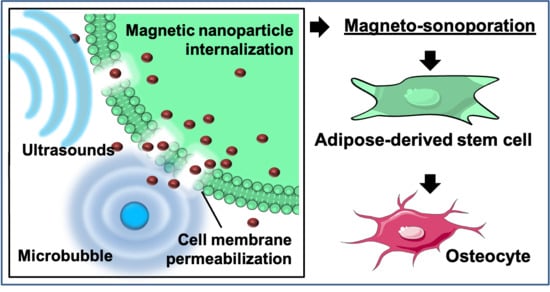
Rapid Magneto-Sonoporation of Adipose-Derived Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipose-Derived Stem Cells (ASC) Culture
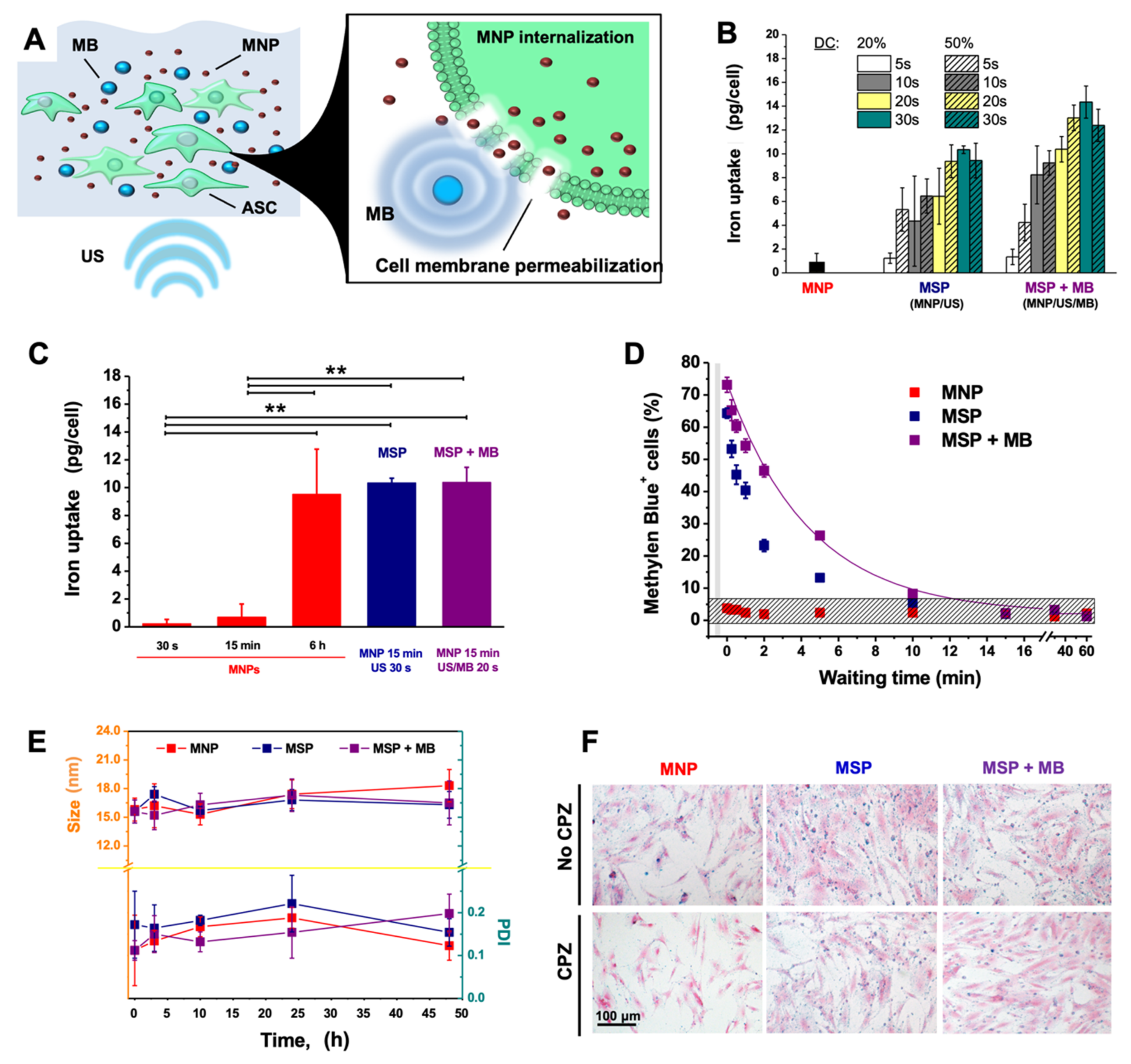
2.2. Magneto-Sonoporation
2.3. Iron Content
2.4. Stability of Nanoparticles
2.5. Prussian Blue Staining of Cells
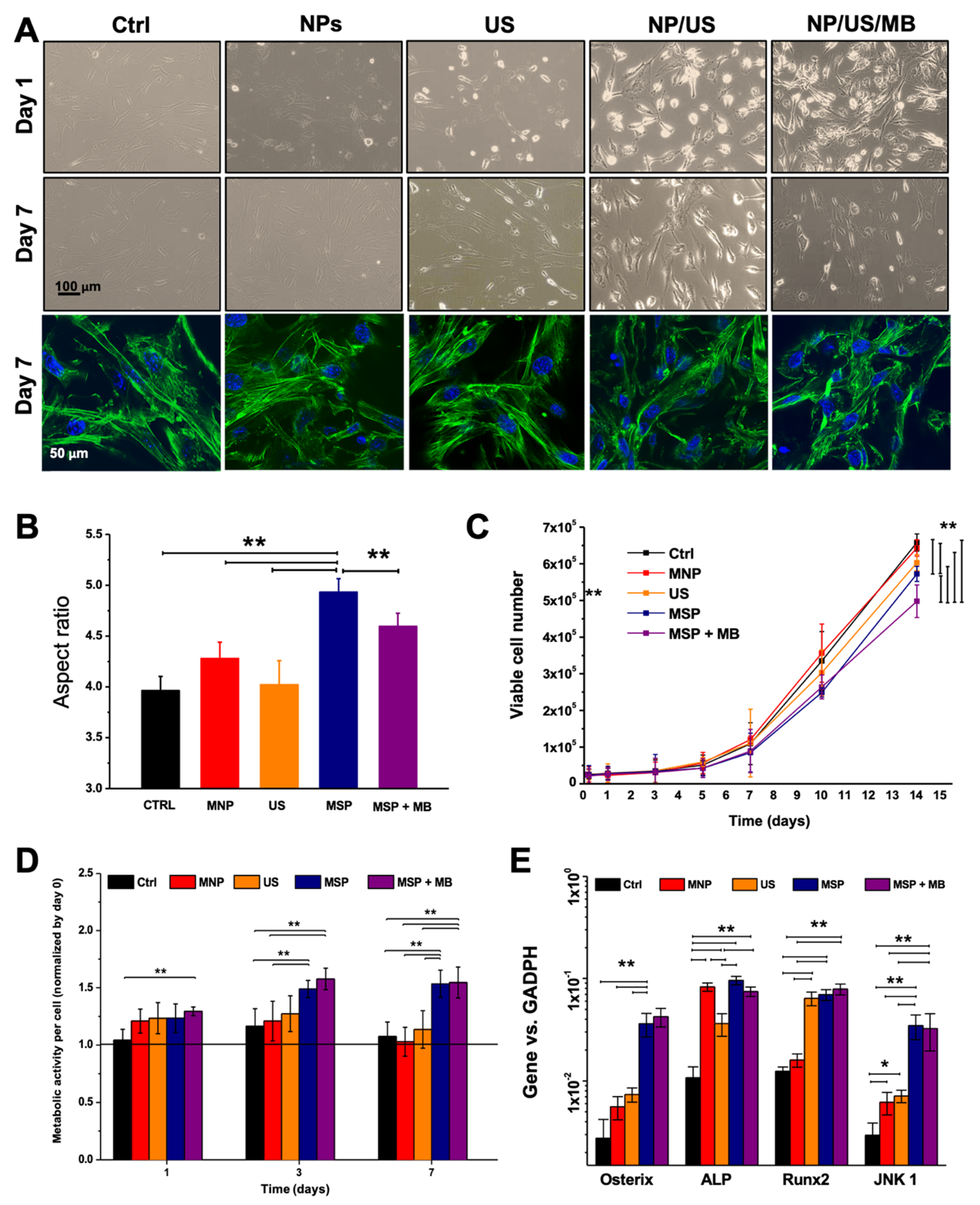
2.6. Cell Viability and Proliferation
2.7. Morphology Analysis
2.8. Evaluation of the Membrane Resealing Time
2.9. Gene Expression Analysis
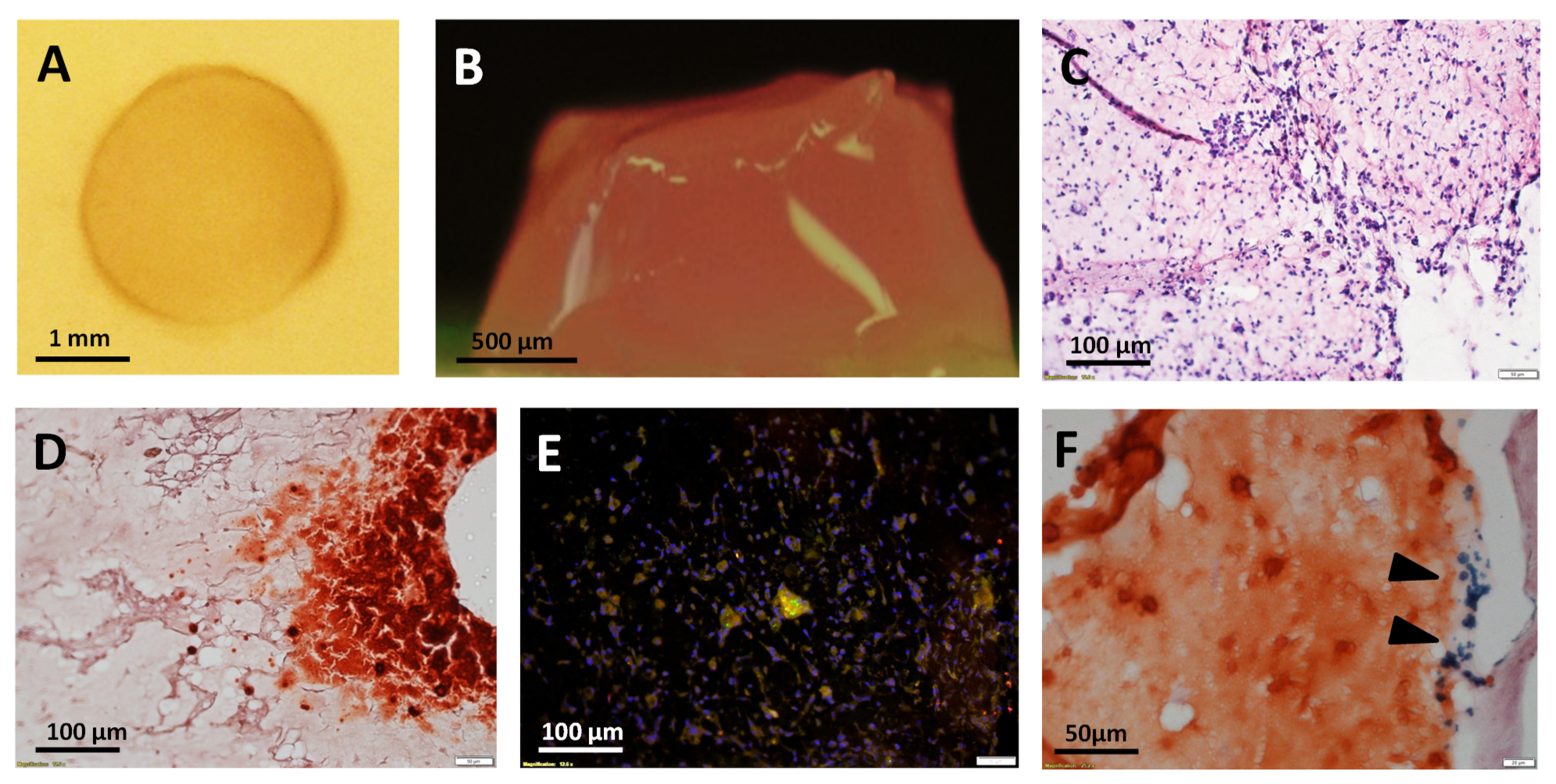
2.10. Magnetized Hydrogel Preparation
2.11. Histological Staining
3. Results
3.1. Magneto-Sonoporation (MSP) Optimization
3.2. Biological Effects of MSP
3.3. Ultrasound (US)-Activated 3D Matrices
4. Discussion
- i.
- a better definition of the mechanobiological activity of sonicated MNPs and the mechanisms triggering the biological alterations observed in the cells;
- ii.
- the influence of the biophysical properties of the specific MNP formulation on the MSP process;
- iii.
- the understanding of the interaction of US-mediated physical forces with human tissues and bodies;
- iv.
- the inherent optimization of the sonication setting.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: Present and future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- Ni, J.S.; Li, Y.; Yue, W.; Liu, B.; Li, K. Nanoparticle-based cell trackers for biomedical applications. Theranostics 2020, 10, 1923–1947. [Google Scholar] [CrossRef]
- Farzamfar, S.; Nazeri, N.; Salehi, M.; Valizadeh, A.; Marashi, S.M.; Savari Kouzehkonan, G.; Ghanbari, H. Will nanotechnology bring new hope for stem cell therapy? Cells Tissues Organs 2018, 206, 229. [Google Scholar] [CrossRef]
- Sun, J.H.; Zhang, Y.L.; Qian, S.P.; Yu, X.B.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Assessment of biological characteristics of mesenchymal stem cells labeled with superparamagnetic iron oxide particles in vitro. Mol. Med. Rep. 2012, 5, 317. [Google Scholar]
- Nakamae, T.; Adachi, N.; Kobayashi, T.; Nagata, Y.; Nakasa, T.; Tanaka, N.; Ochi, M. The effect of an external magnetic force on cell adhesion and proliferation of magnetically labeled mesenchymal stem cells. Sports Med. Arthrosc. Rehabil. Technol. 2010, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Farrell, E.; Wielopolski, P.; Pavljasevic, P.; van Tiel, S.; Jahr, H.; Verhaar, J.; Weinans, H.; Krestin, G.; O’Brien, F.J.; van Osch, G.; et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2008, 369, 1076. [Google Scholar] [CrossRef]
- Huang, D.M.; Hsiao, J.K.; Chen, Y.C.; Chien, L.Y.; Yao, M.; Chen, Y.K.; Ko, B.S.; Hsu, S.C.; Tai, L.A.; Cheng, H.Y.; et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Zhang, S.; Kundu, P.; Yeung, A.C.; Robbins, R.C.; Yang, P.C. In vitro comparison of the biological effects of three transfection methods for magnetically labeling mouse embryonic stem cells with ferumoxides. Magn. Reson. Med. 2007, 57, 1173–1179. [Google Scholar] [CrossRef]
- Hoehn, M.; Kustermann, E.; Blunk, J.; Wiedermann, D.; Trapp, T.; Wecker, S.; Focking, M.; Arnold, H.; Hescheler, J.; Fleischmann, B.K.; et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. USA 2002, 99, 16267–16272. [Google Scholar] [CrossRef] [Green Version]
- Daldrup-Link, H.E.; Meier, R.; Rudelius, M.; Piontek, G.; Piert, M.; Metz, S.; Settles, M.; Uherek, C.; Wels, W.; Schlegel, J.; et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur. Radiol. 2005, 15, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Arbab, A.S.; Yocum, G.T.; Kalish, H.; Jordan, E.K.; Anderson, S.A.; Khakoo, A.Y.; Read, E.J.; Frank, J.A. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 2004, 104, 1217–1223. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Rudelius, M.; Oostendorp, R.A.; Settles, M.; Piontek, G.; Metz, S.; Rosenbrock, H.; Keller, U.; Heinzmann, U.; Rummeny, E.J.; et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology 2003, 228, 760–767. [Google Scholar] [CrossRef]
- Walczak, P.; Kedziorek, D.A.; Gilad, A.A.; Lin, S.; Bulte, J.W. Instant MR labeling of stem cells using magnetoelectroporation. Magn. Reson. Med. 2005, 54, 769–774. [Google Scholar] [CrossRef]
- Tai, J.H.; Foster, P.; Rosales, A.; Feng, B.; Hasilo, C.; Martinez, V.; Ramadan, S.; Snir, J.; Melling, C.W.; Dhanvantari, S.; et al. Imaging islets labeled with magnetic nanoparticles at 1.5 tesla. Diabetes 2006, 55, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Gilad, A.A.; Walczak, P.; McMahon, M.T.; Na, H.B.; Lee, J.H.; An, K.; Hyeon, T.; van Zijl, P.C.; Bulte, J.W. MR tracking of transplanted cells with “positive contrast” using manganese oxide nanoparticles. Magn. Reson. Med. 2008, 60, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellott, A.J.; Forrest, M.L.; Detamore, M.S. Physical non-viral gene delivery methods for tissue engineering. Ann. Biomed. Eng. 2013, 41, 446–468. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wang, J.; Zhou, Q.; Zhang, L.; Wang, S.; Zhang, Z.; Yao, C. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018, 25, 1516–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delalande, A.; Postema, M.; Mignet, N.; Midoux, P.; Pichon, C. Ultrasound and microbubble-assisted gene delivery: Recent advances and ongoing challenges. Ther. Deliv. 2012, 3, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Bouakaz, A.; Zeghimi, A.; Doinikov, A.A. Sonoporation: Concept and mechanisms. Adv. Exp. Med. Biol. 2016, 880, 175–189. [Google Scholar]
- Sun, R.R.; Noble, M.L.; Sun, S.S.; Song, S.; Miao, C.H. Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery. J. Control. Release 2014, 182, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Delalande, A.; Leduc, C.; Midoux, P.; Postema, M.; Pichon, C. Efficient gene delivery by sonoporation is associated with microbubble entry into cells and the clathrin-dependent endocytosis Pathway. Ultrasound Med. Biol. 2015, 41, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Nan, X.; Wang, Z.; Gao, L.; Xie, L.; Zou, C.; Wan, Q.; Pan, D.; Beauchamp, N.; Yang, X.; et al. Stem cell labeling with superparamagnetic iron oxide nanoparticles using focused ultrasound and magnetic resonance imaging tracking. J. Nanosci. Nanotechnol. 2015, 15, 2605–2612. [Google Scholar] [CrossRef]
- Qiu, B.; Xie, D.; Walczak, P.; Li, X.; Ruiz-Cabello, J.; Minoshima, S.; Bulte, J.W.; Yang, X. Magnetosonoporation: Instant magnetic labeling of stem cells. Magn. Reson. Med. 2010, 63, 1437–1441. [Google Scholar] [CrossRef]
- Qin, P.; Xu, L.; Han, T.; Du, L.; Yu, A.C. Effect of non-acoustic parameters on heterogeneous sonoporation mediated by single-pulse ultrasound and microbubbles. Ultrason. Sonochem. 2016, 31, 107–115. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.C.; Moonen, C.T. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, Y.; Cai, C.; Tu, J.; Guo, X.; Zhang, D. Sonoporation-induced cell membrane permeabilization and cytoskeleton disassembly at varied acoustic and microbubble-cell parameters. Sci. Rep. 2018, 8, 3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [Green Version]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; Ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-responsive nanocarriers in cancer treatment: A review. ACS Pharm. Transl. Sci. 2021, 4, 589–612. [Google Scholar] [CrossRef]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef]
- Qiu, B.; Yang, X. Molecular MRI of hematopoietic stem-progenitor cells: In vivo monitoring of gene therapy and atherosclerosis. Nat. Clin. Pr. Cardiovasc. Med. 2008, 5, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Lin, S.; Wang, G.; Wang, Y.; Wu, E.X. Preliminary in vitro study of ultrasound sonoporation cell labeling with superparamagnetic iron oxide particles for MRI cell tracking. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 367–370. [Google Scholar]
- Wang, Y.X.; Leung, K.C.; Cheung, W.H.; Wang, H.H.; Shi, L.; Wang, D.F.; Qin, L.; Ahuja, A.T. Low-intensity pulsed ultrasound increases cellular uptake of superparamagnetic iron oxide nanomaterial: Results from human osteosarcoma cell line U2OS. J. Magn. Reson. Imaging 2010, 31, 1508–1513. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-derived stem cells: Current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Farinazzo, A.; Turano, E.; Marconi, S.; Bistaffa, E.; Bazzoli, E.; Bonetti, B. Murine adipose-derived mesenchymal stromal cell vesicles: In vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy 2015, 17, 571–578. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Schaakxs, D.; Kingham, P.J.; Wiberg, M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011, 344, 251–260. [Google Scholar] [CrossRef]
- Erba, P.; Terenghi, G.; Kingham, P.J. Neural differentiation and therapeutic potential of adipose tissue derived stem cells. Curr. Stem. Cell Res. Ther. 2010, 5, 153–160. [Google Scholar] [CrossRef]
- Mantovani, C.; Terenghi, G.; Shawcross, S.G. Isolation of adult stem cells and their differentiation to Schwann cells. Methods Mol. Biol. 2012, 916, 47–57. [Google Scholar]
- Faroni, A.; Terenghi, G.; Reid, A.J. Adipose-derived stem cells and nerve regeneration: Promises and pitfalls. Int. Rev. Neurobiol. 2013, 108, 121–136. [Google Scholar]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef]
- Kappy, N.S.; Chang, S.; Harris, W.M.; Plastini, M.; Ortiz, T.; Zhang, P.; Hazelton, J.P.; Carpenter, J.P.; Brown, S.A. Human adipose-derived stem cell treatment modulates cellular protection in both in vitro and in vivo traumatic brain injury models. J. Trauma Acute Care Sur 2018, 84, 745–751. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Cangemi, G.C.; Kruzliak, P.; Corazza, G.R. Concise review: Cellular therapies: The potential to regenerate and restore tolerance in immune-mediated intestinal diseases. Stem Cells 2016, 34, 1474–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. 2010, 5, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Tomás, A.R.; Gonçalves, A.I.; Paz, E.; Freitas, P.; Domingues, R.M.A.; Gomes, M.E. Magneto-mechanical actuation of magnetic responsive fibrous scaffolds boosts tenogenesis of human adipose stem cells. Nanoscale 2019, 11, 18255–18271. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef]
- Labusca, L.; Herea, D.D.; Danceanu, C.M.; Minuti, A.E.; Stavila, C.; Grigoras, M.; Gherca, D.; Stoian, G.; Ababei, G.; Chiriac, H.; et al. The effect of magnetic field exposure on differentiation of magnetite nanoparticle-loaded adipose-derived stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110652. [Google Scholar] [CrossRef]
- Papadimitropoulos, A.; Scherberich, A.; Güven, S.; Theilgaard, N.; Crooijmans, H.J.; Santini, F.; Scheffler, K.; Zallone, A.; Martin, I. A 3D in vitro bone organ model using human progenitor cells. Eur. Cell Mater. 2011, 21, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Pigeot, S.; Müller, J.; Schaefer, D.J.; Martin, I.; Scherberich, A. Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification. Acta Biomater. 2018, 77, 142–154. [Google Scholar] [CrossRef]
- Kim, S.J.; Lewis, B.; Steiner, M.S.; Bissa, U.V.; Dose, C.; Frank, J.A. Superparamagnetic iron oxide nanoparticles for direct labeling of stem cells and in vivo MRI tracking. Contrast Media Mol. Imaging 2016, 11, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Schugar, H.J.; Rossman, G.R.; Thibeault, J.; Gray, H.B. Simultaneous pair electronic excitations in a binuclear iron(III) complex. Chem. Phys. Lett. 1970, 6, 26–28. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for biomedical applications: Production, characterisations, recent trends and difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Control. Release 2021, 332, 460–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Im, G.B.; Kim, Y.J.; Kim, Y.H.; Lee, T.J.; Bhang, S.H. Bio-application of inorganic nanomaterials in tissue engineering. Adv. Exp. Med. Biol. 2020, 1249, 115–130. [Google Scholar]
- Clasky, A.J.; Watchorn, J.D.; Chen, P.Z.; Gu, F.X. From prevention to diagnosis and treatment: Biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomater. 2021, 122, 1–25. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Liu, W.; Chen, X.; Song, Z.; Wang, X.; Deng, Y.; Tang, X.; Jiang, Z. The applications of magnetic particle imaging: From cell to body. Diagnostics 2020, 10, 800. [Google Scholar] [CrossRef]
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as novel cardiovascular theranostics. Pharmaceutics 2021, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Siemer, S.; Wünsch, D.; Khamis, A.; Lu, Q.; Scherberich, A.; Filippi, M.; Krafft, M.P.; Hagemann, J.; Weiss, C.; Ding, G.B.; et al. Nano meets micro-translational nanotechnology in medicine: Nano-based applications for early tumor detection and therapy. Nanomaterials 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanzadeh, P. The biomedical significance of multifunctional nanobiomaterials: The key components for site-specific delivery of therapeutics. Life Sci. 2021, 277, 119400. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Felder-Flesch, D.; Scherberich, A. Use of nanoparticles in skeletal tissue regeneration and engineering. Histol. Histopathol. 2020, 35, 331–350. [Google Scholar] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Hajalilou, A.; Ahsanul, K. Synthesis, characterization, and cytotoxicity of iron oxide nanoparticles. Adv. Mater. Sci. Eng. 2013, 2013, 710432. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V.; et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part. Fibre Toxicol. 2009, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Videla, L.A.; Fernández, V.; Tapia, G.; Varela, P. Oxidative stress-mediated hepatotoxicity of iron and copper: Role of Kupffer cells. Biometals 2003, 16, 103–111. [Google Scholar] [CrossRef]
- Wu, X.; Tan, Y.; Mao, H.; Zhang, M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int. J. Nanomed. 2010, 5, 385–399. [Google Scholar] [CrossRef] [Green Version]
- Nejadnik, H.; Jung, K.O.; Theruvath, A.J.; Kiru, L.; Liu, A.; Wu, W.; Sulchek, T.; Pratx, G.; Daldrup-Link, H.E. Instant labeling of therapeutic cells for multimodality imaging. Theranostics 2020, 10, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Pislaru, S.V.; Greenleaf, J.E. Sonoporation: Mechanical DNA delivery by ultrasonic cavitation. Somat. Cell Mol. Genet. 2002, 27, 115–134. [Google Scholar] [CrossRef]
- Nomikou, N.; Feichtinger, G.A.; Saha, S.; Nuernberger, S.; Heimel, P.; Redl, H.; McHale, A.P. Ultrasound-responsive gene-activated matrices for osteogenic gene therapy using matrix-assisted sonoporation. J. Tissue Eng. Regen. Med. 2018, 12, e250–e260. [Google Scholar] [CrossRef]
- Nomikou, N.; Feichtinger, G.A.; Redl, H.; McHale, A.P. Ultrasound-mediated gene transfer (sonoporation) in fibrin-based matrices: Potential for use in tissue regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 29–39. [Google Scholar] [CrossRef]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schuetzenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; van Griensven, M.; et al. Sonoporation increases therapeutic efficacy of inducible and constitutive BMP2/7 in vivo gene delivery. Hum. Gene Methods 2014, 25, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Kaipel, M.; Schützenberger, S.; Hofmann, A.T.; Ferguson, J.; Nau, T.; Redl, H.; Feichtinger, G.A. Evaluation of fibrin-based gene-activated matrices for BMP2/7 plasmid codelivery in a rat nonunion model. Int. Orthop. 2014, 38, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.; Atluri, K.; Geary, S.M.; Hong, L.; Elangovan, S.; Salem, A.K. Bone regeneration using gene-activated matrices. AAPS J. 2017, 19, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, M.; Dasen, B.; Scherberich, A. Rapid Magneto-Sonoporation of Adipose-Derived Cells. Materials 2021, 14, 4877. https://doi.org/10.3390/ma14174877
Filippi M, Dasen B, Scherberich A. Rapid Magneto-Sonoporation of Adipose-Derived Cells. Materials. 2021; 14(17):4877. https://doi.org/10.3390/ma14174877
Chicago/Turabian StyleFilippi, Miriam, Boris Dasen, and Arnaud Scherberich. 2021. "Rapid Magneto-Sonoporation of Adipose-Derived Cells" Materials 14, no. 17: 4877. https://doi.org/10.3390/ma14174877
APA StyleFilippi, M., Dasen, B., & Scherberich, A. (2021). Rapid Magneto-Sonoporation of Adipose-Derived Cells. Materials, 14(17), 4877. https://doi.org/10.3390/ma14174877