Microstructure, Mechanical, and Corrosion Behavior of Al2O3 Reinforced Mg2Zn Matrix Magnesium Composites
Abstract
:1. Introduction
2. Materials and Methods
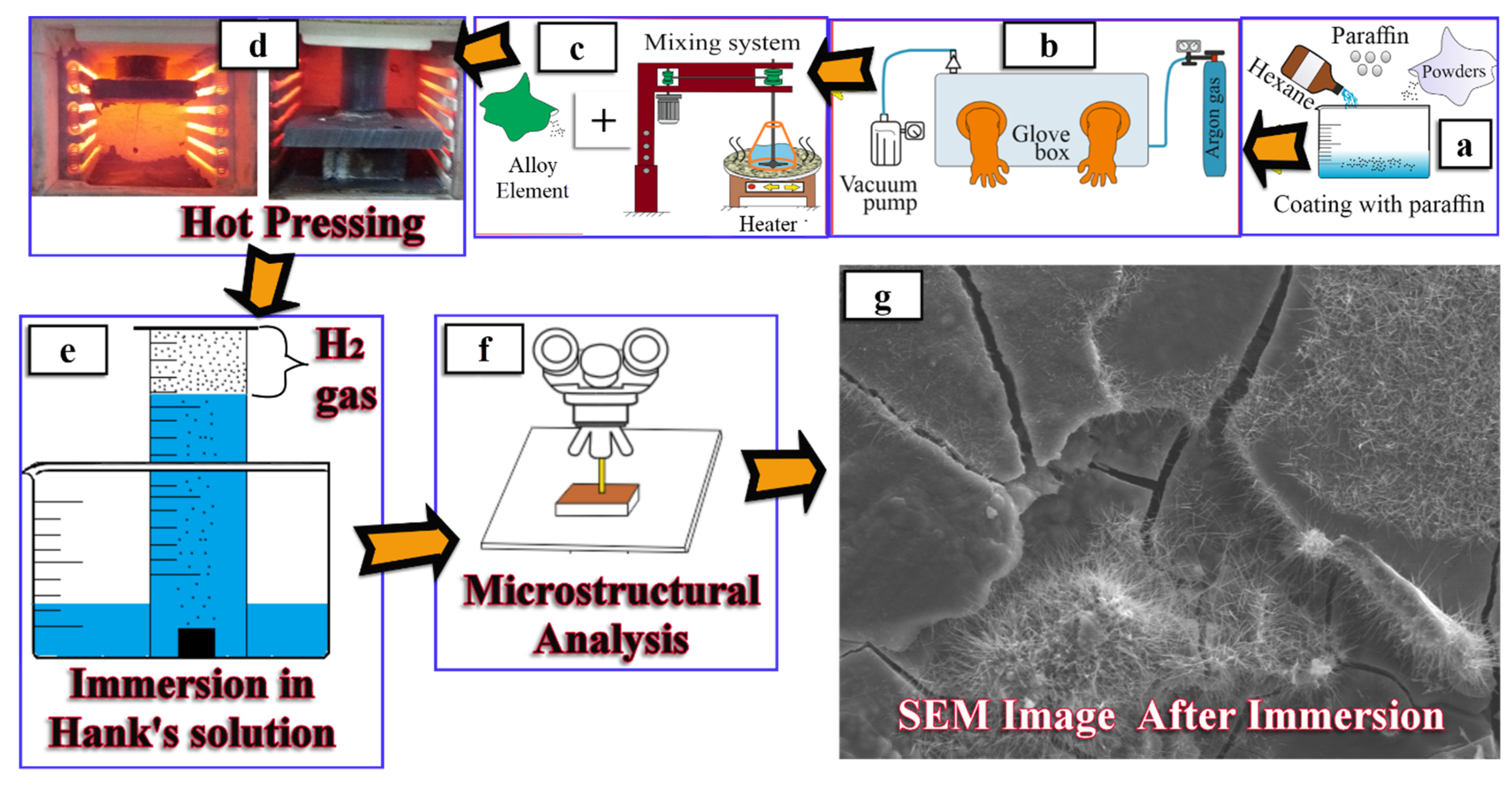
2.1. Mixing Process, Production, and Microstructural Investigation
2.2. Mechanical and Corrosion Test
3. Results and Discussions
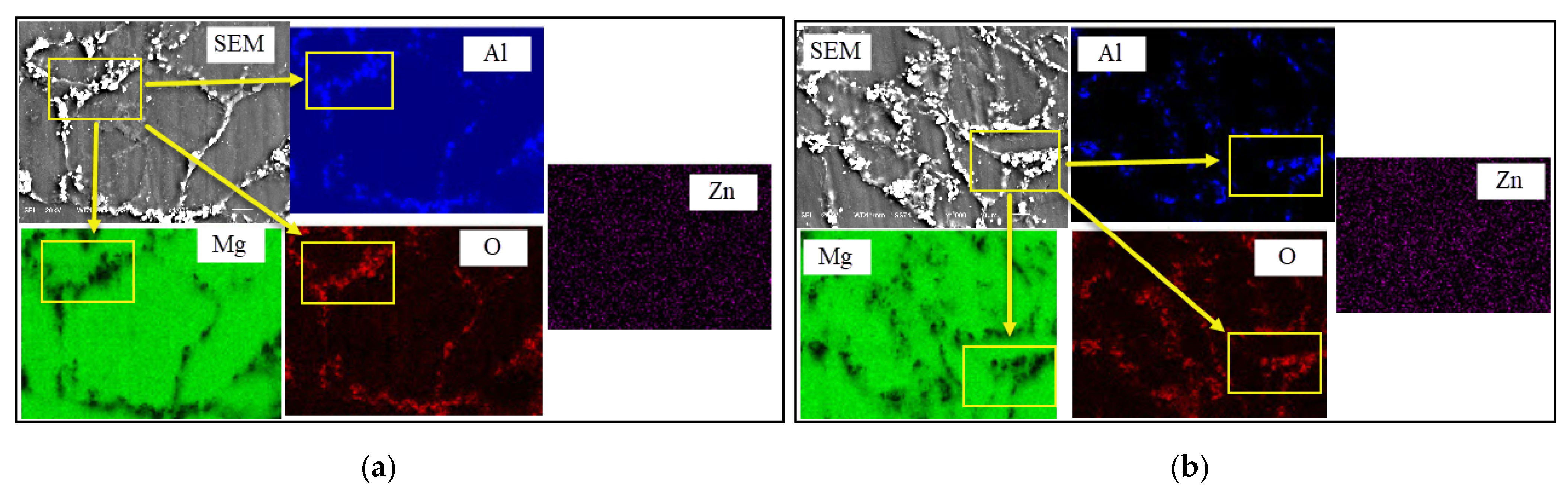
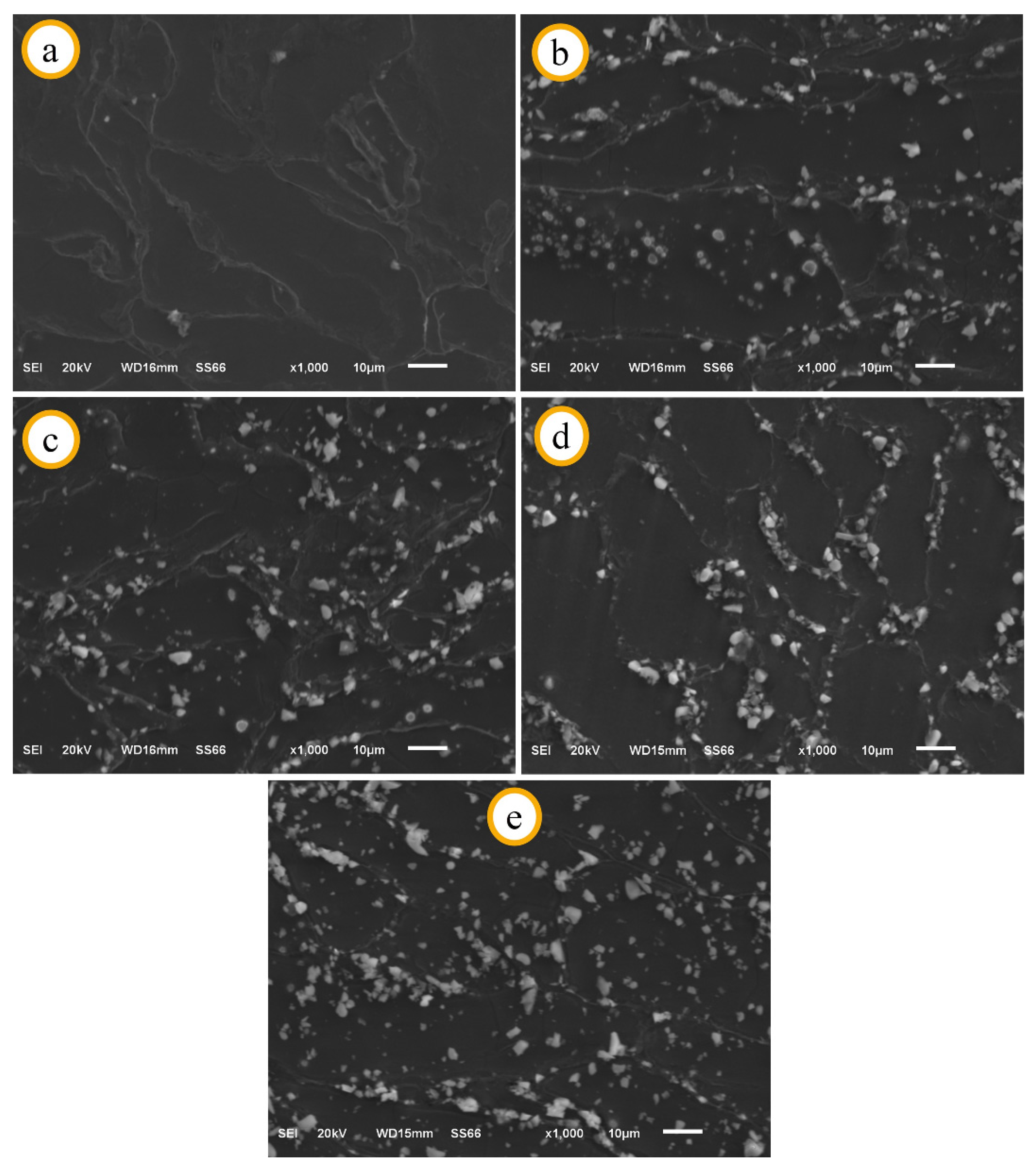
3.1. SEM and EDS Analysis of Specimens before Immersion
3.2. Corrosion Properties of Mg2Zn-xAl2O3 Composites
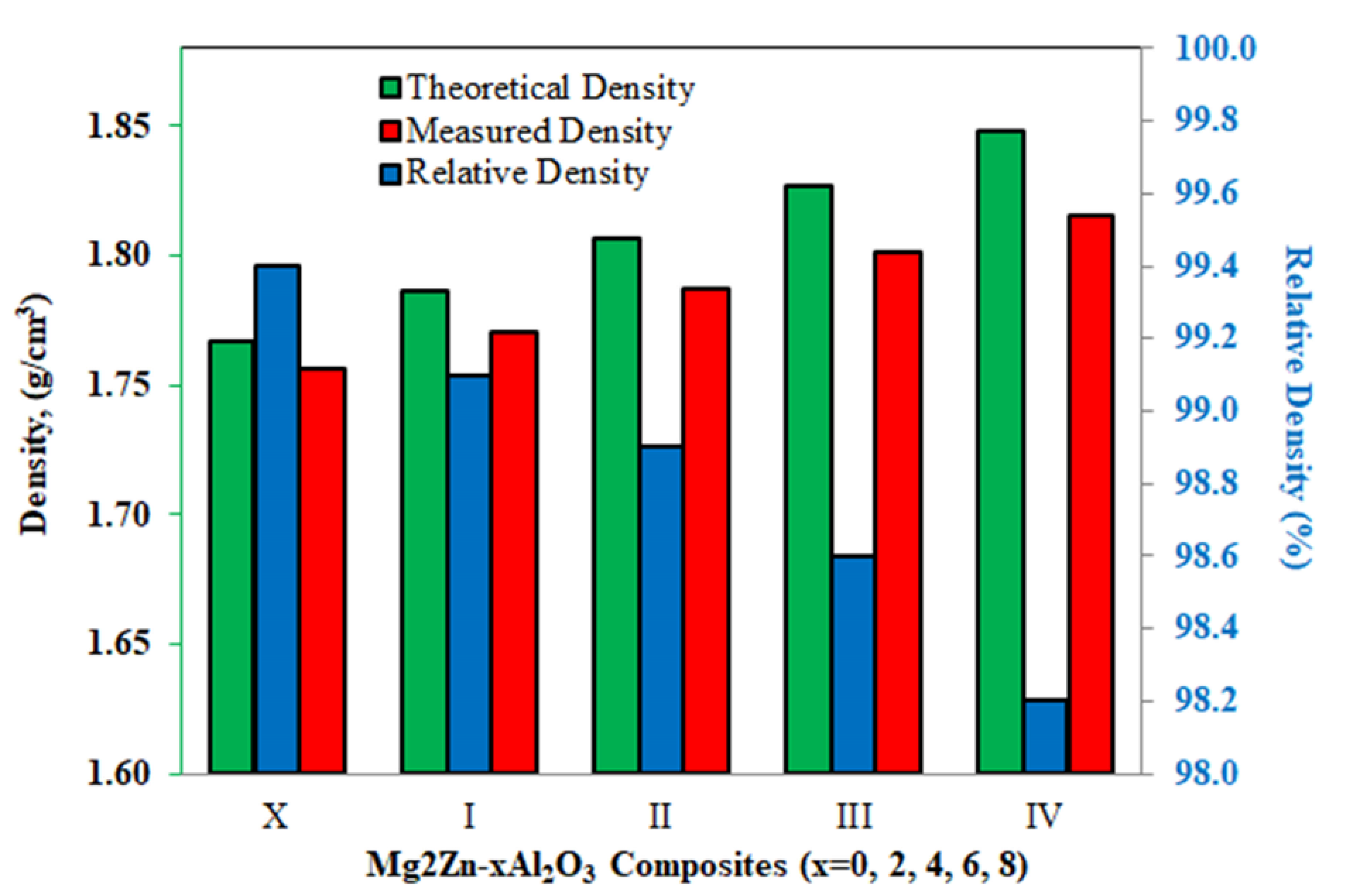
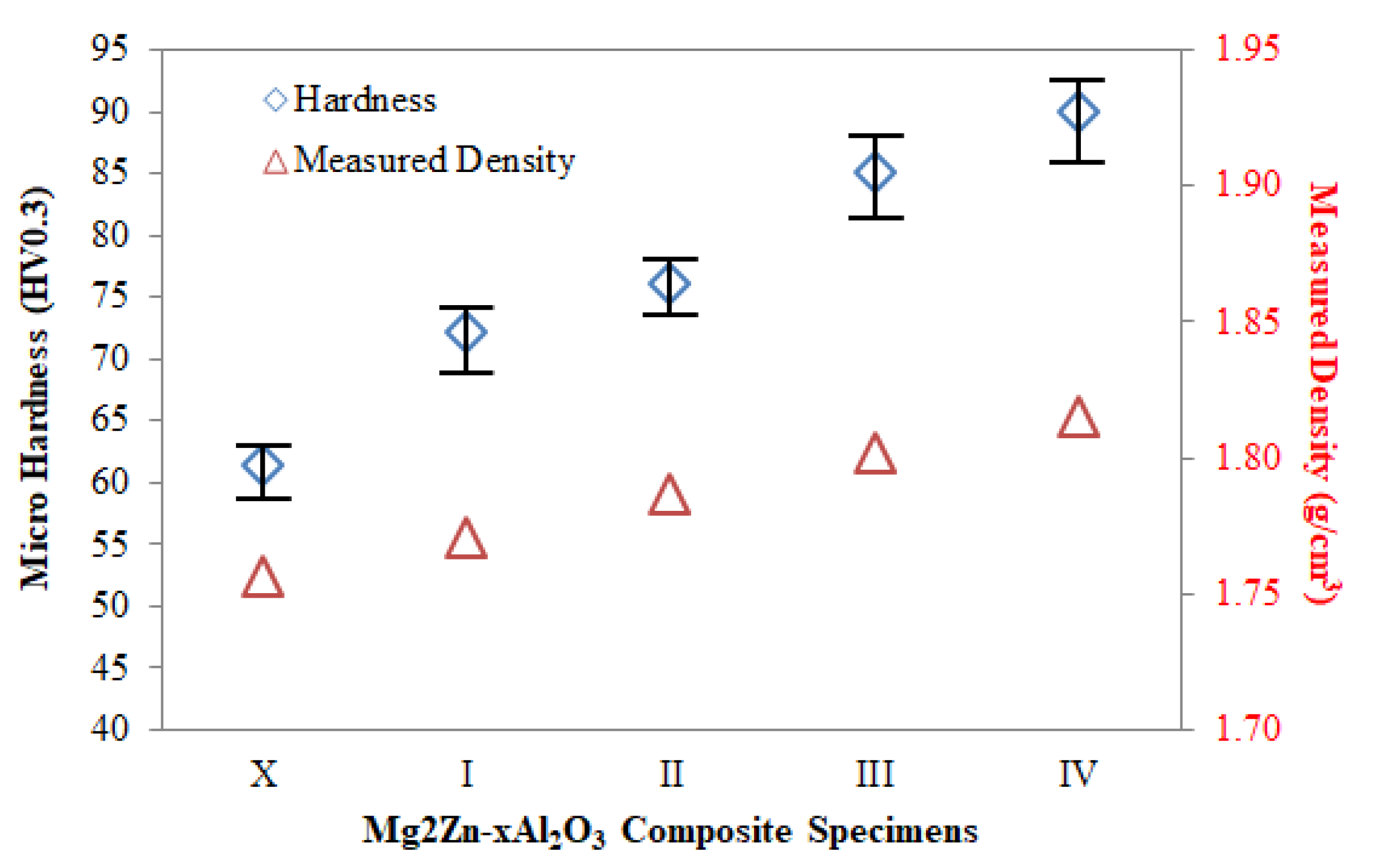
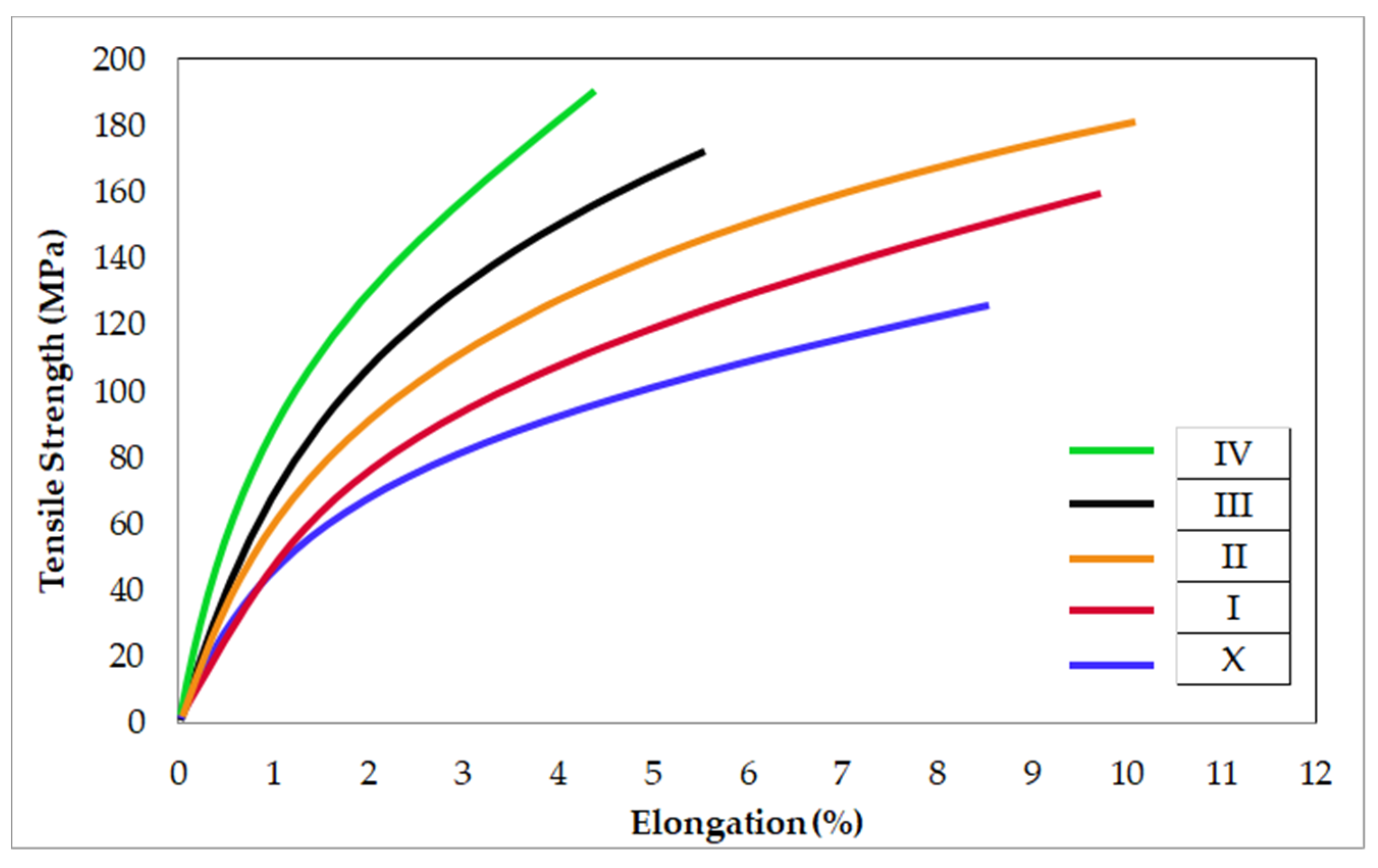
3.3. Mechanical Properties of Mg2Zn-xAl2O3 Composite Specimens
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suresh Kumar, N.; Padma Suvarna, R.; Chandra Babu Naidu, K.; Banerjee, P.; Ratnamala, A.; Manjunatha, H. A review on biological and biomimetic materials and their applications. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–18. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Shanmugam, L.; Kazemi, M.E.; Qiu, C.; Rui, M.; Yang, L.; Yang, J. Influence of UHMWPE fiber and Ti6Al4V metal surface treatments on the low-velocity impact behavior of thermoplastic fiber metal laminates. Adv. Compos. Hybrid Mater. 2020, 3, 508–521. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Venezuela, J.; Wen, C.; Dargusch, M.S.; Atrens, A. Investigating Mg biocorrosion in vitro: Lessons learned and recommendations. JOM 2019, 71, 1406–1413. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 7, 432–440. [Google Scholar] [CrossRef]
- Yun, Y.H.; Dong, Z.; Yang, D.; Schulz, M.J.; Shanov, V.N.; Yarmolenko, S.; Xu, Z.; Kumta, P.; Sfeir, C. Biodegradable Mg corrosion and osteoblast cell culture studies. Mater. Sci. Eng. C 2009, 29, 1814–1821. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Aljihmani, L.; Alic, L.; Boudjemline, Y.; Hijazi, Z.M.; Mansoor, B.; Serpedin, E.; Qaraqe, K. Magnesium-based bioresorbable stent materials: Review of reviews. J. Bio-Tribo-Corros. 2019, 5, 26. [Google Scholar] [CrossRef]
- Mahapatro, A.; Arshanapalli, S.A. Bioceramic coatings on magnesium alloys. J. Bio- Tribo-Corros. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Mokhtari, S.; Eftekhari Yekta, B.; Marghussian, V.; Ahmadi, P.T. Synthesis and characterization of biodegradable AZ31/calcium phosphate glass composites for orthopedic applications. Adv. Compos. Hybrid Mater. 2020, 3, 390–401. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Hu, X.; Wu, K.; Zheng, M. Effects of hot rolling on microstructure, macrotexture and mechanical properties of pre-extruded AZ31/SiC nanocomposite sheets. Mater. Sci. Eng. A 2017, 683, 15–23. [Google Scholar] [CrossRef]
- Abbas, A.T.; Pimenov, D.Y.; Erdakov, I.N.; Taha, M.A.; Soliman, M.S.; El Rayes, M.M. ANN surface roughness optimization of AZ61 magnesium alloy finish turning: Minimum machining times at prime machining costs. Materials 2018, 11, 808. [Google Scholar] [CrossRef] [Green Version]
- Ercetin, A.; Özgün, Ö.; Aslantas, K.; Aykutoğlu, G. The microstructure, degradation behavior and cytotoxicity effect of Mg–Sn–Zn alloys in vitro tests. SN Appl. Sci. 2020, 2, 173. [Google Scholar] [CrossRef] [Green Version]
- Aboudzadeh, N.; Dehghanian, C.; Shokrgozar, M.A. Synthesis, microstructure and mechanical properties of Mg-5Zn-0.3Ca/nHA nanocomposites. Iran. J. Mater. Sci. Eng. 2017, 14, 58–68. [Google Scholar] [CrossRef]
- Dubey, A.; Jaiswal, S.; Lahiri, D. Mechanical integrity of biodegradable Mg–HA composite during in vitro exposure. J. Mater. Eng. Perform. 2019, 28, 800–809. [Google Scholar] [CrossRef]
- Kumar, A.M.; Hassan, S.F.; Sorour, A.A.; Paramsothy, M.; Gupta, M. Investigation on the controlled degradation and invitro mineralization of carbon nanotube reinforced AZ31 nanocomposite in simulated body fluid. Met. Mater. Int. 2019, 25, 105–116. [Google Scholar] [CrossRef]
- Shahin, M.; Wen, C.; Munir, K.; Li, Y. Mechanical and corrosion properties of graphene nanoplatelet–reinforced Mg–Zr and Mg–Zr–Zn matrix nanocomposites for biomedical applications. J. Magnes. Alloy 2021. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Singh, R.A.; Chen, X.; Konovalov, S.; Srivatsan, T.S.; Seshan, S.; Gupta, M. Role of matrix microstructure in governing the mechanical behavior and corrosion response of two magnesium alloy metal matrix composites. JOM 2020, 72, 2882–2891. [Google Scholar] [CrossRef]
- Gu, X.; Cheng, W.; Cheng, S.; Liu, Y.; Wang, Z.; Yu, H.; Cui, Z.; Wang, L.; Wang, H. Tailoring the microstructure and improving the discharge properties of dilute Mg-Sn-Mn-Ca alloy as anode for Mg-air battery through homogenization prior to extrusion. J. Mater. Sci. Technol. 2021, 60, 77–89. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Xiong, H.; Zhou, J.; Yu, H.; Qin, C.; Wang, Z. Stearic acid coated MgO nanoplate arrays as effective hydrophobic films for improving corrosion resistance of Mg-based metallic glasses. Nanomaterials 2020, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.C.; Zhang, J.; Huang, W.J.; Dietzel, W.; Kainer, K.U.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferrous Met. Soc. China Engl. Ed. 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg-Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Kevorkov, D.; Pekguleryuz, M. Experimental study of the Ce-Mg-Zn phase diagram at 350 °C via diffusion couple techniques. J. Alloys Compd. 2009, 478, 427–436. [Google Scholar] [CrossRef]
- Ramesh, S.; Anne, G.; Nayaka, H.S.; Sahu, S.; Ramesh, M.R. Influence of multidirectional forging on microstructural, mechanical, and corrosion behavior of Mg-Zn alloy. J. Mater. Eng. Perform. 2019, 28, 2053–2062. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, F.; Li, Y.; Song, L.; Jiang, D.; Zeng, R.C.; Tjong, S.C.; Chen, D.C. Corrosion resistance of dodecanethiol-modified magnesium hydroxide coating on AZ31 magnesium alloy. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–11. [Google Scholar] [CrossRef]
- Castro, J.; Gokula Krishnan, K.; Jamaludeen, S.; Venkataragavan, P.; Gnanavel, S. Degradation and corrosion behavior of electrospun PHBV coated AZ-31 magnesium alloy for biodegradable implant applications. J. Bio-Tribo-Corros. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Nie, K.B.; Zhu, Z.H.; Deng, K.K.; Guo, Y.C.; Han, J.G. Microstructure and tensile strength of nano-TiCp/Mg–Zn–Ca magnesium matrix nanocomposites processed by multidirectional forging. Met. Mater. Int. 2019, 1–11. [Google Scholar] [CrossRef]
- Ercetin, A. Application of the hot press method to produce new Mg alloys: Characterization, mechanical properties, and effect of Al addition. J. Mater. Eng. Perform. 2021, 30, 4254–4262. [Google Scholar] [CrossRef]
- Ercetin, A.; Özgün, Ö.; Aslantas, K. Investigation of mechanical properties of Mg5Sn-xZn alloys produced through new method in powder metallurgy. J. Test. Eval. 2021, 49, 3506–3518. [Google Scholar] [CrossRef]
- Kandemir, S. Development of graphene nanoplatelet-reinforced AZ91 magnesium alloy by solidification processing. J. Mater. Eng. Perform. 2018, 27, 3014–3023. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Kalsi, N.S.; Sharma, S.; Pruncu, C.I.; Pimenov, D.Y.; Rao, K.V.; Kaplonek, W. Comparative study on the mechanical, tribological, morphological and structural properties of vortex casting processed, Al-SiC-Cr hybrid metal matrix composites for high strength wear-resistant applications: Fabrication and characterizations. J. Mater. Res. Technol. 2020, 9, 13607–13615. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, J.; Gupta, M.K.; Mia, M.; Dwivedi, S.P.; Saxena, A.; Chattopadhyaya, S.; Singh, R.; Pimenov, D.Y.; Korkmaz, M.E. Investigation on mechanical, tribological and microstructural properties of Al-Mg-Si-T6/SiC/muscovite-hybrid metal-matrix composites for high strength applications. J. Mater. Res. Technol. 2021, 12, 1564–1581. [Google Scholar] [CrossRef]
- Donnadieu, P.; Benrhaiem, S.; Renou, G.; Zhang, C.; Tassin, C.; Blandin, J.-J. Deformation of Mg-γMg17Al12 in situ composites: Room temperature mechanical behaviour, microstructures and mechanisms. Intermetallics 2021, 132, 107127. [Google Scholar] [CrossRef]
- Shen, M.J.; Ying, W.F.; Wang, X.J.; Zhang, M.F.; Wu, K. Development of high performance magnesium matrix nanocomposites using nano-SiC particulates as reinforcement. J. Mater. Eng. Perform. 2015, 24, 3798–3807. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Li, G.; Liu, S.; Xiang, Y.; Cheng, H. Sintering temperature and interphase effects on mechanical properties of an oxide fiber-reinforced Al2O3-SiO2 composite fabricated by Sol–Gel method. Appl. Compos. Mater. 2021, 28, 321–339. [Google Scholar] [CrossRef]
- Dehghanian, C.; Lotfpour, M.; Emamy, M. The microstructure, and mechanical and corrosion properties of as-cast and as-extruded Mg-2%Zn-x%Cu alloys after solution and aging heat treatments. J. Mater. Eng. Perform. 2019, 28, 2305–2315. [Google Scholar] [CrossRef]
- Lotfpour, M.; Emamy, M.; Dehghanian, C. Influence of Cu addition on the microstructure, mechanical, and corrosion properties of extruded Mg-2%Zn alloy. J. Mater. Eng. Perform. 2020, 29, 2991–3003. [Google Scholar] [CrossRef]
- Lotfpour, M.; Emamy, M.; Dehghanian, C.; Tavighi, K. Influence of Cu addition on the structure, mechanical and corrosion properties of cast Mg-2%Zn alloy. J. Mater. Eng. Perform. 2017, 26, 2136–2150. [Google Scholar] [CrossRef]
- Xu, H.; Zou, N.; Li, Q. Effect of ball milling time on microstructure and hardness of porous magnesium/carbon nanofiber composites. JOM 2017, 69, 1236–1243. [Google Scholar] [CrossRef]
- Wang, T.; Huang, Y.; Yang, L.; Ma, Y.; Wu, L.; Yan, H.; Liu, Y.; Liu, W. Preparation of 2024-Al/AZ31-Mg laminated composite by powder metallurgy integrated forming and sintering. JOM 2020, 72, 3547–3557. [Google Scholar] [CrossRef]
- Erçetin, A.; Aslantaş, K.; Perçin, M. Micro milling of tungsten-copper composite materials produced through powder metallurgy method: Effect of composition and sintering temperature. J. Fac. Eng. Archit. Gazi Univ. 2018, 33, 1369–1381. [Google Scholar] [CrossRef]
- Erçetin, A.; Aslantas, K.; Özgün, Ö. Micro-end milling of biomedical TZ54 magnesium alloy produced through powder metallurgy. Mach. Sci. Technol. 2020, 24, 924–947. [Google Scholar] [CrossRef]
- Nayyeri, G.; Mahmudi, R. Enhanced creep properties of a cast Mg-5Sn alloy subjected to aging-treatment. Mater. Sci. Eng. A 2010, 527, 4613–4618. [Google Scholar] [CrossRef]
- Özgün, Ö.; Aslantaş, K.; Erçetin, A. Powder metallurgy Mg-Sn alloys: Production and characterization. Sci. Iran. 2020, 27, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, B.; Cavaliere, P.; Roeen, G.A.; Nosko, M.; Shamanian, M.; Trembošová, V.; Nagy, Š.; Ebrahimzadeh, N. Hot rolling of MWCNTs reinforced Al matrix composites produced via spark plasma sintering. Adv. Compos. Hybrid Mater. 2019, 2, 549–570. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Saba, F.; Sajjadi, S.A.; Heydari, S.; Haddad-Sabzevar, M.; Salehi, J.; Babayi, H. A novel approach to the uniformly distributed carbon nanotubes with intact structure in aluminum matrix composite. Adv. Compos. Hybrid Mater. 2019, 2, 540–548. [Google Scholar] [CrossRef]
- Shouren, W.; Haoran, G.; Jingchun, Z.; Yingzi, W. Interpenetrating microstructure and properties of Si3N4/Al-Mg composites fabricated by pressureless infiltration. Appl. Compos. Mater. 2006, 13, 115–126. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.J.; Xue, Y.Z.B.; Liang, X.Y.; Tan, Z.J.; Tang, B. Novel PEEK/nHA composites fabricated by hot-pressing of 3D braided PEEK matrix. Adv. Compos. Hybrid Mater. 2020, 3, 156–166. [Google Scholar] [CrossRef]
- Suresh, S.; Gowd, G.H.; Kumar, M.L.S.D. Mechanical and wear behavior of Al 7075/Al2O3/SiC/mg metal matrix nanocomposite by liquid state process. Adv. Compos. Hybrid Mater. 2019, 2, 530–539. [Google Scholar] [CrossRef]
- Shuai, C.; Zhou, Y.; Lin, X.; Yang, Y.; Gao, C.; Shuai, X.; Wu, H.; Liu, X.; Wu, P.; Feng, P. Preparation and characterization of laser-melted Mg–Sn–Zn alloys for biomedical application. J. Mater. Sci. Mater. Med. 2017, 28, 13. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon nanotubes (CNTs)-reinforced magnesium-based matrix composites: A comprehensive review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Ercetin, A. A novel Mg-Sn-Zn-Al-Mn magnesium alloy with superior corrosion properties. Metall. Res. Technol. 2021. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, M.; Wen, C.; Li, Y. Microstructure, mechanical and corrosion properties of hot-pressed graphene nanoplatelets-reinforced Mg matrix nanocomposites for biomedical applications. J. Alloys Compd. 2021, 887, 161379. [Google Scholar] [CrossRef]
- Munir, K.; Wen, C.; Li, Y. Graphene nanoplatelets-reinforced magnesium metal matrix nanocomposites with superior mechanical and corrosion performance for biomedical applications. J. Magnes. Alloy. 2020, 8, 269–290. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Corrosion characterisation of alumina–magnesium metal matrix composites. Corros. Sci. 2007, 49, 1110–1130. [Google Scholar] [CrossRef]
- Packia Antony Amalan, A.; Sivaram, N.M.; Bavatharani, C.; Ragupathy, D. A study on the effect of ageing heat treatment on hardness, tensile and corrosion behaviour of stir-cast AZ91D–5SiC–1Gr hybrid magnesium composite. Int. J. Met. 2021. [Google Scholar] [CrossRef]
- Say, Y.; Guler, O.; Dikici, B. Carbon nanotube (CNT) reinforced magnesium matrix composites: The effect of CNT ratio on their mechanical properties and corrosion resistance. Mater. Sci. Eng. A 2020, 798, 139636. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Mukhopadhyay, N.K.; Yadav, A.; Winczek, J. Effect of SiC reinforcement and its variation on the mechanical characteristics of AZ91 composites. Materials 2020, 13, 4913. [Google Scholar] [CrossRef] [PubMed]
- Luo, A. Processing, microstructure, and mechanical behavior of cast magnesium metal matrix composites. Metall. Mater. Trans. A 1995, 26, 2445–2455. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium-based composites reinforced with graphene nanoplatelets as biodegradable implant materials. J. Alloys Compd. 2020, 828, 154461. [Google Scholar] [CrossRef]
- Braszczyńska-Malik, K.N.; Malik, M.A. Microstructure and mechanical properties of hypo- and hypereutectic cast Mg/Mg2Si composites. Materials 2020, 13, 3591. [Google Scholar] [CrossRef] [PubMed]
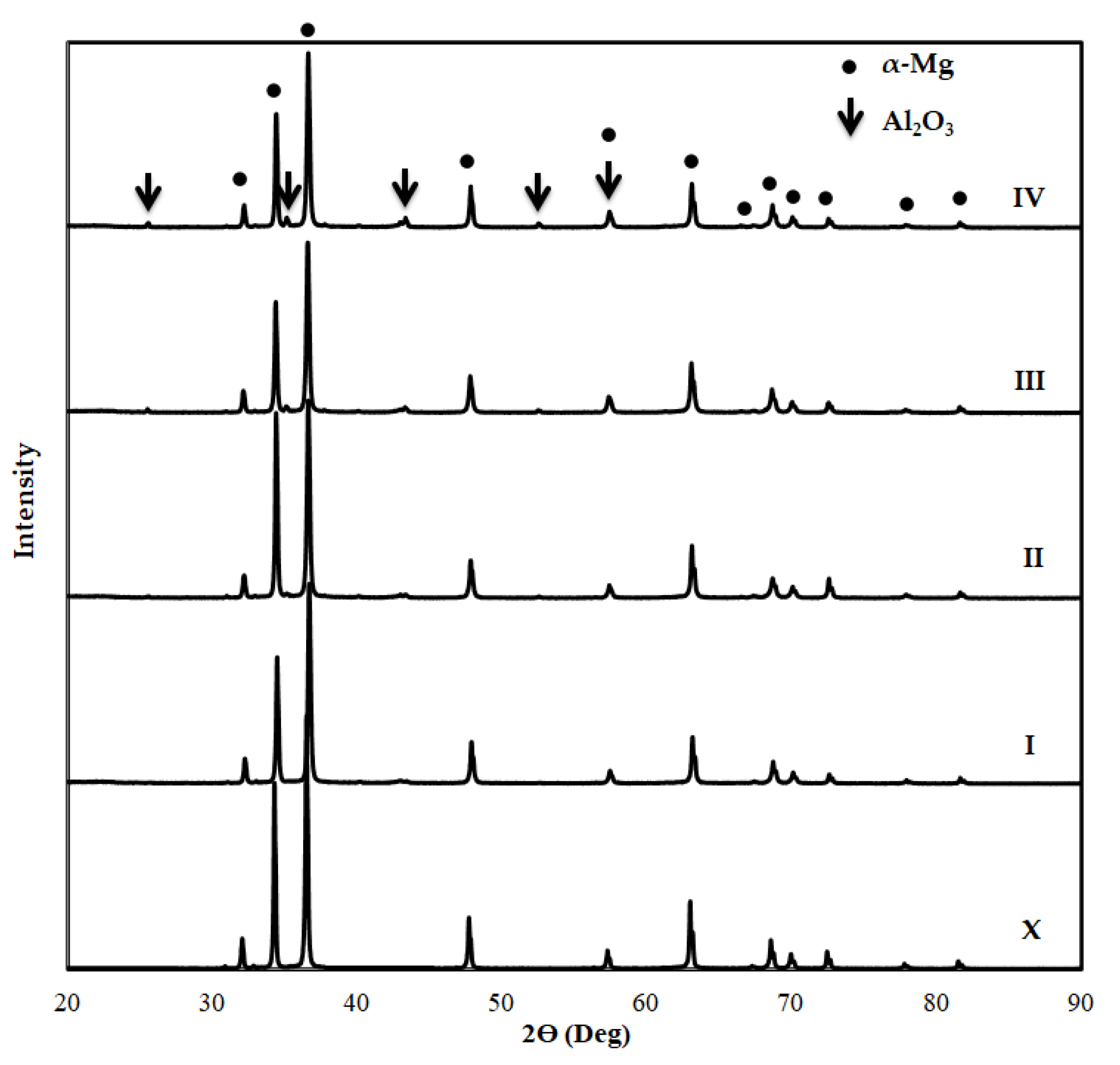
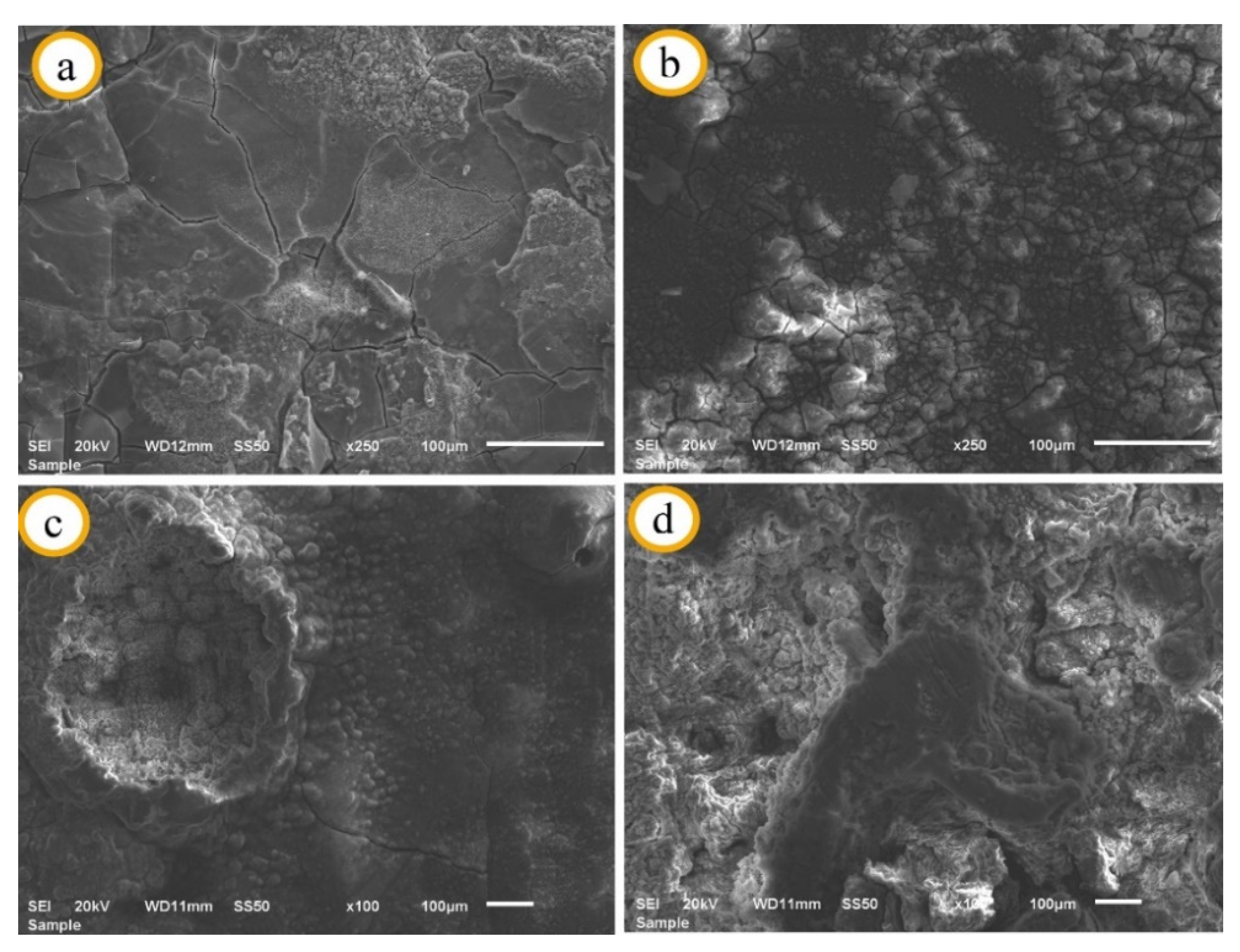
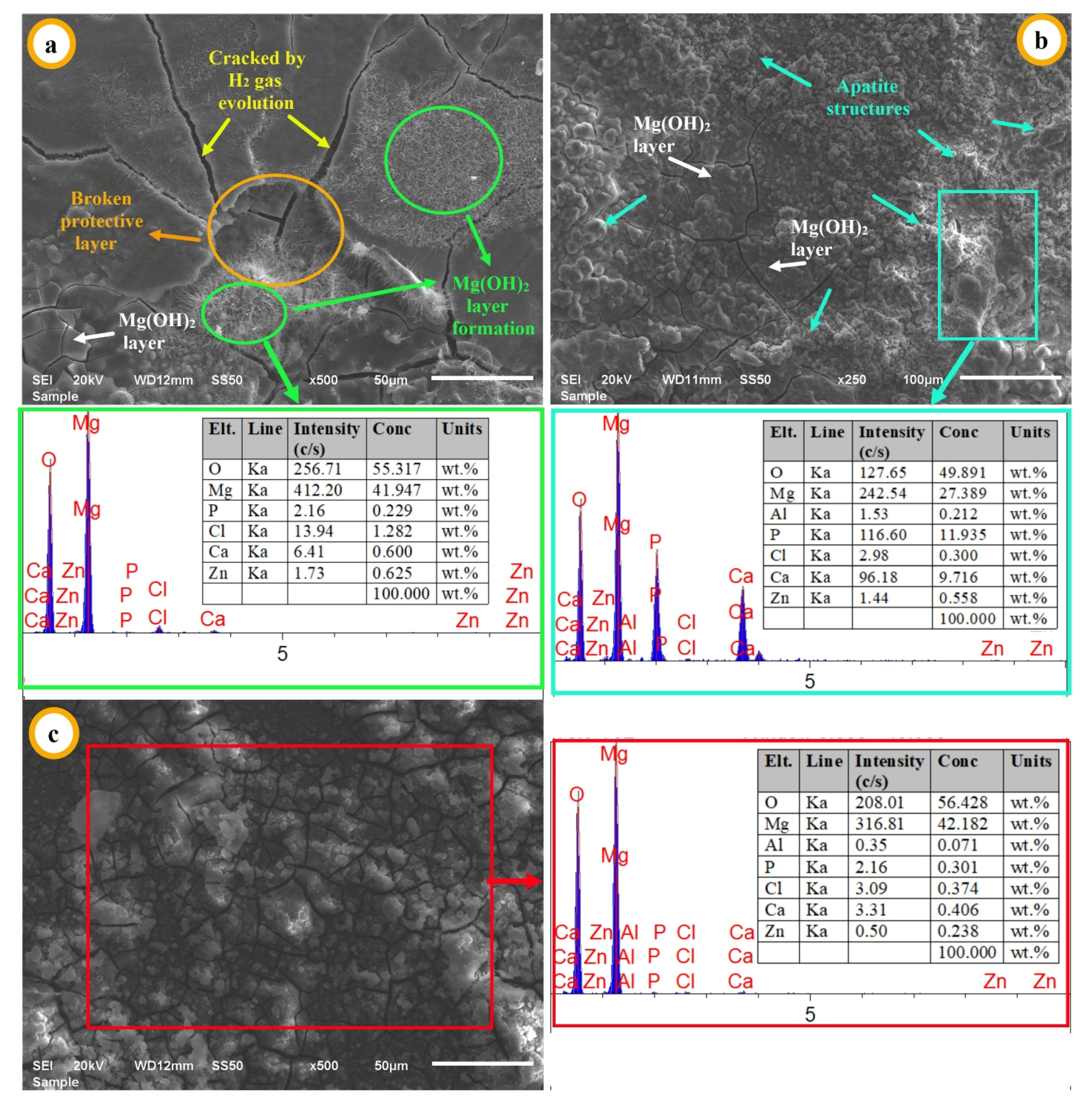
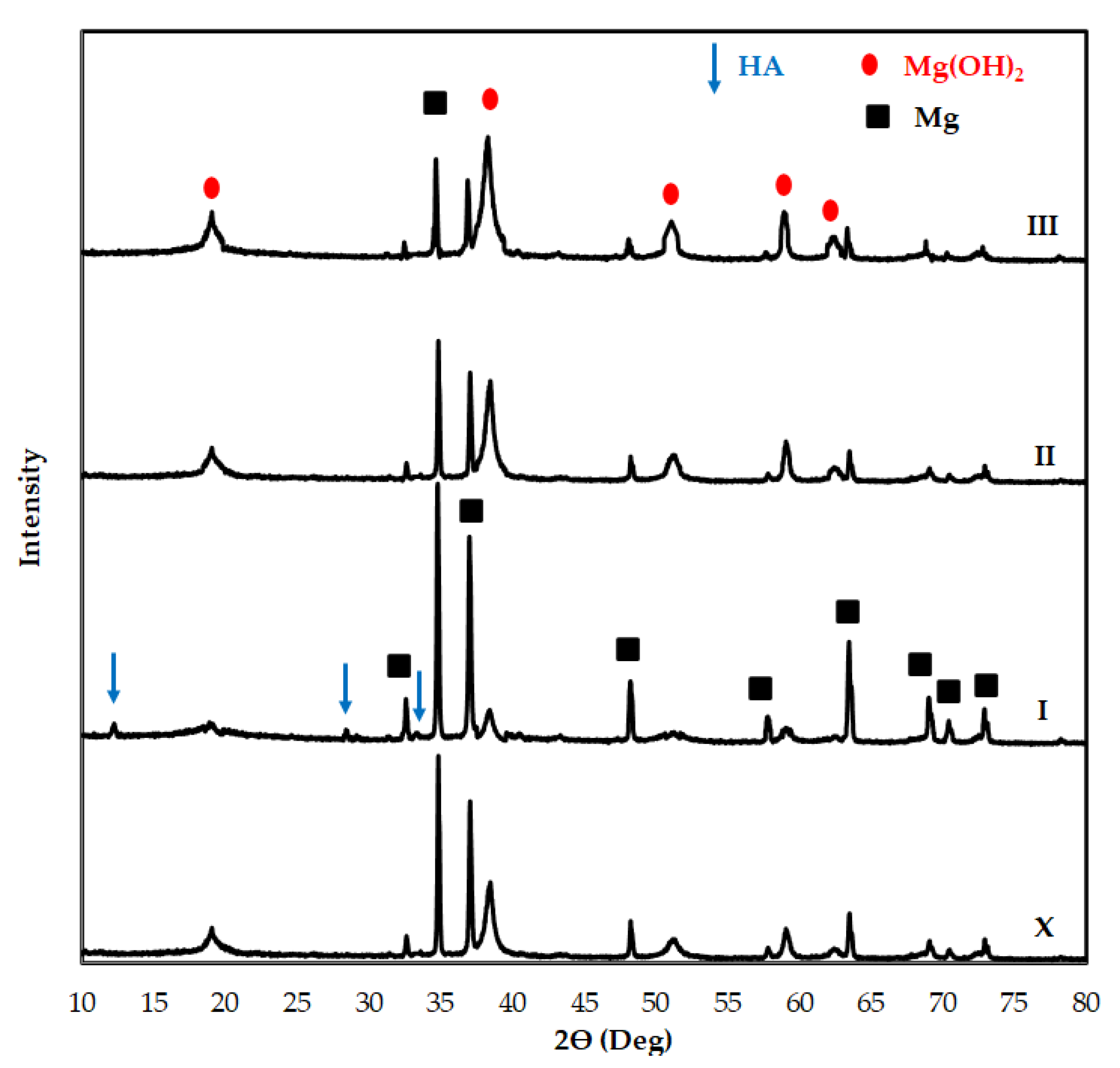

| Matrix Code | Matrix Nomenclature | Mg (wt.%) Purity: 99.8% Size: <45 µm | Zn (wt.%) Purity: 99.9% Size: <10 µm |
|---|---|---|---|
| X | Mg2Zn | 98 | 2 |
| Composite Code | Composite Nomenclature | Mg2Zn (wt.%) | Al2O3 (wt.%) Purity: 99.8% Size: <5 µm |
|---|---|---|---|
| X | Mg2Zn-0 wt.% Al2O3 | 100 | 0 |
| I | Mg2Zn-2 wt.% Al2O3 | 98 | 2 |
| II | Mg2Zn-4 wt.% Al2O3 | 96 | 4 |
| III | Mg2Zn-6 wt.% Al2O3 | 94 | 6 |
| IV | Mg2Zn-8 wt.% Al2O3 | 92 | 8 |
| Material | Corrosion Rate (mm/Year) | Weight Loss (%) | Ref |
|---|---|---|---|
| Mg2Zn + 2 wt.% Al2O3 | 2.5 | 9 | PS |
| AM100 + 25 vol.% Al2O3 | 7 | - | [20] |
| ZC63 + 25 vol.% Al2O3 | 9.5 | - | [20] |
| Mg3Zn + 5HA | - | 27 | [17] |
| Mg0.3GNP | 4 | - | [57] |
| Mg0.5Zr0.5GNP | 18 | - | [19] |
| Material | Hardness (HV) | Tensile Strength (MPa) | Elongation (%) | Ref |
|---|---|---|---|---|
| Mg2Zn-2 wt.% Al2O3 | 72.2 | 160 | 9.8 | PS |
| Mg2Zn-8 wt.% Al2O3 | 89.9 | 191 | 4.4 | PS |
| AZ91-2 wt.% SiC | 75 | 120 | 4 | [62] |
| AZ91-8 wt.% SiC | 85 | 145 | 3.5 | [62] |
| Mg/Mg2Si-HO | 145 | [65] | ||
| Mg/Mg2Si-HR | 155 | [65] | ||
| Mg0.5Zr0.5GNP | 53 | - | - | [19] |
| Mg3Zn-0.5Cu | 62 | 171 | 15 | [41] |
| Mg3Zn-5Cu | 80 | 135 | 6 | [41] |
| AZ91-0.5 wt.% GNP | 69 | - | - | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ercetin, A.; Pimenov, D.Y. Microstructure, Mechanical, and Corrosion Behavior of Al2O3 Reinforced Mg2Zn Matrix Magnesium Composites. Materials 2021, 14, 4819. https://doi.org/10.3390/ma14174819
Ercetin A, Pimenov DY. Microstructure, Mechanical, and Corrosion Behavior of Al2O3 Reinforced Mg2Zn Matrix Magnesium Composites. Materials. 2021; 14(17):4819. https://doi.org/10.3390/ma14174819
Chicago/Turabian StyleErcetin, Ali, and Danil Yurievich Pimenov. 2021. "Microstructure, Mechanical, and Corrosion Behavior of Al2O3 Reinforced Mg2Zn Matrix Magnesium Composites" Materials 14, no. 17: 4819. https://doi.org/10.3390/ma14174819







