Agar Acts as Cathode Microskin to Extend the Cycling Life of Zn//α-MnO2 Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.1.1. Preparation of α-MnO2
2.1.2. Modification of α-MnO2 Electrode by Agar Coating
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
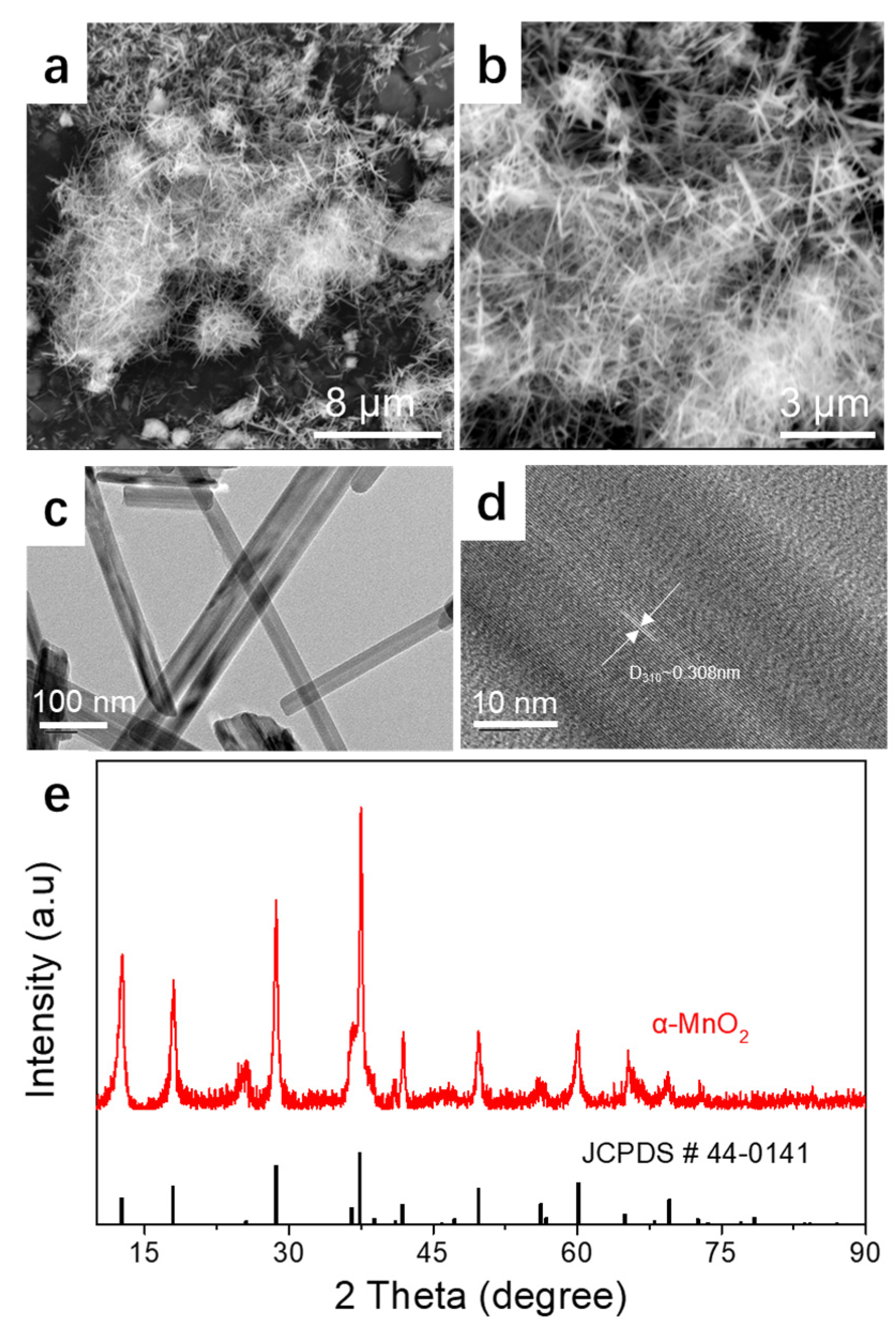
3.1. Characterization of the Physical Properties of the Prepared α-MnO2
3.2. Characterization of the Agar-Modified Cathodes
3.3. Electrochemical Performance of Zn//α-MnO2 Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.Q.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric vehicles batteries: Requirements and challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.E.; Xia, Y.; Gorji, M.B.; Wierzbicki, T. Data-driven safety envelope of lithium-ion batteries for electric vehicles. Joule 2019, 3, 2703–2715. [Google Scholar] [CrossRef]
- Chen, S.R.; Dai, F.; Cai, M. Opportunities and challenges of high-energy lithium metal batteries for electric vehicle applications. ACS Energy Lett. 2020, 5, 3140–3151. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous-electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Wang, G.J.; Zhao, N.H.; Yang, L.C.; Wu, Y.P.; Wu, H.Q.; Holze, R. Characteristics of an aqueous rechargeable lithium battery (arlb). Electrochim. Acta 2007, 52, 4911–4915. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.P.; Deng, Q.; Mo, J.; Xie, H.; Liu, Z.C.; Xiong, Y.F.; Wu, X.W.; Wu, Y.P. Aqueous rechargeable batteries for large-scale energy storage. Isr. J. Chem. 2015, 55, 521–536. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, Y.S.; Hou, Y.Y.; Liu, L.L.; Wu, Y.P.; Loh, K.P.; Zhang, H.P.; Zhu, K. Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy Environ. Sci. 2013, 6, 2093–2104. [Google Scholar] [CrossRef]
- Yuan, X.H.; Ma, F.X.; Zuo, L.Q.; Wang, J.; Yu, N.F.; Chen, Y.H.; Zhu, Y.S.; Huang, Q.H.; Holze, R.; Wu, Y.P.; et al. Latest advances in high-voltage and high-energy-density aqueous rechargeable batteries. Electrochem. Energy Rev. 2021, 4, 1–34. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, F.; Chen, X.; Sun, R.; Chen, Y.; Fu, L.; Zhu, Y.; Liu, L.; Yu, F.; Wang, J.; et al. Aqueous zinc-sodium hybrid battery based on high crystallinity sodium-iron hexacyanoferrate. Mater. Today Energy 2021, 20, 100660. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.X.; Lee, W.S.V.; Xue, J.M. Defect engineering in manganese-based oxides for aqueous rechargeable zinc-ion batteries: A review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, X.H.; Xia, Y.B.; Zhang, Y.; Fu, L.J.; Liu, L.L.; Yu, N.F.; Huang, Q.H.; Wang, B.; Hu, X.W.; et al. An artificial polyacrylonitrile coating layer confining zinc dendrite growth for highly reversible aqueous zinc-based batteries. Adv. Sci. 2021, 8, 2100309. [Google Scholar] [CrossRef]
- Kim, S.J.; Wu, D.R.; Sadique, N.; Quilty, C.D.; Wu, L.J.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S.; Zhu, Y.M. Unraveling the dissolution-mediated reaction mechanism of alpha-MnO2 cathodes for aqueous Zn-ion batteries. Small 2020, 16, 2005406. [Google Scholar] [CrossRef]
- Gao, Y.N.; Yang, H.Y.; Bai, Y.; Wu, C. Mn-based oxides for aqueous rechargeable metal ion batteries. J. Mater. Chem. A 2021, 9, 11472–11500. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; He, Z.X.; Dai, L.; Wang, H.Y.; Wang, L. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries. J. Energy Chem. 2021, 59, 134–159. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.R.; Zhong, Y.W.; Li, Y.; Sun, C.B.; Han, X.P.; Deng, Y.D.; Zhao, N.Q.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, J.; Sedighi, M.; Han, M.; Shi, Q.Y.; Wu, Y.; Chen, P. Mn2+ ions confined by electrode microskin for aqueous battery beyond intercalation capacity. Adv. Energy Mater. 2020, 10, 2002578. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- Lee, W.H.; Choi, S.R.; Kim, J.G. Effect of agar as electrolyte additive on the aluminum-air batteries. J. Electrochem. Soc. 2020, 167, 110503. [Google Scholar] [CrossRef]
- Liew, S.Y.; Juan, J.C.; Lai, C.W.; Pan, G.T.; Yang, T.C.K.; Lee, T.K. An eco-friendly water-soluble graphene-incorporated agar gel electrolyte for magnesium-air batteries. Ionics 2019, 25, 1291–1301. [Google Scholar] [CrossRef]
- Lu, C.H.; Li, W.Y.; Subburaj, T.; Ou, C.Y.; Kumar, P.S. Influence of bio-derived agar addition on the electrochemical performance of LiFePO4 cathode powders for Li-ion batteries. Ceram. Int. 2019, 45, 12218–12224. [Google Scholar] [CrossRef]
- Menzel, J.; Frackowiak, E.; Fic, K. Agar-based aqueous electrolytes for electrochemical capacitors with reduced self-discharge. Electrochim. Acta 2020, 332, 135435. [Google Scholar] [CrossRef]
- Chotkowski, M.; Rogulski, Z.; Czerwinski, A. Spectroelectrochemical investigation of MnO2 electro-generation and electro-reduction in acidic media. J. Electroanal. Chem. 2011, 651, 237–242. [Google Scholar] [CrossRef]
- Su, D.; Ahn, H.J.; Wang, G. Hydrothermal synthesis of alpha-MnO2 and beta-MnO2 nanorods as high capacity cathode materials for sodium ion batteries. J. Mater. Chem. A 2013, 1, 4845–4850. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.S.; Liao, T.L.; Wang, Y.Y.; Wan, C.C. Assessment of the wettability of porous electrodes for lithium-ion batteries. J. Appl. Electrochem. 2004, 34, 797–805. [Google Scholar] [CrossRef]
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147. [Google Scholar] [CrossRef]
- Zhang, X.T.; Li, J.X.; Ao, H.S.; Liu, D.Y.; Shi, L.; Wang, C.M.; Zhu, Y.C.; Qian, Y.T. Appropriately hydrophilic/hydrophobic cathode enables high-performance aqueous zinc-ion batteries. Energy Storage Mater. 2020, 30, 337–345. [Google Scholar] [CrossRef]
- Delay, A.; Infelta, P.P. On the impedance of an interface between a pt electrode and a naf solution. Electrochim. Acta 1990, 35, 1177–1184. [Google Scholar] [CrossRef]
- Ruetschi, P.; Giovanoli, R. Cation vacancies in MnO2 and their influence on electrochemical reactivity. J. Electrochem. Soc. 1988, 135, 2663–2669. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.Y.; Yang, C.Y.; Fan, X.L.; Ma, Z.H.; Gao, T.; Han, F.D.; Hu, R.Z.; Zhu, M.; et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.X.; Chen, J.Z.; Zhou, W.J.; Wang, A.R.; Chen, M.F.; Tian, Q.H.; Xu, J.L. Electrodeposition of MnO2 nanoflakes onto carbon nanotube film towards high-performance flexible quasi-solid-state Zn-MnO2 batteries. J. Electroanal. Chem. 2020, 873, 114392. [Google Scholar] [CrossRef]
- Chen, S.G.; Lan, R.; Humphreys, J.; Tao, S.W. Salt-concentrated acetate electrolytes for a high voltage aqueous Zn/MnO2 battery. Energy Storage Mater. 2020, 28, 205–215. [Google Scholar] [CrossRef]
- Ghosh, M.; Vijayakumar, V.; Anothumakkool, B.; Kurungot, S. Nafion lonomer-based single component electrolytes for aqueous Zn/MnO2 batteries with long cycle life. ACS Sustain. Chem. Eng. 2020, 8, 5040–5049. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.D.; Zhang, X.Y.; Wang, S.M.; Han, J.T.; Zhao, Y.S.; Huang, Y.H.; Qin, J.Q. Mechanochemical reactions of MnO2 and graphite nanosheets as a durable zinc ion battery cathode. Appl. Surf. Sci. 2020, 534, 147630. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wang, A.R.; Huang, A.X.; Chen, M.F.; Tian, Q.H.; Chen, J.Z.; Xu, X.W. Hybridizing delta-type MnO2 with lignin-derived porous carbon as a stable cathode material for aqueous Zn-MnO2 batteries. Front. Energy Res. 2020, 8, 182. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.X.; Jin, S.Y.; Zhang, S.D.; Wang, H.L.; Hiralal, P.; Amaratunga, G.A.J.; Zhou, H. Carbon nanohorns/nanotubes: An effective binary conductive additive in the cathode of high energy-density zinc-ion rechargeable batteries. Carbon 2020, 167, 431–438. [Google Scholar] [CrossRef]
- Liu, N.N.; Mohanapriya, K.; Pan, J.; Hu, Y.; Sun, Y.Z.; Liu, X.G. A facile preparation of lambda-MnO2 as cathode material for high-performance zinc-manganese redox flow battery. J. Electrochem. Soc. 2020, 167, 040517. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, M.Q.; He, Z.C.; Liu, L.; Huang, Y.D. Rechargeable aqueous zinc-manganese dioxide/graphene batteries with high rate capability and large capacity. ACS Appl. Energy Mater. 2020, 3, 1742–1748. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.W.; Li, W.J.; Tian, Y.; Zhang, Y.; Wu, H.; Yang, L.; Zou, G.Q.; Hou, H.S.; Ji, X.B. H+ insertion boosted alpha-MnO2 for an aqueous Zn-ion battery. Small 2020, 16, 1905842. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wu, F.F.; Shi, W.H.; Liu, W.X.; Xu, X.L.; Cai, G.F.; Li, Y.W.; Cao, X.H. Preparation of polyaniline-coated composite aerogel of MnO2 and reduced graphene oxide for high-performance zinc-ion battery. Chin. J. Polym. Sci. 2020, 38, 514–521. [Google Scholar] [CrossRef]
- Palaniyandy, N.; Kebede, M.A.; Raju, K.; Ozoemena, K.I.; le Roux, L.; Mathe, M.K.; Jayaprakasam, R. Alpha-MnO2 nanorod/onion-like carbon composite cathode material for aqueous zinc-ion battery. Mater. Chem. Phys. 2019, 230, 258–266. [Google Scholar] [CrossRef]
- Wu, B.K.; Zhang, G.B.; Yan, M.Y.; Xiong, T.F.; He, P.; He, L.; Xu, X.; Mai, L.Q. Graphene scroll-coated alpha-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]






| Cathode | Anode | Electrolyte | Electrolyte Voltage (V) | Capacity (mAh g−1) | Retention/Cycles | Ref. |
|---|---|---|---|---|---|---|
| CNT@MnO2 | zinc foil | 2 M ZnSO4/0.2 M MnSO4 | 1–1.85 | 105.6 mAh g−1 (3 mA cm−2) | 85.72%/1000 cycles | [33] |
| α-MnO2-TiN/TiO | zinc foil | 1 M Zn(OAc)/31 M KOAc | 0.8–2.0 | 304.6 mAh·g−1 (100 mA g−1) | 79.7%/600 cycles | [34] |
| MnCP-X | zinc foil | 1 M Zn(CF3SO3)2 | 0.8–2.0 | 151 mAh g−1 (3 A g−1) | 89%/500 cycles | [35] |
| MnO2/graphite | zinc foil | 2 M ZnSO4/0.5 M MnSO4 | 0.8–1.8 | 80 mAh g−1 (1 A g−1) | 80.8%/1000 cycles | [36] |
| LPC/δ-MnO2 | zinc foil | 2 M ZnSO4/0.2 M MnSO4 | 1.0–1.85 | 196.1 mAh g–1 (5 A g–1) | 82%/1000 cycles | [37] |
| MnO2-CNTs/CNHs | zinc foil | 2 M ZnSO4/0.1 M MnSO4 | 1.0–1.9 | 168.1 mAh g−1 (3 A g−1) | 96.5%/500 cycles | [38] |
| λ-MnO2 | zinc foil | 1 M Li2SO4/1 M ZnSO4 | 1.5–2.1 | 128 mAh g−1 (2 C) | 83%/1,000 cycles | [39] |
| MnO2/GO | zinc foil | 2 M ZnSO4/0.2 M MnSO4 | 0.9–1.8 | 190mAh g−1 (3C) | 91%/cycles | [40] |
| MnO2/carbon | zinc foil | 2 M ZnSO4/0.2 M MnSO4 | 0.9–1.8 | 240 mAh g−1 (0.1 A g−1) | 58.33%/300 cycles | [41] |
| MnO2/rGO/PANI | zinc foil | 2 M ZnSO4 | 0.8–1.8 | 100.6 mAh·g−1 (1 A g−1) | 82.7%/600 cycles | [42] |
| MnO2/OLC | zinc foil | 1 M ZnSO4/0.1M MnSO4 | 1.0–1.8 | 168 mAh g−1 (246 mA g−1) | 93%/100 cycles | [43] |
| MnO2–agar | zinc foil | 2 M ZnSO4/0.5 M MnSO4 | 0.1–1.95 | 260.6 mAh g−1 (0.5 A g−1) | 95.4%/500 cycles | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, L.; Sun, H.; Yuan, X.; Wen, J.; Chen, X.; Zhou, S.; Wu, Y.; van Ree, T. Agar Acts as Cathode Microskin to Extend the Cycling Life of Zn//α-MnO2 Batteries. Materials 2021, 14, 4895. https://doi.org/10.3390/ma14174895
Zuo L, Sun H, Yuan X, Wen J, Chen X, Zhou S, Wu Y, van Ree T. Agar Acts as Cathode Microskin to Extend the Cycling Life of Zn//α-MnO2 Batteries. Materials. 2021; 14(17):4895. https://doi.org/10.3390/ma14174895
Chicago/Turabian StyleZuo, Linqing, Haodong Sun, Xinhai Yuan, Juan Wen, Xi Chen, Shiyu Zhou, Yuping Wu, and Teunis van Ree. 2021. "Agar Acts as Cathode Microskin to Extend the Cycling Life of Zn//α-MnO2 Batteries" Materials 14, no. 17: 4895. https://doi.org/10.3390/ma14174895






