminium. Available online: https://www.exxentis.com (accessed on 19 August 2021).














| Sample NaCl | Chemical Composition NaCl of wt% | ||||||
|---|---|---|---|---|---|---|---|
| Cl | Na | Ca | S | Si | K | Br | |
| I. | 58.8 | 40.7 | 0.17 | 0.13 | 0.08 | 0.04 | 0.08 |
| II. | 58.9 | 40.6 | 0.21 | 0.14 | - | - | 0.15 |
| Chemical Composition of Aluminium Alloy AlSi12 (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Mn | Cu | Zn | Ti | V | Mg | Na | Pb | Al |
| According to EN AC 44300 | ||||||||||
| 10.5–13.5 | 0.45–0.90 | 0.55 | 0.08 | 0.15 | 0.15 | - | - | - | - | Balance |
| Real chemical composition of used alloy | ||||||||||
| 12.2 | 0.44 | 0.45 | 0.04 | 0.12 | 0.1 | 0.006 | 0.001 | 0.005 | 0.004 | Balance |
| Calculus of AlSi12 Aluminum Alloy Weight | ||
| Weight AlSi12 mAlSi12 (kg) | Volume AlSi12 VAlSi12 (m3) | Density AlSi12 AlSi12 (kg·m−3) |
| 0.079 | 2.987 × 10−5 | 2650 |
| Calculus of Sodium Chloride Weight | ||
| Weight NaCl mNaCl (kg) | Volume NaCl VNaCl (m3) | Density NaCl NaCl (kg⋅m−3) |
| 0.065 | 2.987 × 10−5 | 2165 |
| No. of Sample | Size of NaCl Particles | Basic Dimensions | Values for Density Computation | |||||
|---|---|---|---|---|---|---|---|---|
| ØD | Ød | Height | Weight | Volume | Density | Relative Density | ||
| h | m | V | ρ | ρREL | ||||
| (mm) | (mm) | (mm) | (mm) | (kg) | (m3) | (kg·m−3) | (1) | |
| SAM1 | 8 to 10 | 45.9 | 40.1 | 27.5 | 0.0390 | 3.997 × 10−5 | 975 | 0.37 |
| SAM2 | 8 to 10 | 45.5 | 41.0 | 28.3 | 0.0411 | 4.159 × 10−5 | 988 | 0.37 |
| SAM3 | 5 to 7 | 45.3 | 42.2 | 24.6 | 0.0367 | 3.678 × 10−5 | 997 | 0.38 |
| SAM4 | 5 to 7 | 46.5 | 42.3 | 25.0 | 0.0389 | 3.870 × 10−5 | 1005 | 0.38 |
| SAM5 | 3 to 5 | 46.2 | 44.1 | 20.5 | 0.0359 | 3.281 × 10−5 | 1094 | 0.41 |
| SAM6 | 3 to 5 | 46.5 | 44.4 | 16.2 | 0.0285 | 2.610 × 10−5 | 1085 | 0.41 |
| SAM7 | 1 to 3 | 46.3 | 42.7 | 22.5 | 0.0415 | 3.500 × 10−5 | 1186 | 0.45 |
| No. of Sample | SAM1 | SAM2 | SAM3 | SAM4 | |
|---|---|---|---|---|---|
| Size of NaCl Particles | (mm) | 8 to 10 | 8 to 10 | 5 to 7 | 5 to 7 |
| Density of AlSi12 (base material) | ρB.M.(AlSi12) | 2650 | 2650 | 2650 | 2650 |
| (kg·m−3) | |||||
| Volume of AlSi12 (base material) | VB.M.(AlSi12) | 3.992 × 10−5 | 4.156 × 10−5 | 3.696 × 10−5 | 3.869 × 10−5 |
| (m3) | |||||
| Weight of AlSi12 (base material) | mB.M.(AlSi12) | 0.106 | 0.110 | 0.098 | 0.103 |
| (kg) | |||||
| Density of porous AlSi12 | ρP.(AlSi12) | 975 | 988 | 997 | 1005 |
| (kg·m−3) | |||||
| Relative density of AlSi12 (porous/base) | ρREL.(AlSi12) | 0.37 | 0.37 | 0.38 | 0.38 |
| (kg·m−3) | |||||
| Volume of porous AlSi12 | VP.(AlSi12) | 4.012 × 10−5 | 4.159 × 10−5 | 3.678 × 10−5 | 3.870 × 10−5 |
| (m3) | |||||
| Weight of porous AlSi12 | mP.(AlSi12) | 0.039 | 0.041 | 0.037 | 0.039 |
| (kg) | |||||
| Porosity of porous AlSi12 | p | 63 | 63 | 62 | 62 |
| (%) | |||||
| MPa | |||||
| No. of Sample | SAM5 | SAM6 | SAM7 | |
|---|---|---|---|---|
| Size of NaCl particles | (mm) | 3 to 5 | 3 to 5 | 1 to 3 |
| Density of AlSi12 (base material) | ρB.M.(AlSi12) | 2650 | 2650 | 2650 |
| (kg·m−3) | ||||
| Volume of AlSi12 (base material) | VB.M.(AlSi12) | 3.281 × 10−5 | 2.627 × 10−5 | 3.500 × 10−5 |
| (m3) | ||||
| Weight of AlSi12 (base material) | mB.M.(AlSi12) | 0.087 | 0.068 | 0.093 |
| (kg) | ||||
| Density of porous AlSi12 | ρP.(AlSi12) | 1094 | 1085 | 1186 |
| (kg·m−3) | ||||
| Relative density of AlSi12 (porous/base) | ρREL.(AlSi12) | 0.41 | 0.41 | 0.45 |
| (kg·m−3) | ||||
| Volume of porous AlSi12 | VP.(AlSi12) | 3.281 × 10−5 | 2.627 × 10−5 | 3.500 × 10−5 |
| (m3) | ||||
| Weight of porous AlSi12 | mP.(AlSi12) | 0.036 | 0.029 | 0.042 |
| (kg) | ||||
| Porosity of porous AlSi12 | p | 59 | 59 | 55 |
| (%) | ||||
| Chemical Composition of wt% (EDX Analysis) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Al | Si | Cl | Na | Zn | Fe | O | Ca | Mg | S | Ar | B | P |
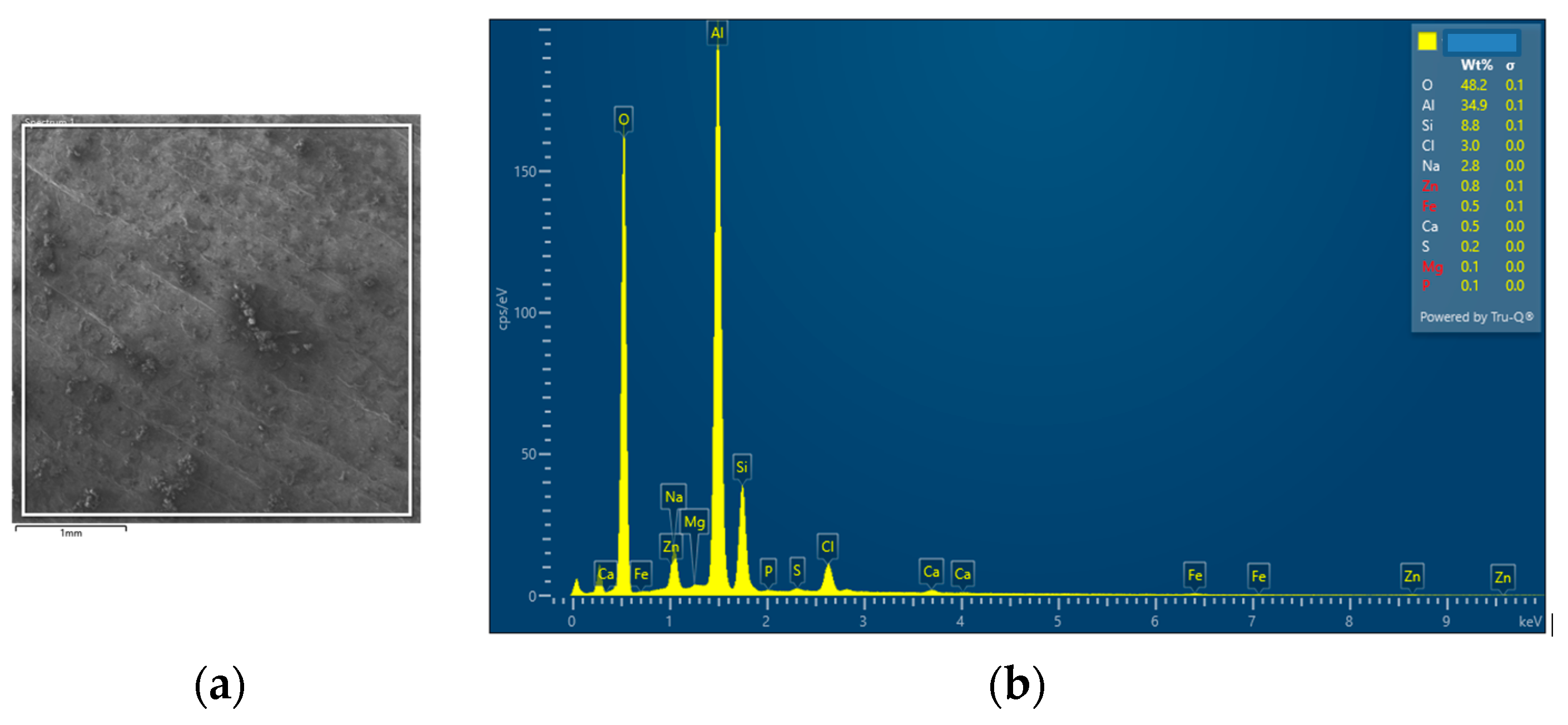
| SAM1 | 34.9 | 8.8 | 3.0 | 2.8 | 0.8 | 0.5 | 48.2 | 0.5 | 0.1 | 0.3 | - | - | 0.1 |
| SAM2 | the sample was not tested | ||||||||||||
| SAM3 | 76.3 | 10.1 | 1.1 | 1.3 | - | - | 11.3 | - | - | - | - | - | - |
| SAM4 | 76.8 | 10.0 | 3.4 | 1.6 | - | 1.1 | 7.1 | - | - | - | - | - | - |
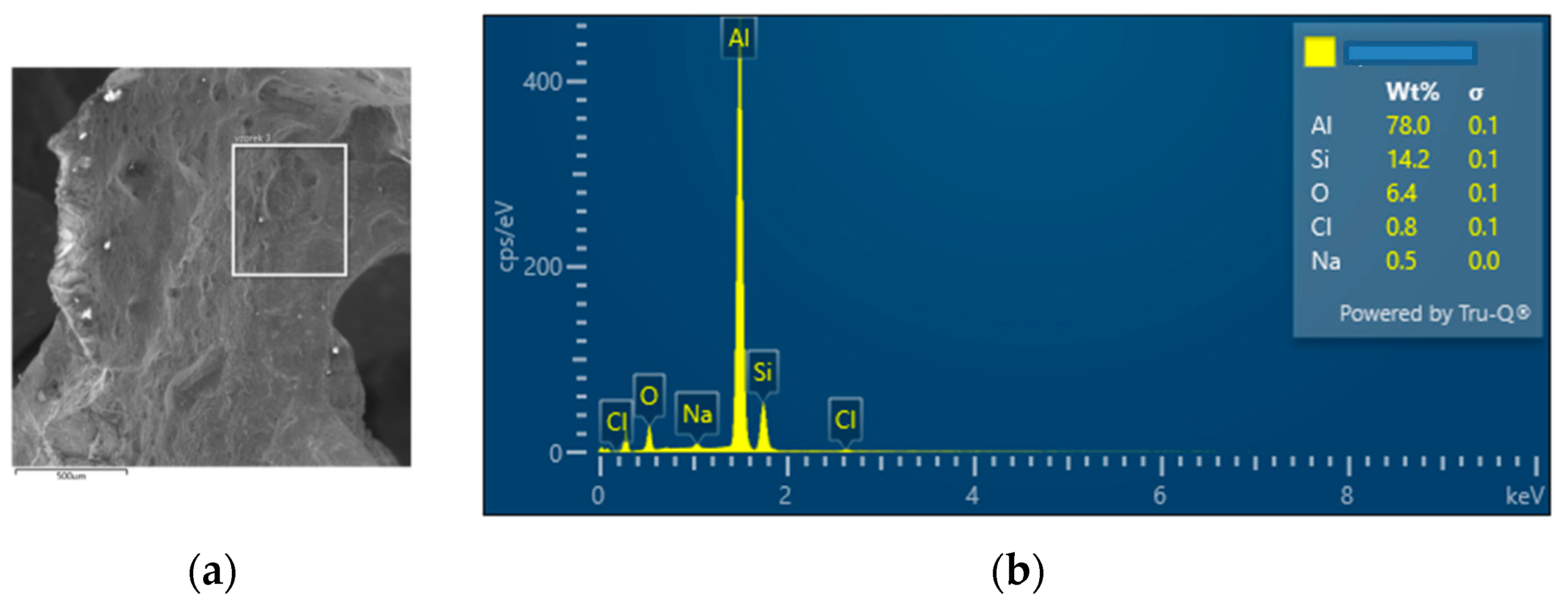
| SAM5 | 78.0 | 14.2 | 0.8 | 0.5 | - | - | 6.4 | - | - | - | - | - | - |
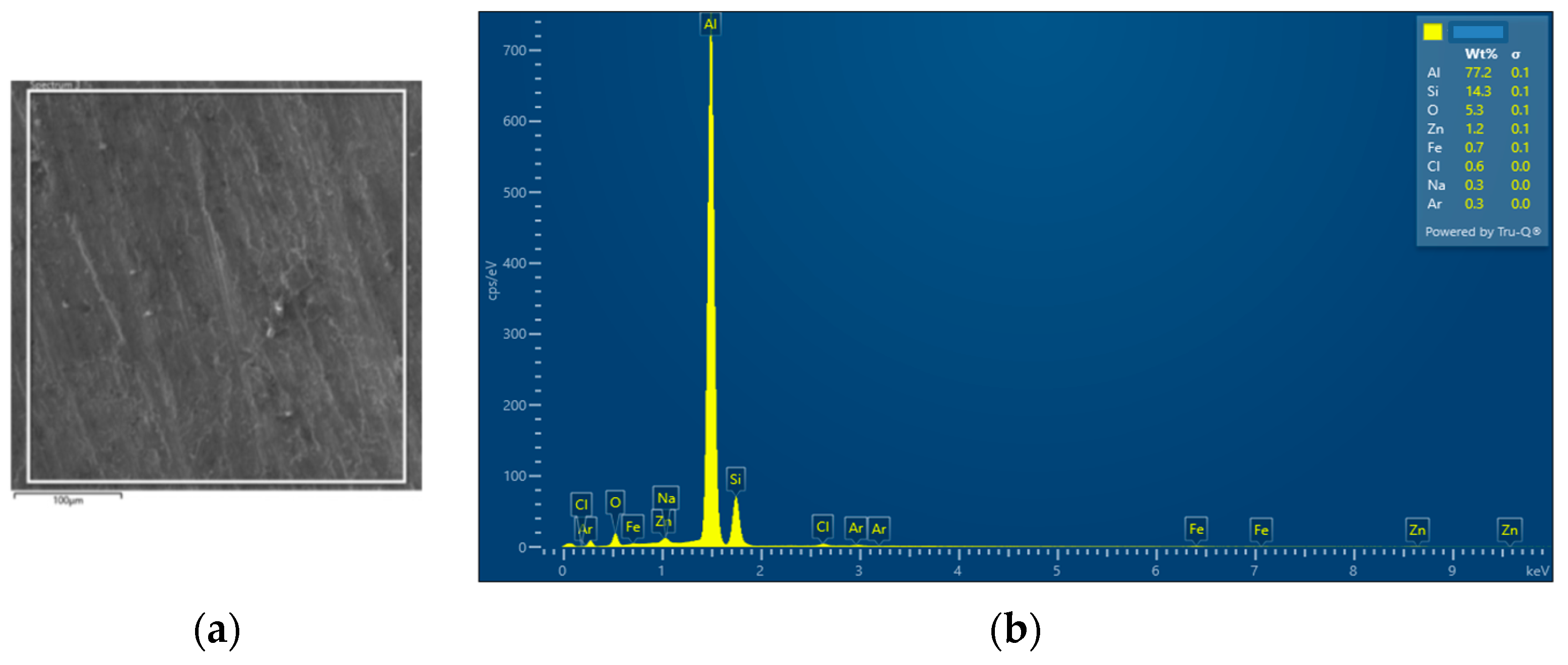
| SAM6 | 77.2 | 14.3 | 0.6 | 0.3 | 1.2 | 0.7 | 5.3 | - | - | - | 0.3 | - | - |
| SAM7 | 57.6 | 11.1 | 1.3 | 1.7 | - | 0.6 | 26.6 | 0.4 | 0.2 | 0.3 | - | 0.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nová, I.; Fraňa, K.; Solfronk, P.; Sobotka, J.; Koreček, D.; Švec, M. Characteristics of Porous Aluminium Materials Produced by Pressing Sodium Chloride into Their Melts. Materials 2021, 14, 4809. https://doi.org/10.3390/ma14174809
Nová I, Fraňa K, Solfronk P, Sobotka J, Koreček D, Švec M. Characteristics of Porous Aluminium Materials Produced by Pressing Sodium Chloride into Their Melts. Materials. 2021; 14(17):4809. https://doi.org/10.3390/ma14174809
Chicago/Turabian StyleNová, Iva, Karel Fraňa, Pavel Solfronk, Jiří Sobotka, David Koreček, and Martin Švec. 2021. "Characteristics of Porous Aluminium Materials Produced by Pressing Sodium Chloride into Their Melts" Materials 14, no. 17: 4809. https://doi.org/10.3390/ma14174809
APA StyleNová, I., Fraňa, K., Solfronk, P., Sobotka, J., Koreček, D., & Švec, M. (2021). Characteristics of Porous Aluminium Materials Produced by Pressing Sodium Chloride into Their Melts. Materials, 14(17), 4809. https://doi.org/10.3390/ma14174809






