Behaviour of Microwave-Heated Al4SiC4 at 2.45 GHz
Abstract
:1. Introduction
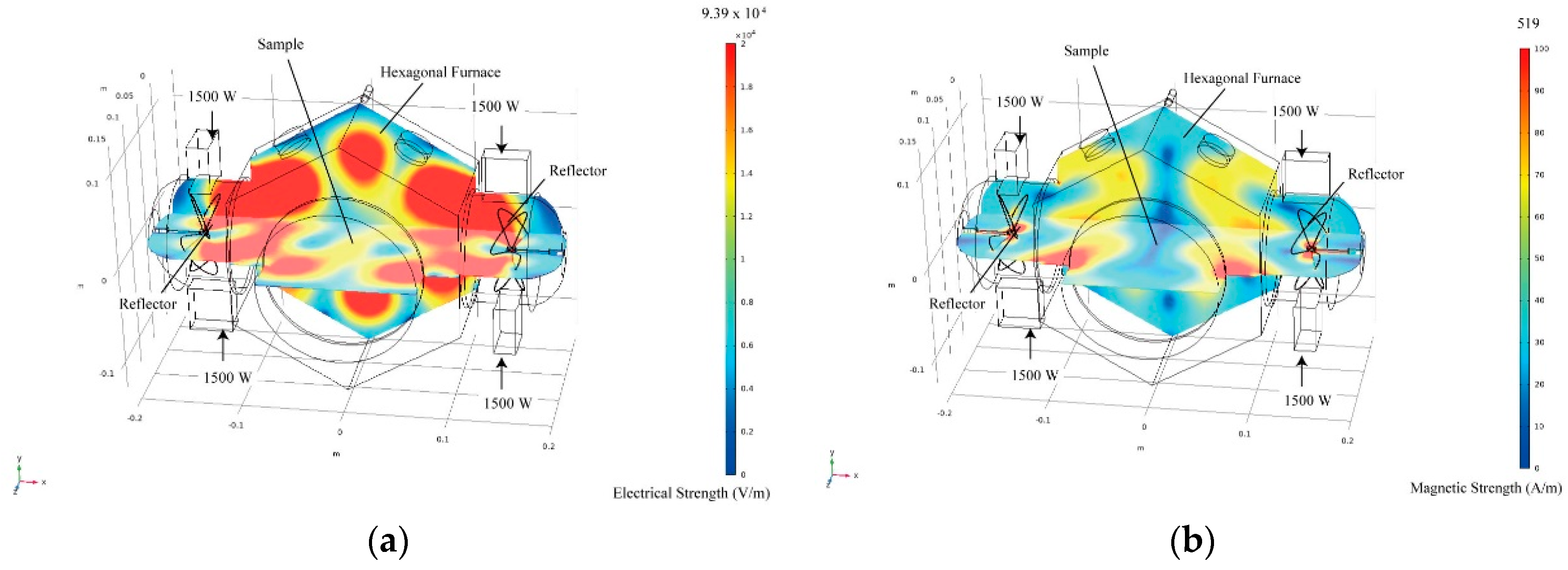
2. Materials and Methods
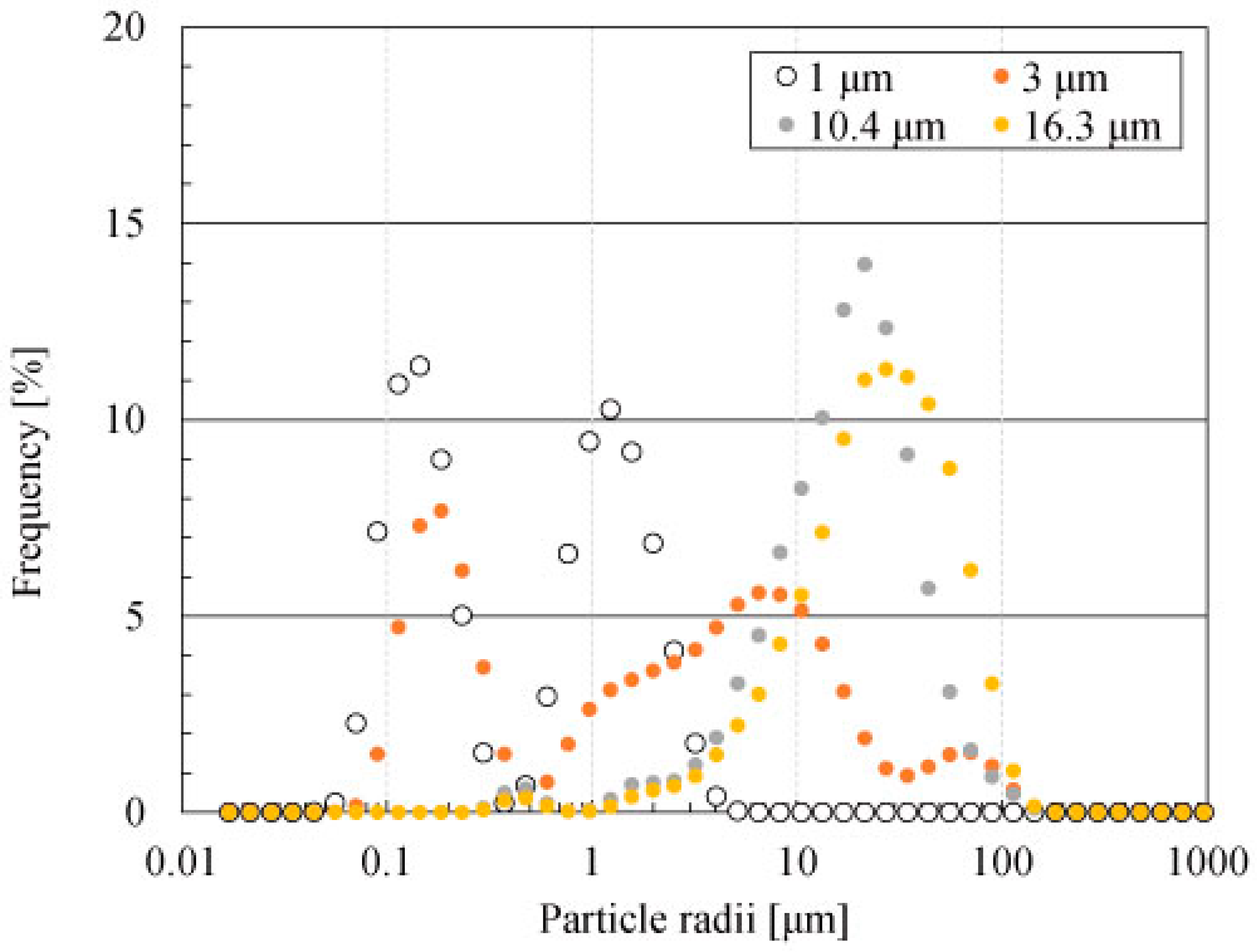
Heating Behaviour and Electrical Permittivity of Al4SiC4 Powders
3. Results and Discussion
4. Conclusions
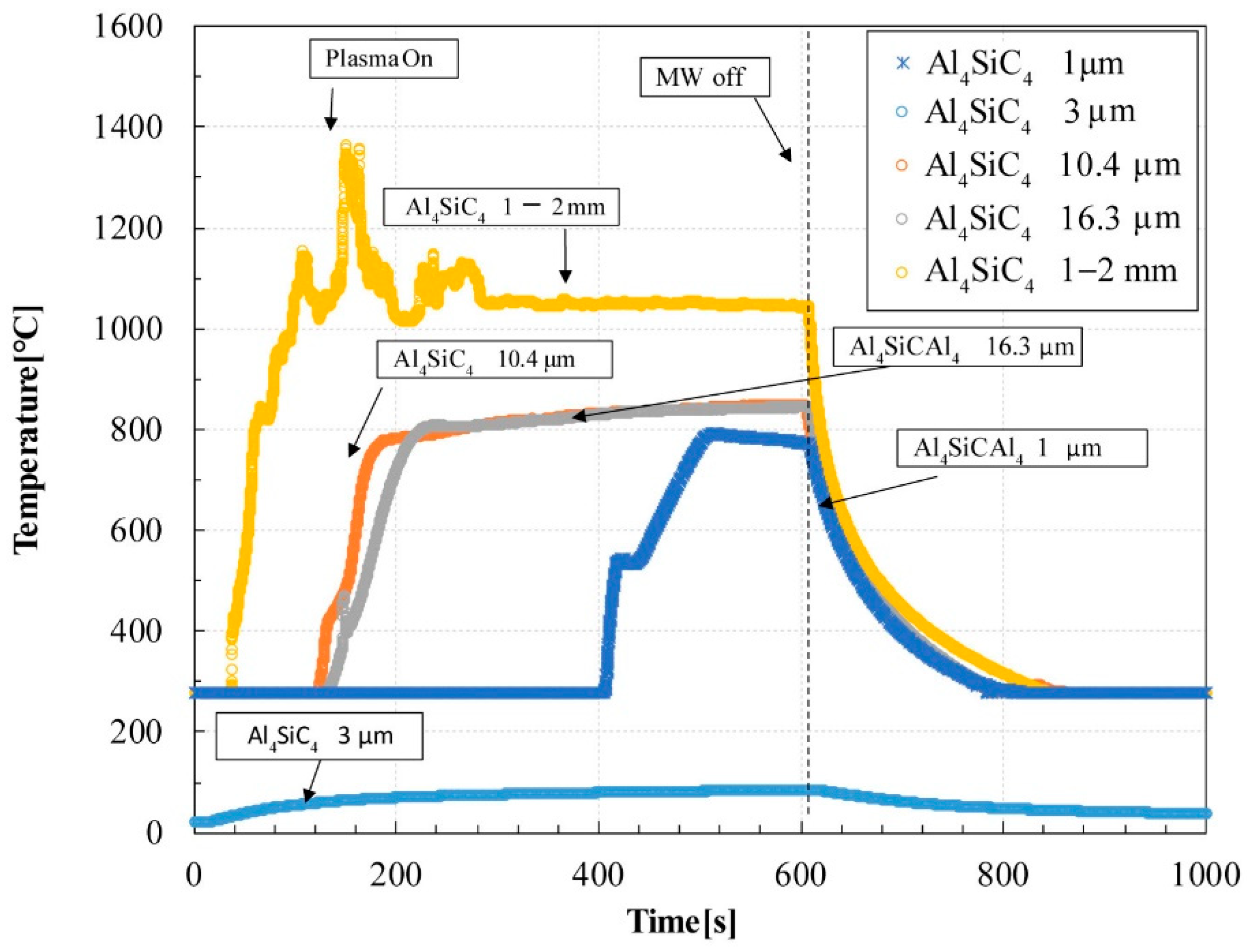
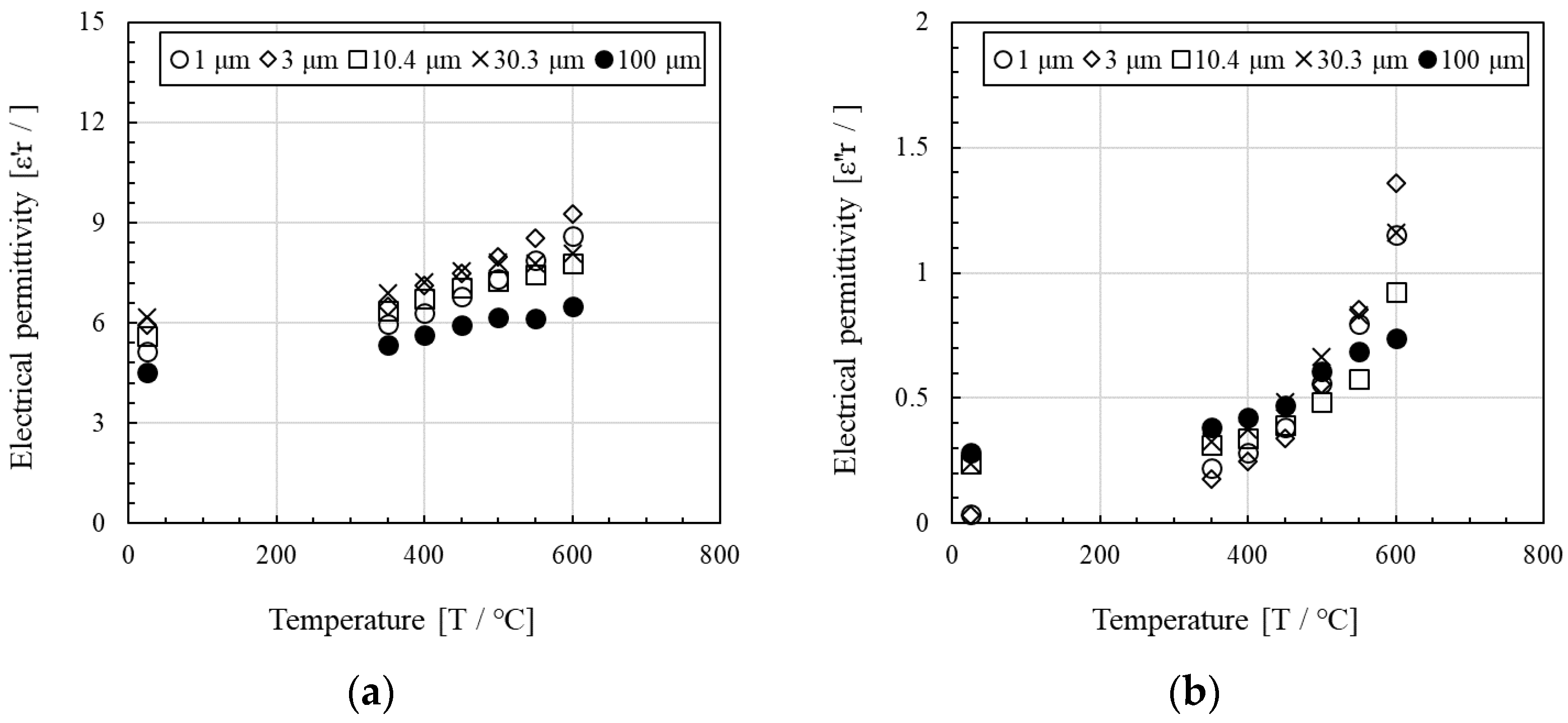
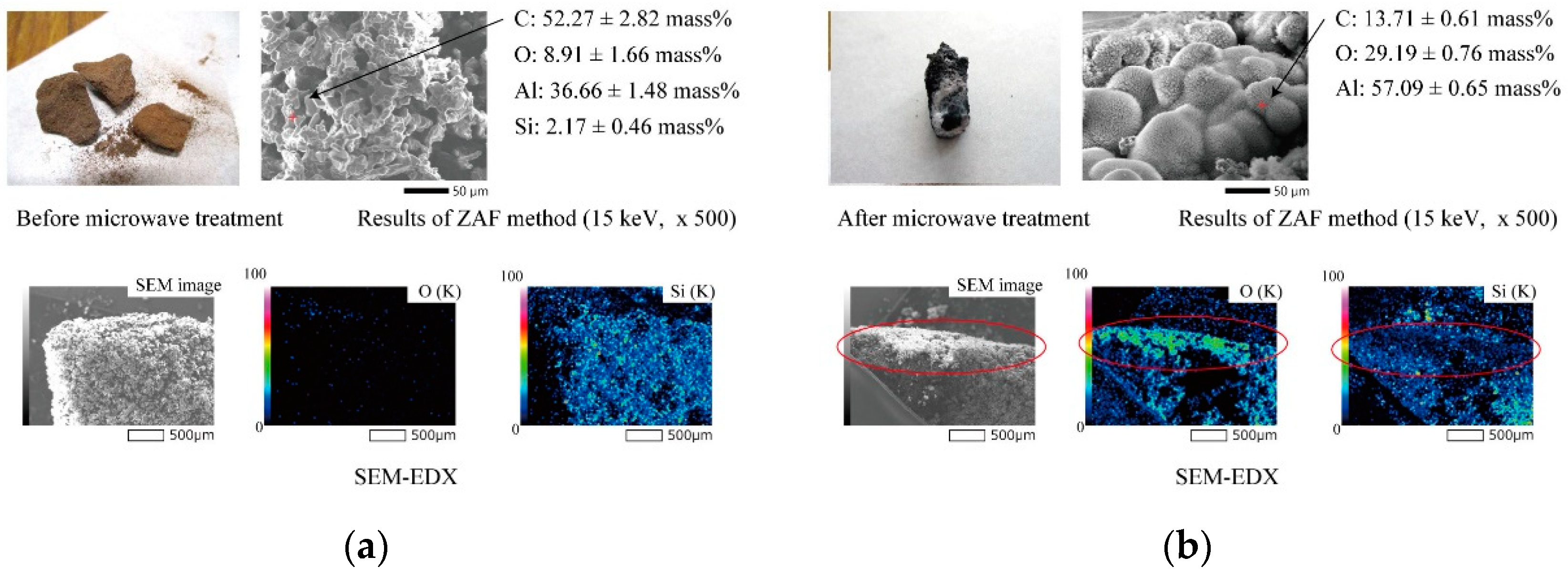
- The Al4SiC4 powders were adequately heated by microwaves. The imaginary parts of their electrical permittivity yielded values in the range of 0.032–0.240. For Al4SiC4 particle sizes in the range of 3 μm–2 mm, larger particles led to higher maximum temperatures during microwave heating and higher imaginary parts of the electrical permittivity.
- In the particle size range of 1 μm–2 mm, the Al4SiC4 powders were adequately heated by microwaves as the particle size increased. This tendency was also consistent with the complex permittivity of the Al4SiC4.
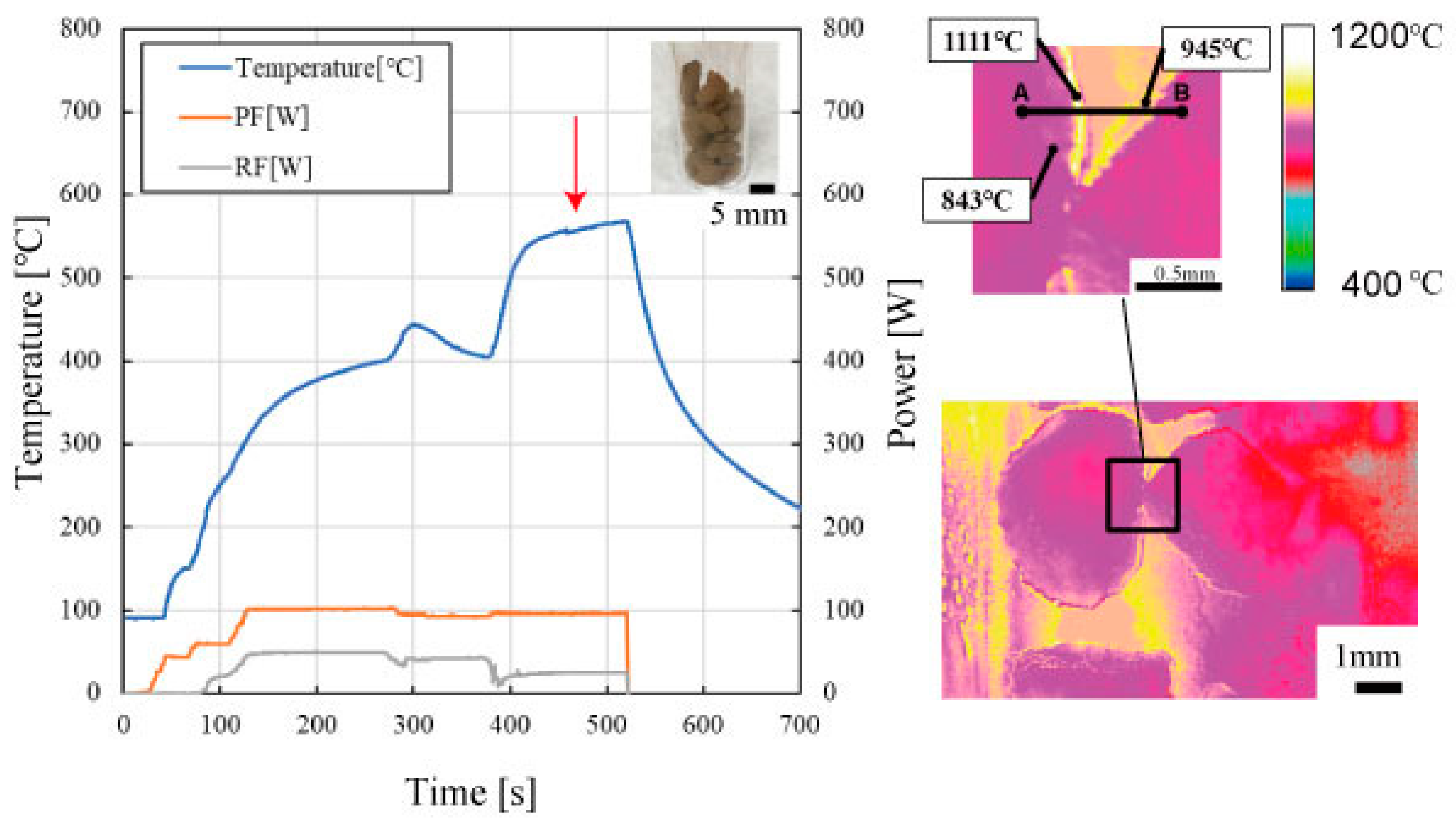
- According to measurements by the resonance perturbation method, the higher the temperature of the Al4SiC4 powders (from 25 °C to 600 °C), the higher the real and imaginary parts of their electrical permittivities.
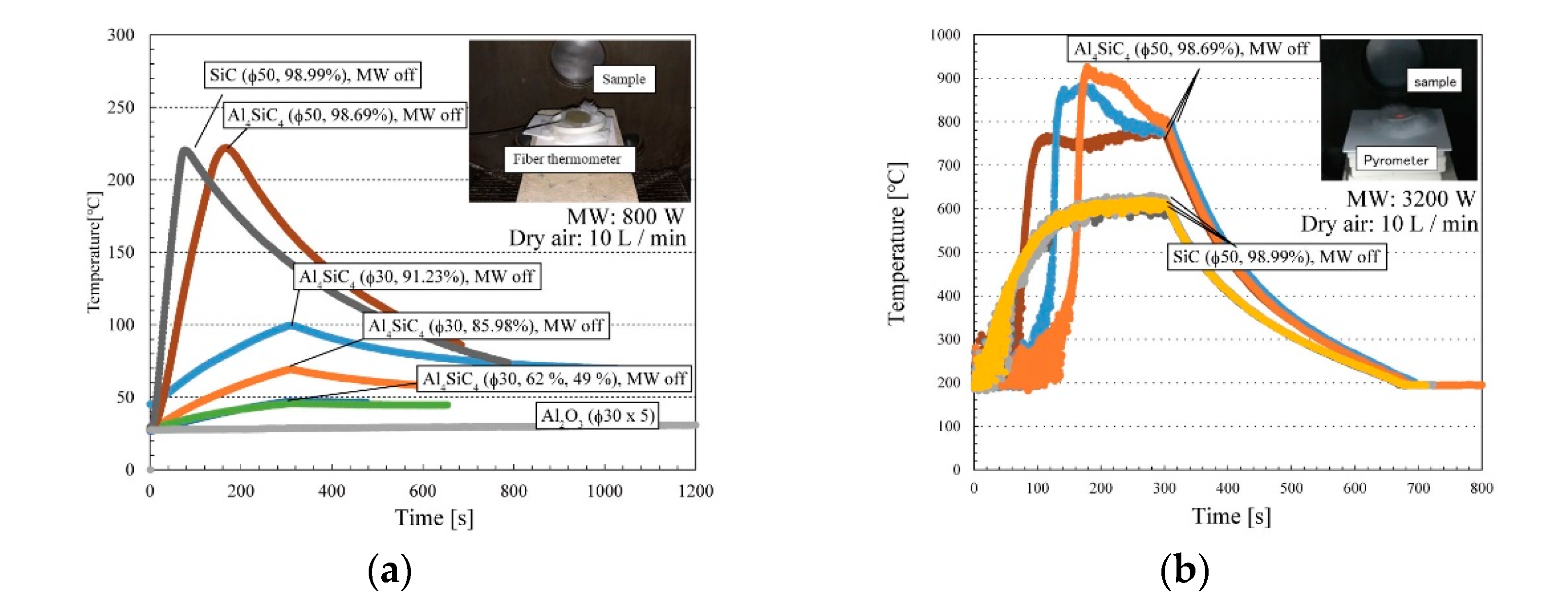
- The sintered Al4SiC4 (∅50 mm × 5 mm) disk could be heated more effectively with microwaves than a sintered SiC disk of the same size. This tendency was presumed to become more pronounced with the increasing size of the sintered body and the rising temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full Sintering of Powdered-Metal Bodies in a Microwave Field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Ishizaki, K.; Nagata, K. Selectivity of Microwave Energy Consumption in the Reduction of Fe3O4 with Carbon Black in Mixed Powder. ISIJ Int. 2007, 47, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Huang, X. Novel Direct Steelmaking by Combining Microwave, Electric Arc and Exothermal Heating Technologies; United States Department of Energy: Washington, DC, USA, 2005; Available online: https://pdfs.semanticscholar.org/49f7/e09a002d967e9741e426b1c3ed49130d2a9b.pdf (accessed on 15 August 2021).
- Amini, A.; Ohno, K.I.; Maeda, T.; Kunitomo, K. Effect of the Ratio of Magnetite Particle Size to Microwave Penetration Depth on Reduction Reaction Behaviour by H2. Sci. Rep. 2018, 8, 15023. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Hayashi, M.; Sato, M.; Nagata, K. Pig Iron Making by Focused Microwave Beams with 20 kW at 2.45 GHz. ISIJ Int. 2012, 52, 2149–2157. [Google Scholar] [CrossRef] [Green Version]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave Thermal Inertisation of Asbestos Containing Waste and Its Recycling in Traditional Ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, D.N.; Leonelli, C.; Rivasi, M.R.; Romagnoli, M.; Veronesi, P.; Pellacani, G.C.; Boccaccini, A.R. Recycling of Microwave Inertised Asbestos Containing Waste in Refractory Materials. J. Eur. Ceram. Soc. 2007, 27, 1855–1858. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Kashimura, K.; Hashiguchi, M.; Sato, M.; Horikoshi, S.; Mitani, T.; Shinohara, N. Detoxification Mechanism of Asbestos Materials by Microwave Treatment. J. Hazard. Mater. 2015, 284, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Clausell, C.; Felíu, C.; Monzó, M.; Nuño, L.; Heras, D.; Balbastre, J.V. Study of NiZn Ferrite Complex Permeability: Effect of Relative Density and Microstructure. J. Am. Ceram. Soc. 2004, 87, 1314–1318. [Google Scholar] [CrossRef]
- Yadoji, P.; Peelamedu, R.; Agrawal, D.; Roy, R. Microwave Sintering of Ni–Zn Ferrites: Comparison with Conventional Sintering. Mater. Sci. Eng. B 2003, 98, 269–278. [Google Scholar] [CrossRef]
- JFE Chemical Corporation. Manufacturing Method of NiCuZn Ferrite Powders for Microwave Well Absorber. Japanese Patent P5912994, 27 April 2016.
- Xi, W.; Tinga, W.R. Microwave: Theory and application in materials processing. Ceram. Trans. 1992, 21, 215. [Google Scholar]
- Sugawara, H.; Kashimura, K.; Hayashi, M.; Ishihara, S.; Mitani, T.; Shinohara, N. Behavior of Microwave-Heated Silicon Carbide Particles at Frequencies of 2.0–13.5 GHz. Appl. Phys. Lett. 2014, 105, 034103. [Google Scholar] [CrossRef]
- Inoue, K.; Mori, S.; Yamaguchi, A. Thermal Conductivity and Temperature Dependence of Linear Thermal Expansion Coefficient of Al4SiC4 Sintered Bodies Prepared by Pulse Electronic Current Sintering. J. Ceram. Soc. Jpn. 2003, 111, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, O.; Ohtani, M.; Sasamoto, T. Preparation and Oxidation of Al4SiC4. J. Mater. Res. 2002, 17, 774–778. [Google Scholar] [CrossRef]
- Wen, G.W.; Huang, X.X. Increased High Temperature Strength and Oxidation Resistance of Al4SiC4 Ceramics. J. Eur. Ceram. Soc. 2006, 26, 1281–1286. [Google Scholar] [CrossRef]
- Kashimura, K.; Hasegawa, N.; Suzuki, S.; Hayashi, M.; Mitani, T.; Nagata, K.; Shinohara, N. Effects of Relative Density on Microwave Heating of Various Carbon Powder Compacts Microwave-Metallic Multi-Particle Coupling Using Spatially Separated Magnetic Fields. J. Appl. Phys. 2013, 113, 024902. [Google Scholar] [CrossRef]
- Pickles, C.A.; Mouris, J.; Hutcheon, R.M. High-Temperature Dielectric Properties of Goethite from 400 to 3000 MHz. J. Mater. Res. 2005, 20, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Hwang, J.Y.; Mouris, J.; Hutcheon, R.; Huang, X. Microwave Penetration Depth in Materials with Non-Zero Magnetic Susceptibility. ISIJ Int. 2010, 50, 1590–1596. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Kawahara, K.; Yoshikawa, N.; Todoroki, H.; Taniguchi, S. Dehydration Behavior of Goethite Blended with Graphite by Microwave Heating. ISIJ Int. 2011, 51, 878–883. [Google Scholar] [CrossRef] [Green Version]
- Ignatenko, M.; Tanaka, M. Effective Permittivity and Permeability of Coated Metal Powders at Microwave Frequency. Physica B 2010, 405, 352–358. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, T.; Oshita, A.; Kashimura, K. Behaviour of Microwave-Heated Al4SiC4 at 2.45 GHz. Materials 2021, 14, 4878. https://doi.org/10.3390/ma14174878
Fujii T, Oshita A, Kashimura K. Behaviour of Microwave-Heated Al4SiC4 at 2.45 GHz. Materials. 2021; 14(17):4878. https://doi.org/10.3390/ma14174878
Chicago/Turabian StyleFujii, Takashi, Akio Oshita, and Keiichiro Kashimura. 2021. "Behaviour of Microwave-Heated Al4SiC4 at 2.45 GHz" Materials 14, no. 17: 4878. https://doi.org/10.3390/ma14174878




