Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
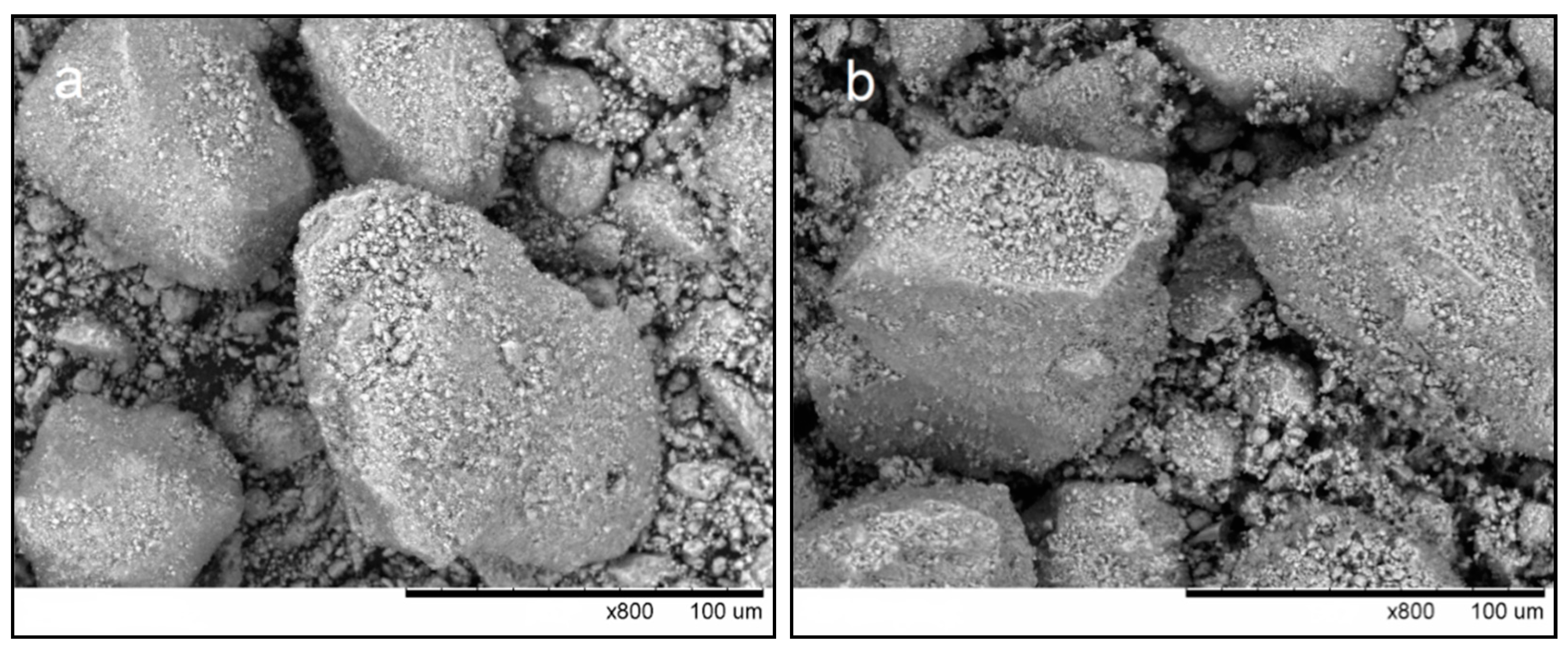
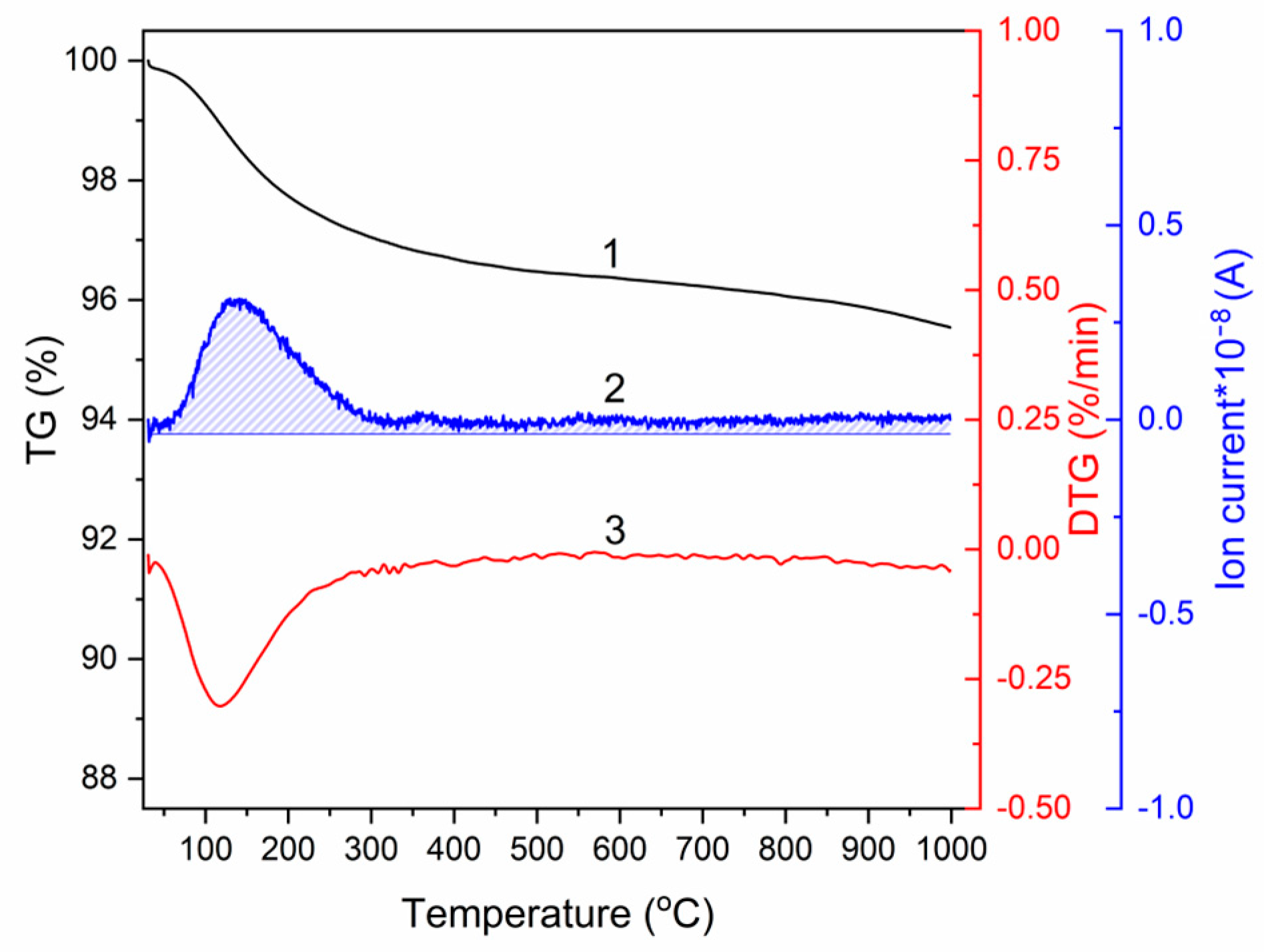
3.1. Characterization of HA Feedstock Powders
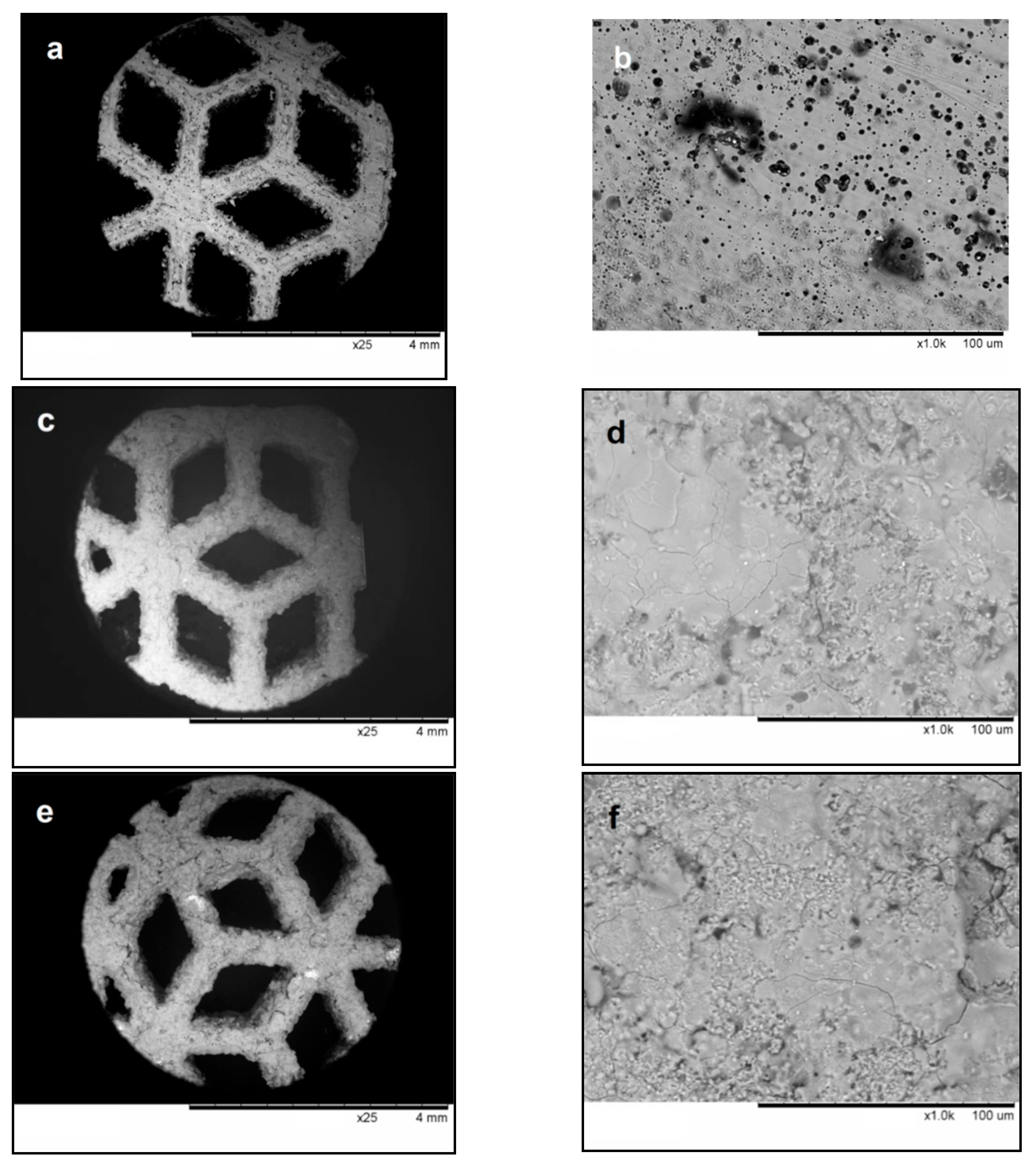
3.2. Characterization of Detonation Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomsia, P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.K.; Saiz, E. Nanotechnology approaches for better dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25–49. [Google Scholar]
- Christenson, E.M.; Anseth, K.S.; van der Beucken, J.J.J.P.; Chan, C.K.; Ercn, B.; Jansen, J.A.; Laurenin, C.T.; Li, W.J.; Murugan, R.; Nair, L.S.; et al. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Fini, M.; Giavaresi, G.; Torricelli, P.; Borsari, V.; Giardino, R.; Nicolini, A.; Carpi, A. Osteoporosis and biomaterial osteointegration. Biomed. Pharmacother. 2004, 58, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Fini, M.; Martitni, D.; Orsini, E.; Leonardi, L.; Ruggeri, A.; Giavaresi, G.; Ottani, V. Biological fixation of endosseous implants. Micron 2005, 36, 630–671. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Ariola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2021, 9, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2019, 35, 1–12. [Google Scholar] [CrossRef]
- Teghil, R.; Curcio, M.; De Bonis, A. Substituted hydroxyapatite, glass, and glass-ceramic thin films deposited by nanosecond pulsed laser deposition (PLD) for biomedical applications: A systematic review. Coatings 2021, 11, 811. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regı, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, Z.E. The effect of heat treatment on the morphology of D-gun sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. 1999, 48, 861–868. [Google Scholar] [CrossRef]
- Gryshkov, O.; Klyui, N.I.; Temchenko, V.P.; Kyselov, V.S.; Chatterjee, A.; Belyaev, A.E.; Lauterboeck, L.; Iarmolenko, D.; Glasmacher, B. Porous biomorphic silicon carbide ceramics coated with hydroxyapatiteas prospective materials for bone implants. Mater. Sci. Eng. C 2016, 68, 143–152. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. Direct morphological comparison of vacuum plasma sprayed and detonation gun sprayed hydroxyapatite coatings for orthopaedic applications. Biomaterials 1999, 20, 315–322. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. In vitro fatigue behavior of vacuum plasma and detonation gun sprayed hydroxyapatite coatings. Biomaterials 2001, 22, 1233–1240. [Google Scholar] [CrossRef]
- Nosenko, V.; Strutynska, N.; Vorona, I.; Zatovsky, I.; Dzhagan, V.; Lemishko, S.; Epple, M.; Prymak, O.; Baran, N.; Ishchenko, S.; et al. Structure of Biocompatible Coatings Produced from Hydroxyapatite Nanoparticles by Detonation Spraying. Nanoscale Res. Lett. 2015, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Ulianitsky, V.; Shtertser, A.; Sadykov, V.; Smurov, I. Development of Catalytic Converters Using Detonation Spraying. Mater. Manuf. Proc. 2016, 31, 1433–1438. [Google Scholar] [CrossRef]
- Russian Federation. A Method of Manufacturing an Individual Implant for the Replacement of Defects in the Bones of the Skull. RU Patent 2644275, 8 February 2018.
- ISO 14630:2012. Non-Active Surgical Implants—General Requirements; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 15223-1:2012. Medical Devices—Symbols to Be Used with Medical Device Labels, Labelling, and Information to Be Supplied—Part 1: General Requirements; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 9585:1990. Implants for Surgery—Determination of Bending Strength and Stiffness of Bone Plates; ISO: Geneva, Switzerland, 1990. [Google Scholar]
- Ulianitsky, V.; Shtertser, A.; Zlobin, S.; Smurov, I. Computer-controlled detonation spraying: From process fundamentals toward advanced applications. J. Therm. Spray Technol. 2011, 20, 791–801. [Google Scholar] [CrossRef]
- Ulianitsky, V.Y.; Dudina, D.V.; Shtertser, A.A.; Smurov, I. Computer-controlled detonation spraying: Flexible control of the coating chemistry and microstructure. Metals 2019, 9, 1244. [Google Scholar] [CrossRef] [Green Version]
- Ulianitsky, V.Y.; Dudina, D.V.; Batraev, I.S.; Rybin, D.K.; Bulina, N.V.; Ukhina, A.V.; Bokhonov, B.B. The influence of the in-situ formed and added carbon on the formation of metastable Ni-based phases during detonation spraying. Mater. Lett. 2016, 181, 127–131. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Plūduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Bulina, N.V.; Titkov, A.I.; Baev, S.G.; Makarova, S.V.; Khusnutdinov, V.R.; Bessmeltsev, V.P.; Lyakhov, N.Z. Selective laser sintering of hydroxyapatite for fabrication of bioceramic scaffolds. Mater. Today Proc. 2021, 37, 4022–4026. [Google Scholar] [CrossRef]

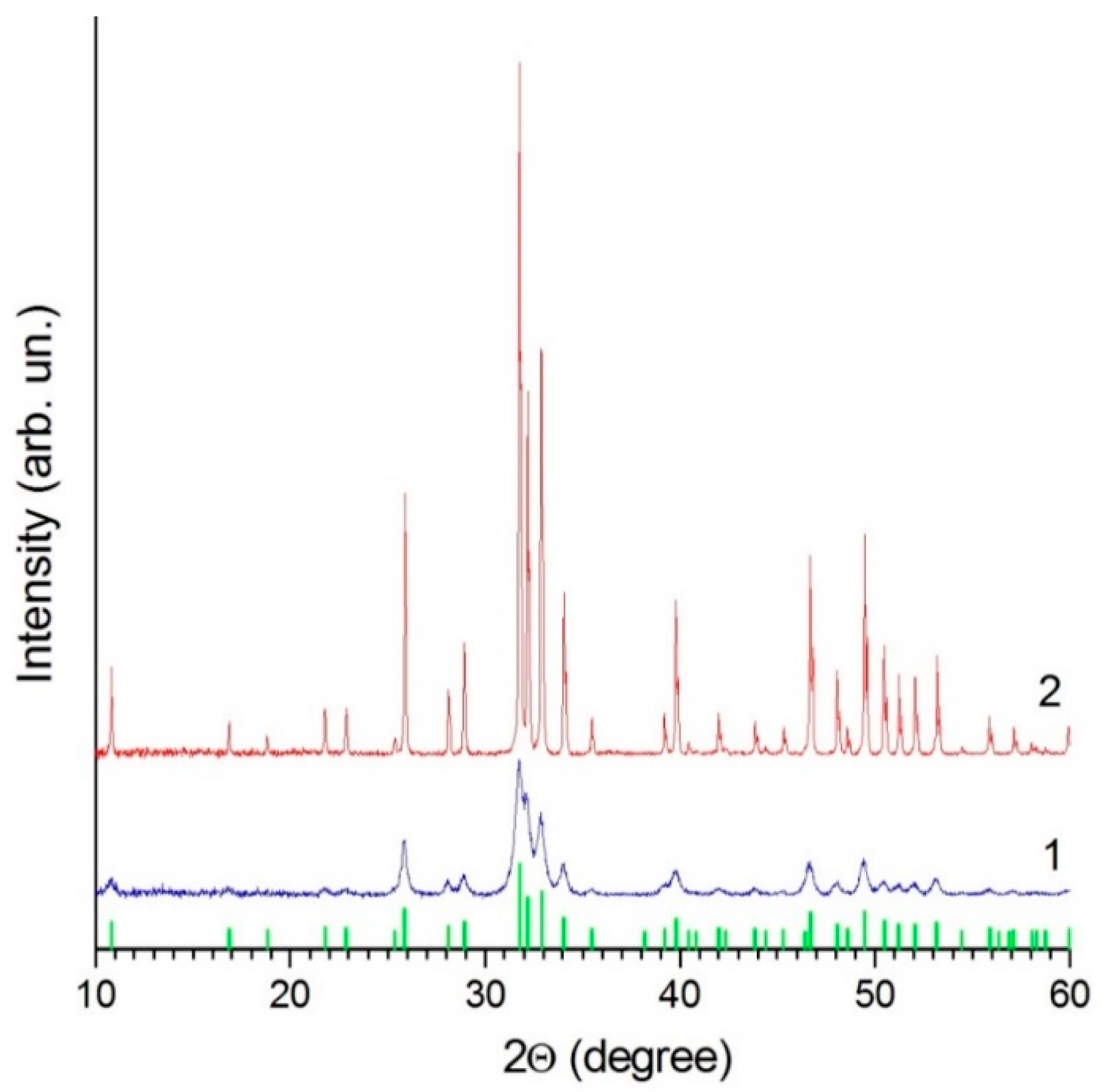
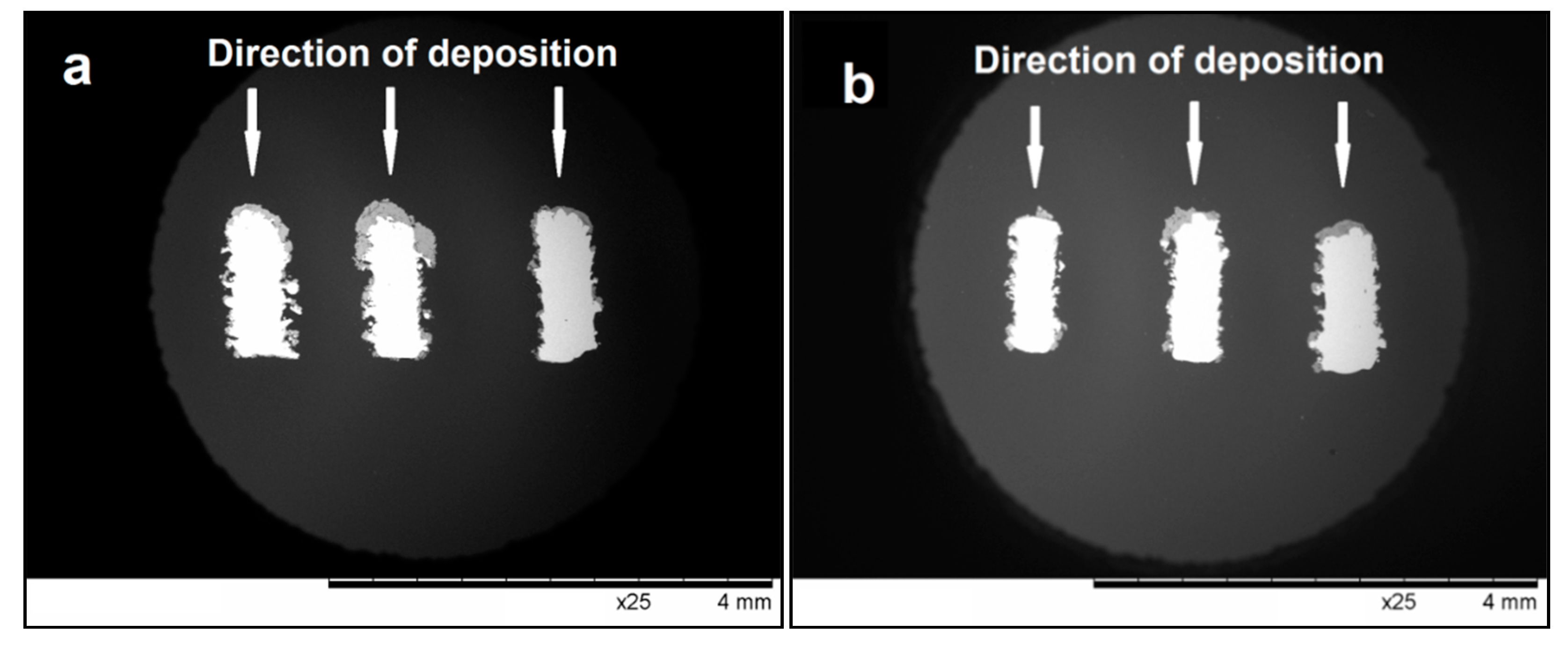

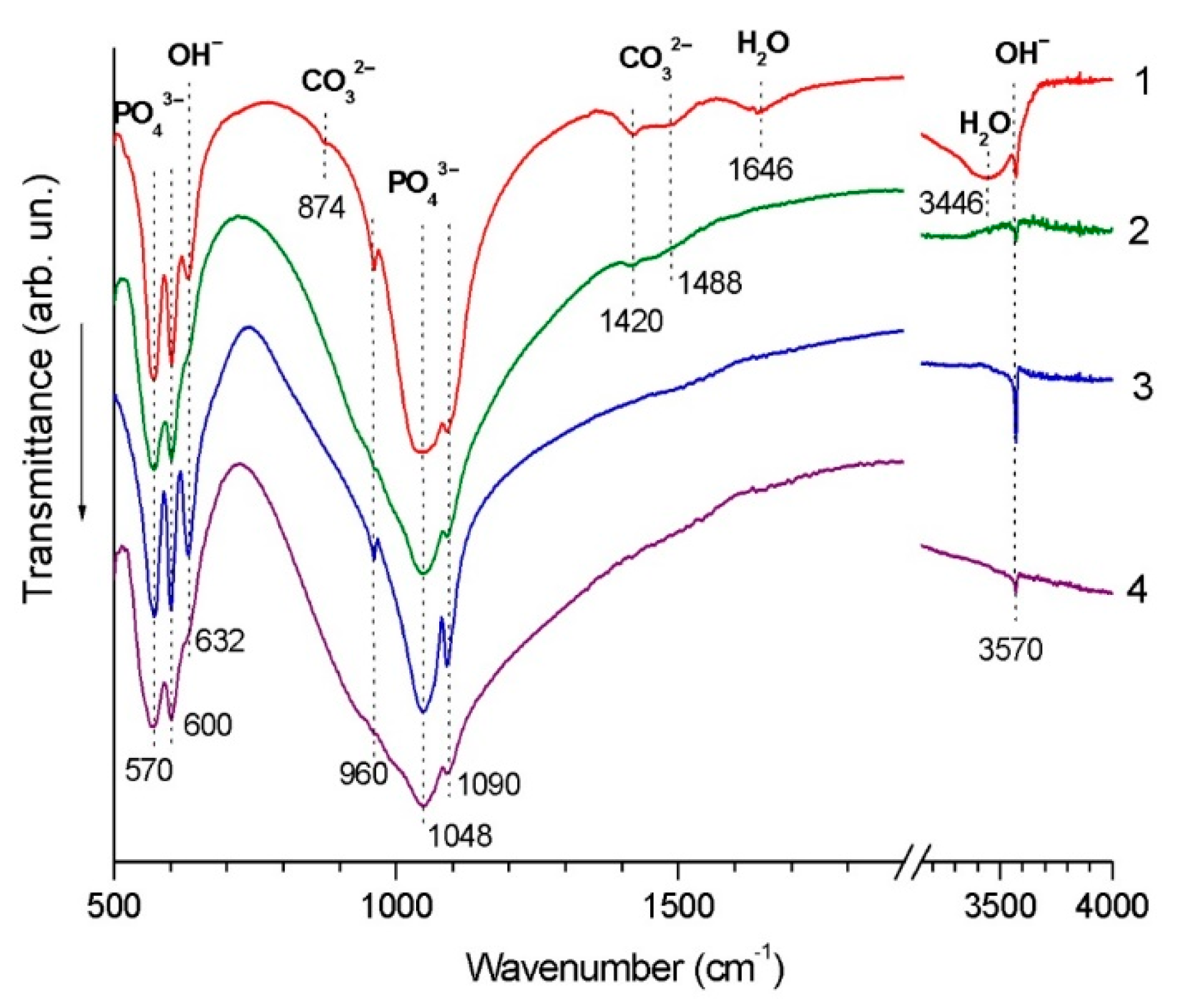
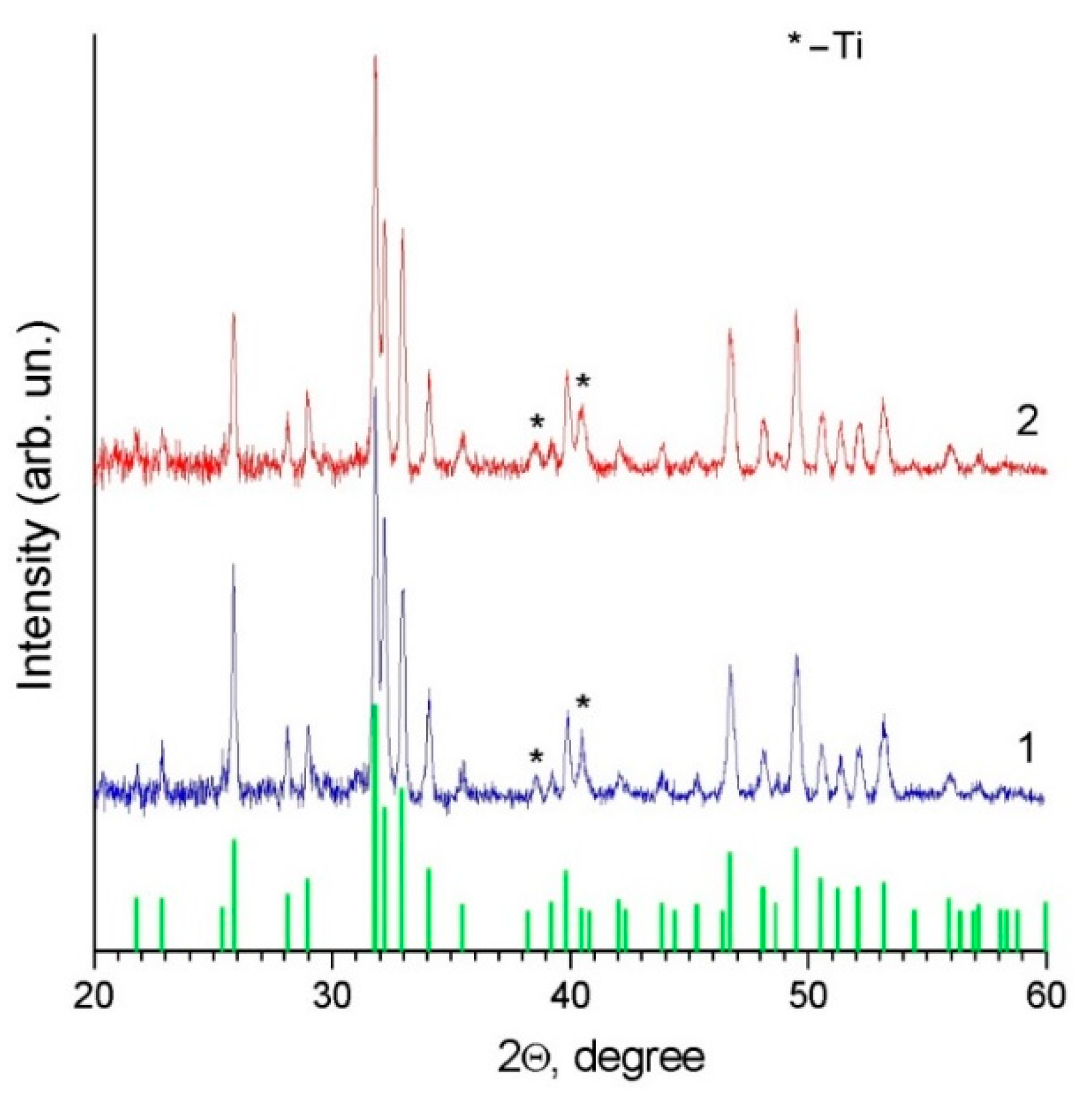
| Powder/Coating | a (Å) | c (Å) | Crystallite Size (nm) |
|---|---|---|---|
| nano-HA powder | 9.434 ± 0.001 | 6.8912 ± 0.0008 | 24.2 ± 0.04 |
| calc-HA powder | 9.4243 ± 0.0002 | 6.8816 ± 0.0002 | 234 ± 6 |
| coating from nano-HA powder | 9.410 ± 0.002 | 6.890 ± 0.002 | 64 ± 3 |
| coating from calc-HA powder | 9.411 ± 0.003 | 6.891 ± 0.002 | 66 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulina, N.V.; Rybin, D.K.; Makarova, S.V.; Dudina, D.V.; Batraev, I.S.; Utkin, A.V.; Prosanov, I.Y.; Khvostov, M.V.; Ulianitsky, V.Y. Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant. Materials 2021, 14, 4852. https://doi.org/10.3390/ma14174852
Bulina NV, Rybin DK, Makarova SV, Dudina DV, Batraev IS, Utkin AV, Prosanov IY, Khvostov MV, Ulianitsky VY. Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant. Materials. 2021; 14(17):4852. https://doi.org/10.3390/ma14174852
Chicago/Turabian StyleBulina, Natalia V., Denis K. Rybin, Svetlana V. Makarova, Dina V. Dudina, Igor S. Batraev, Alexey V. Utkin, Igor Yu. Prosanov, Mikhail V. Khvostov, and Vladimir Yu. Ulianitsky. 2021. "Detonation Spraying of Hydroxyapatite on a Titanium Alloy Implant" Materials 14, no. 17: 4852. https://doi.org/10.3390/ma14174852






