Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Surface Coatings
2.2.1. Magnesium Hydroxide Slurry
2.2.2. Coating Application
2.3. Scanning Electron Microscopy Analysis
2.4. Accelerated Spraying Tests
2.4.1. Stoichiometry Calculations
2.4.2. 4-Days Accelerated Acid Spraying Test
2.4.3. 4-Months Accelerated Acid Spraying Test
2.5. X-ray Diffraction (XRD) Analysis and Attenuated Total Reflectance (ATR)
3. Results
3.1. Adhesion Measurements
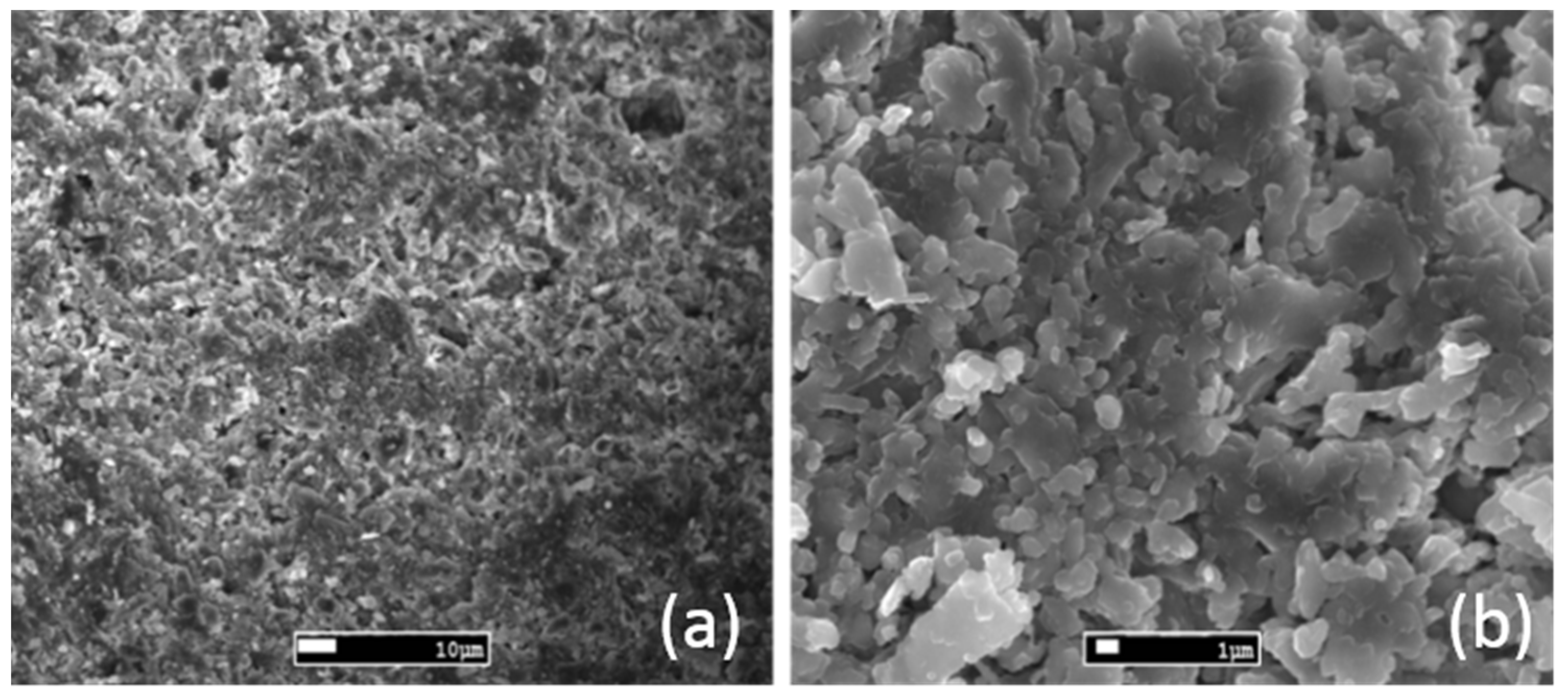
3.2. SEM Analysis
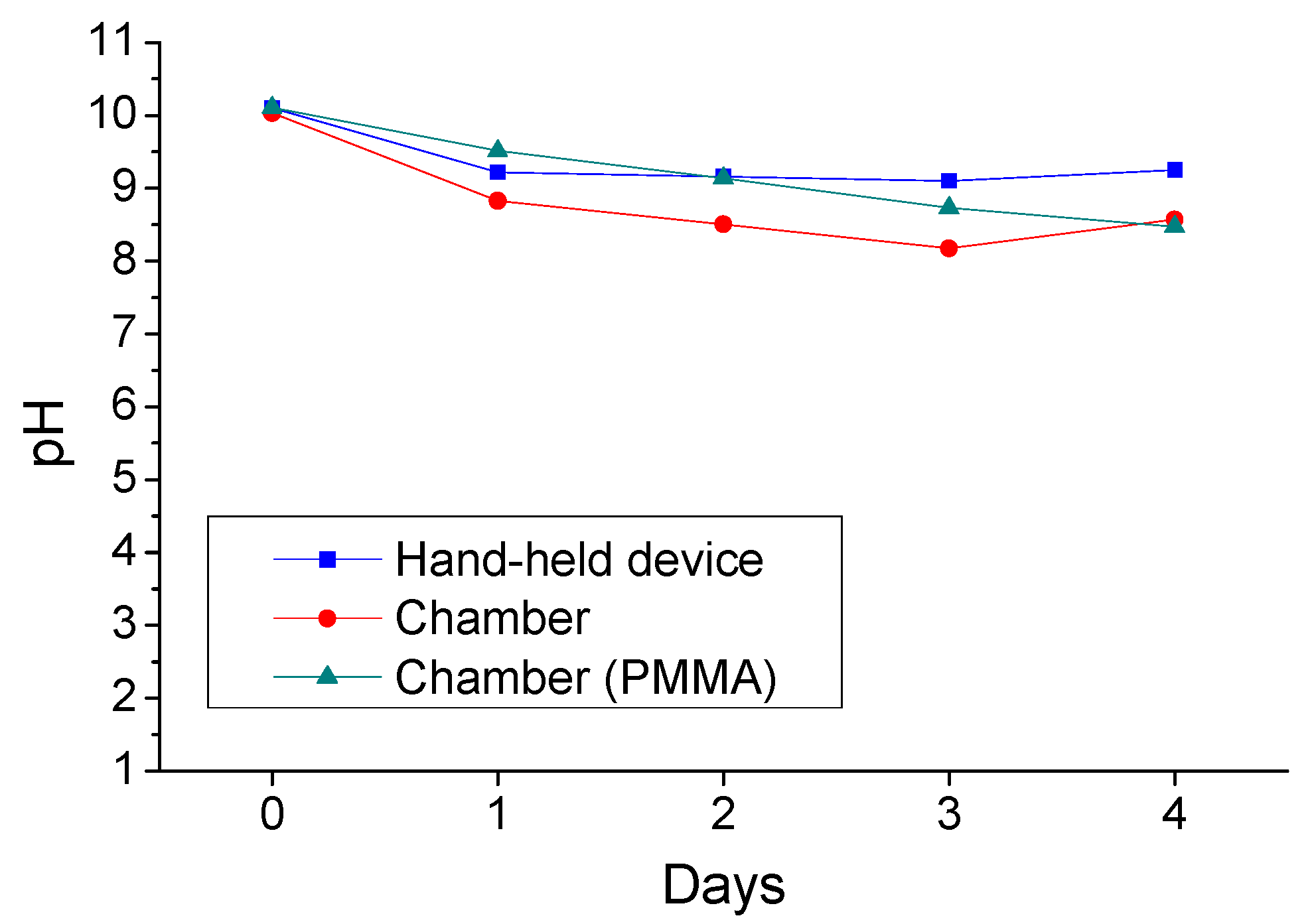
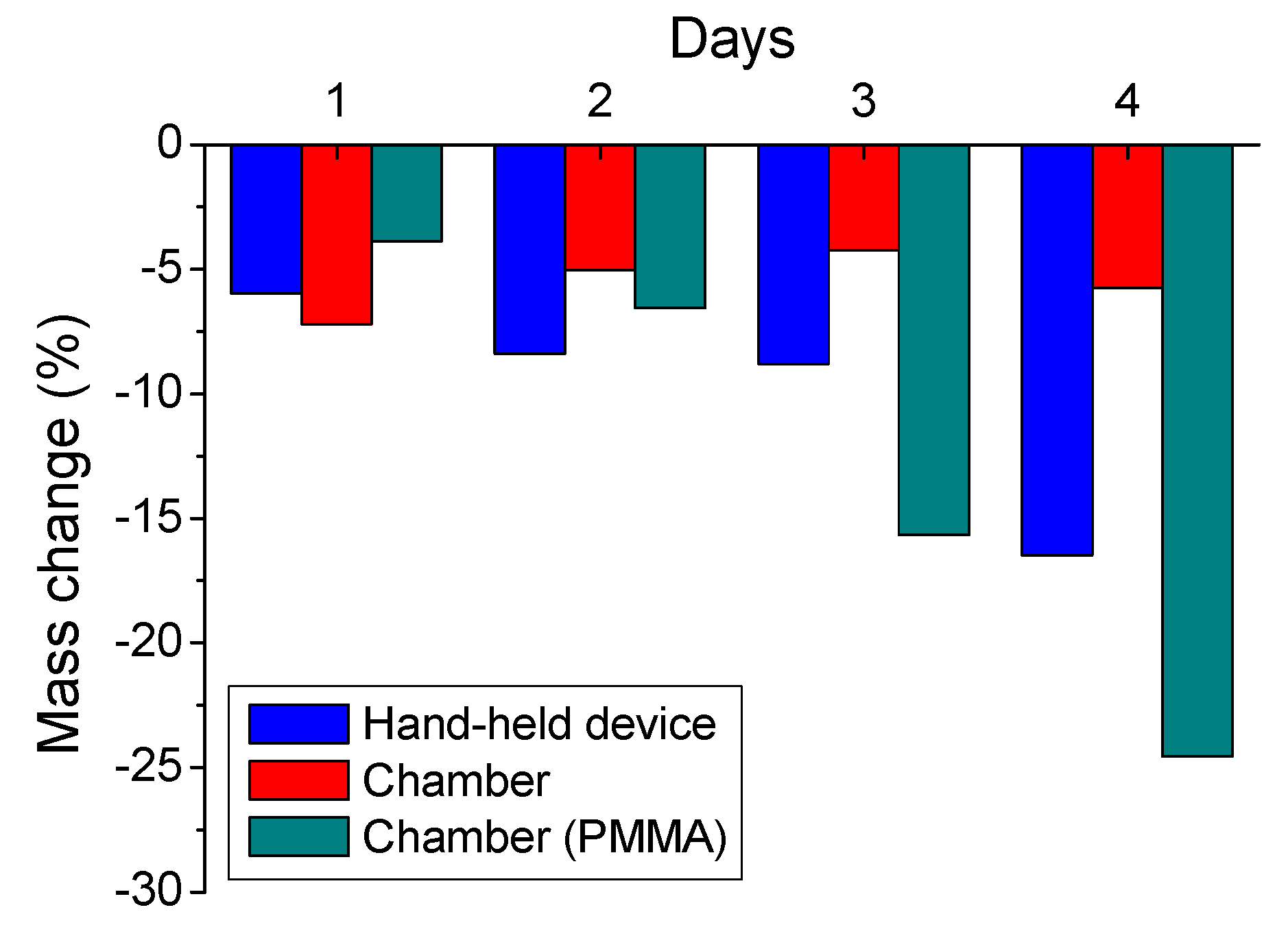
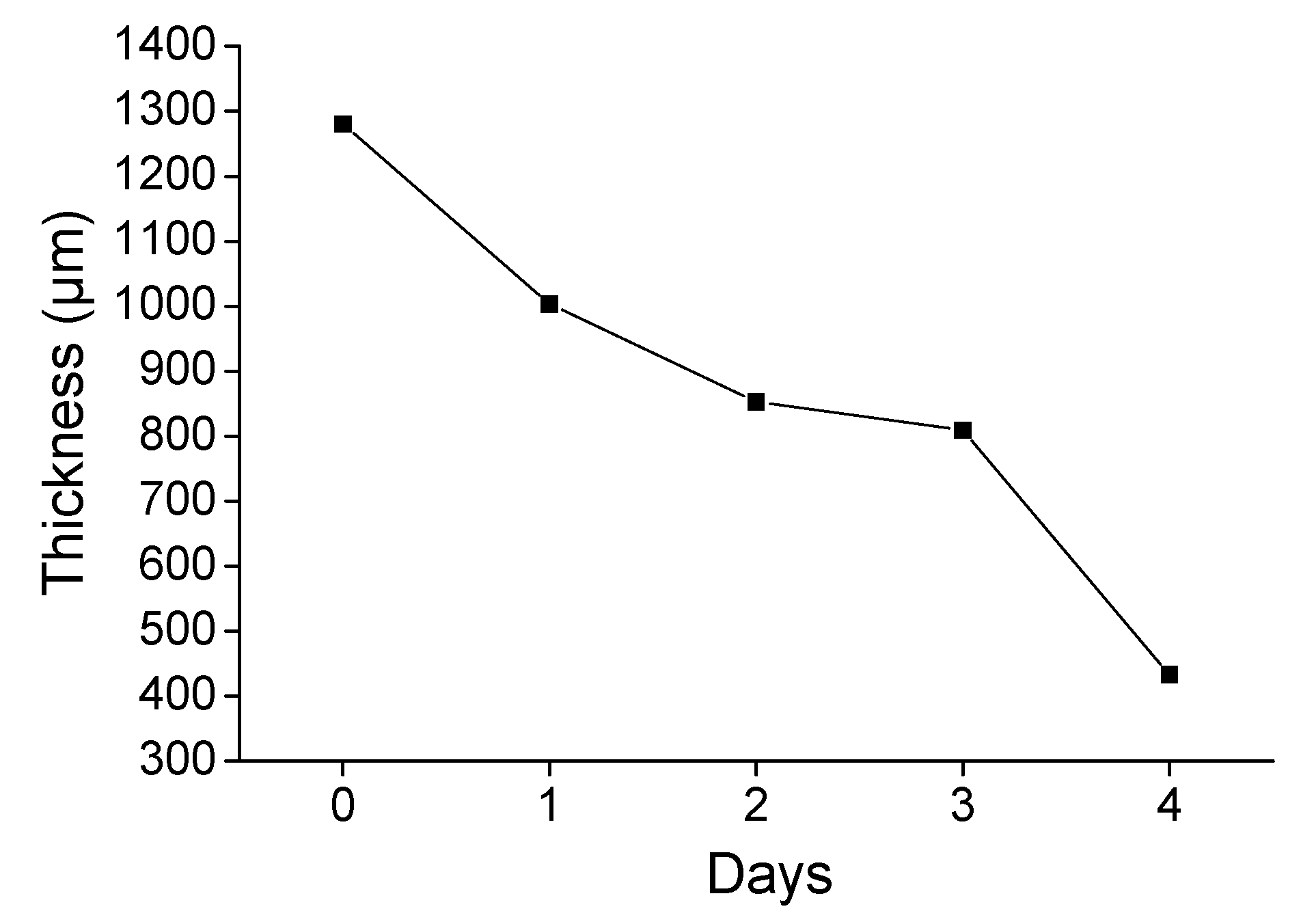
3.3. Accelerated Sulfuric Acid Spraying Tests
3.3.1. 4-Days Accelerated Acid Spraying Tests
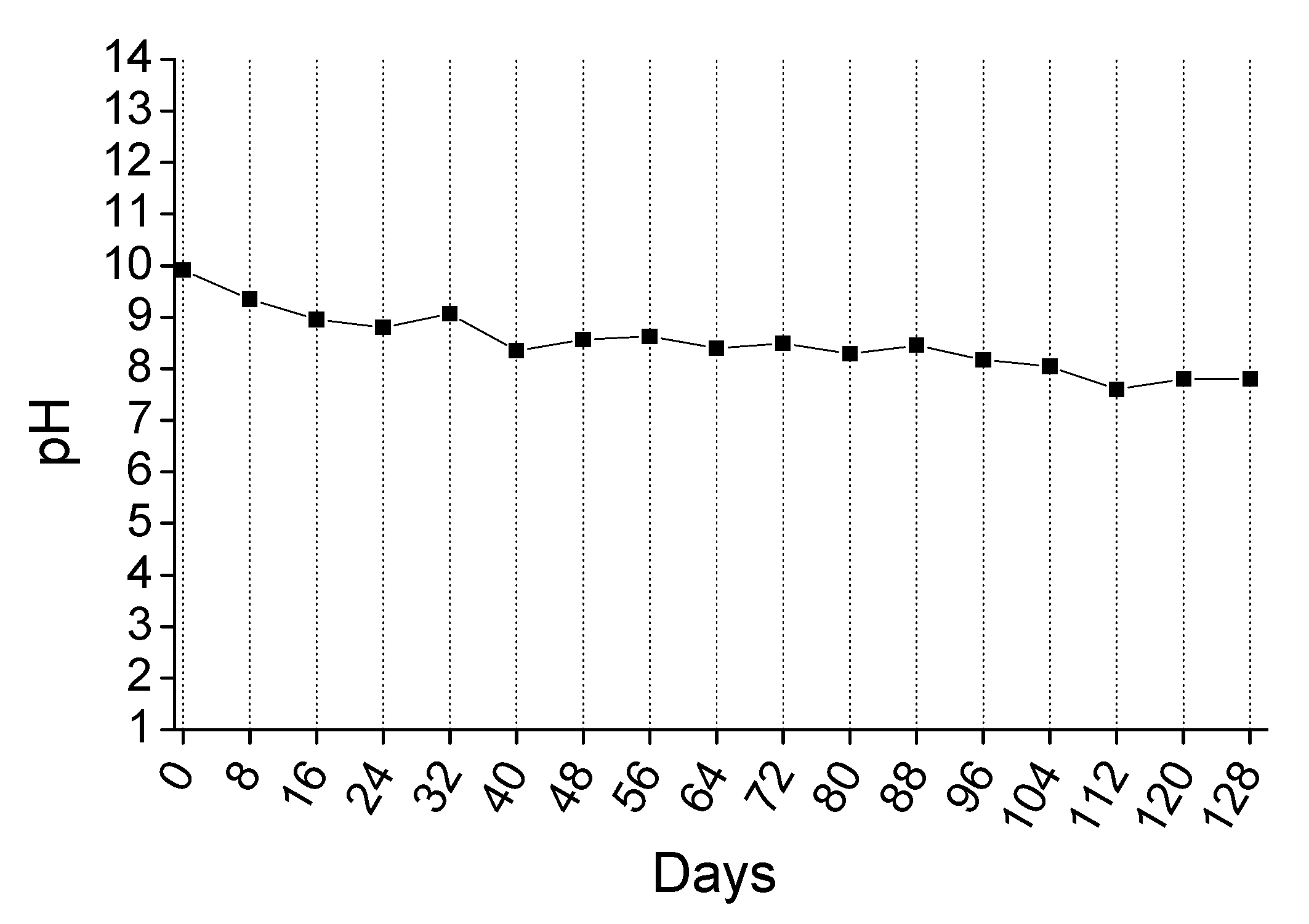
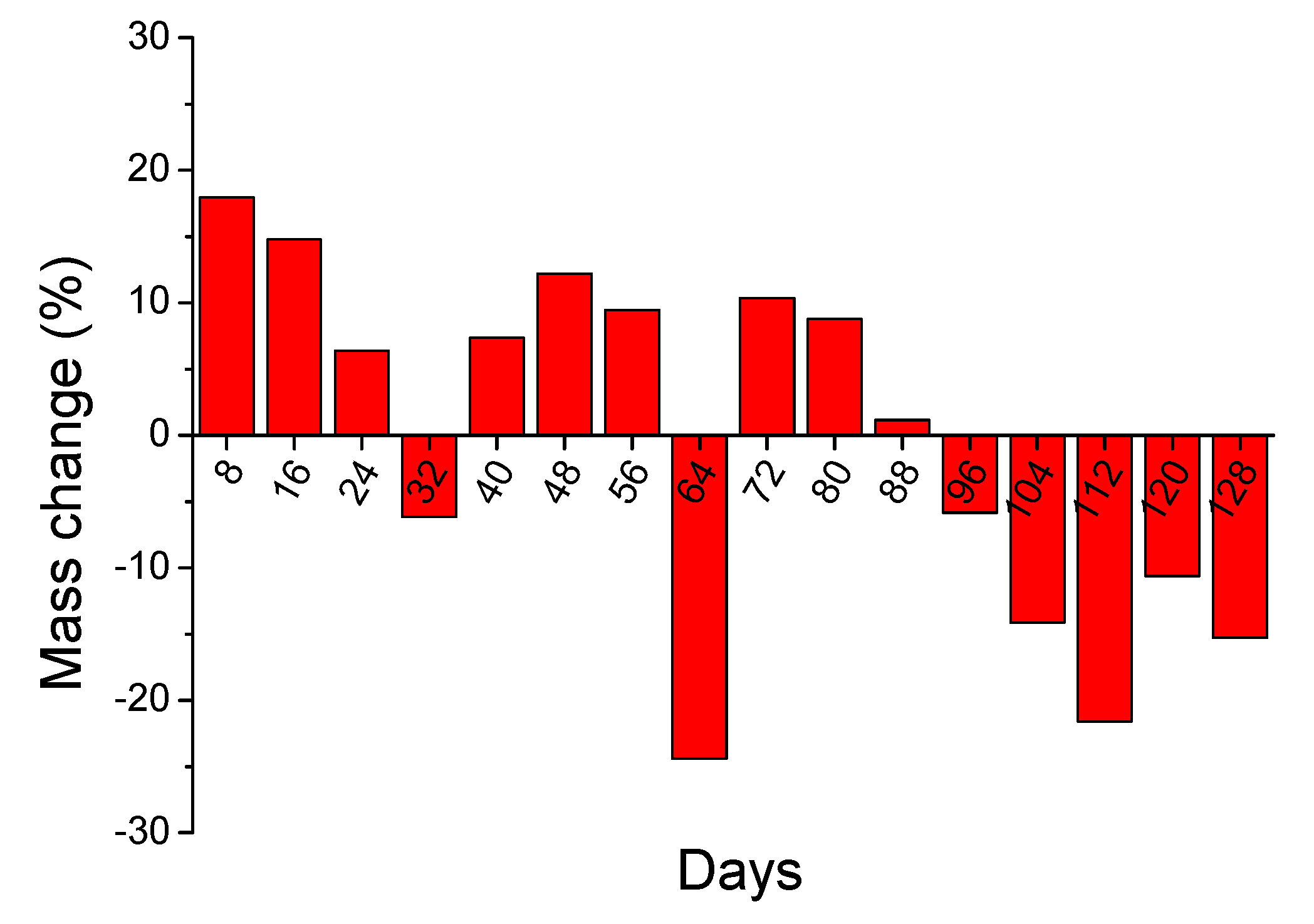
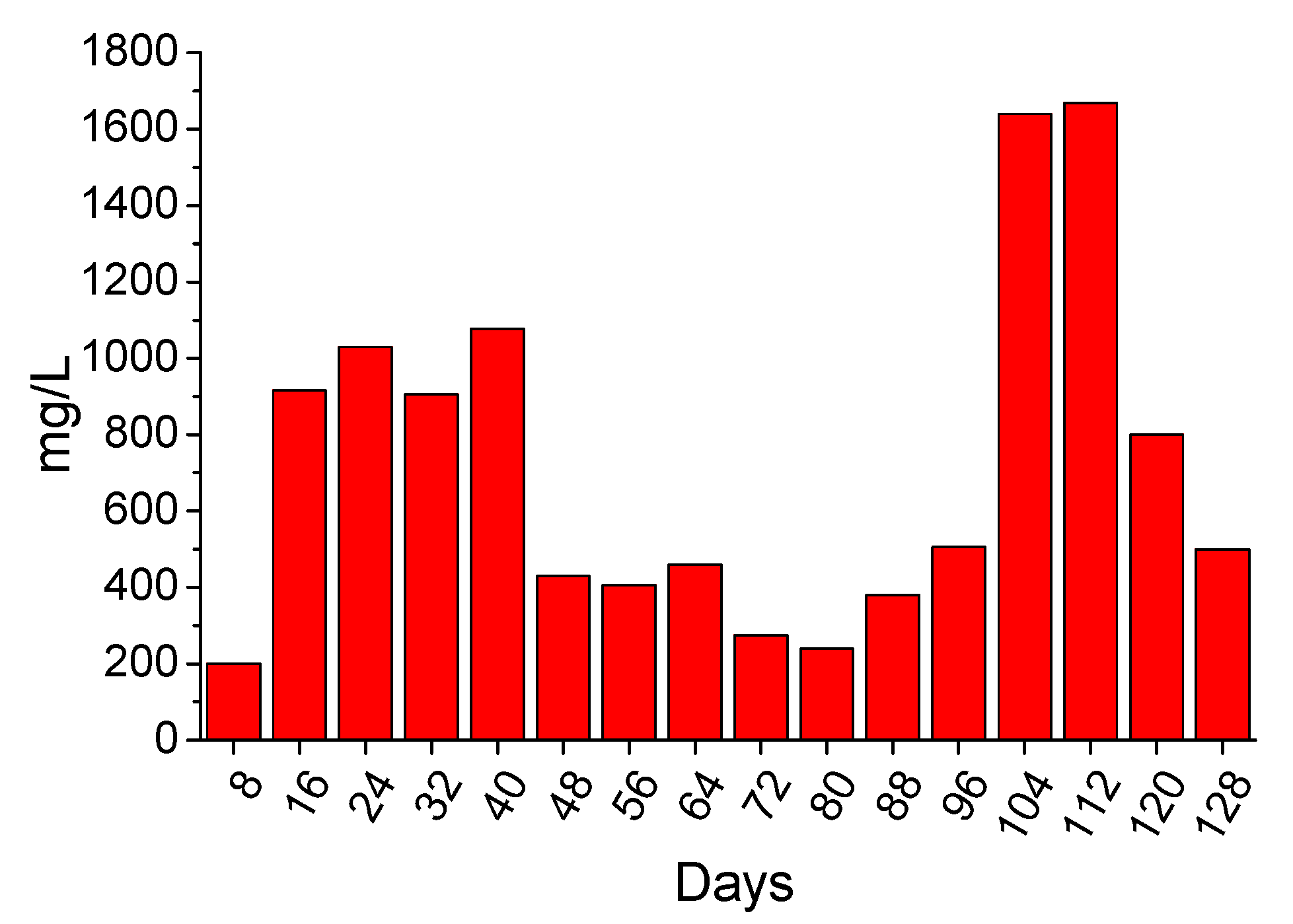
3.3.2. 4-Months Accelerated Acid Spraying Test
3.4. XRD Analysis
3.4.1. 4-Days Accelerated Acid Spraying Tests
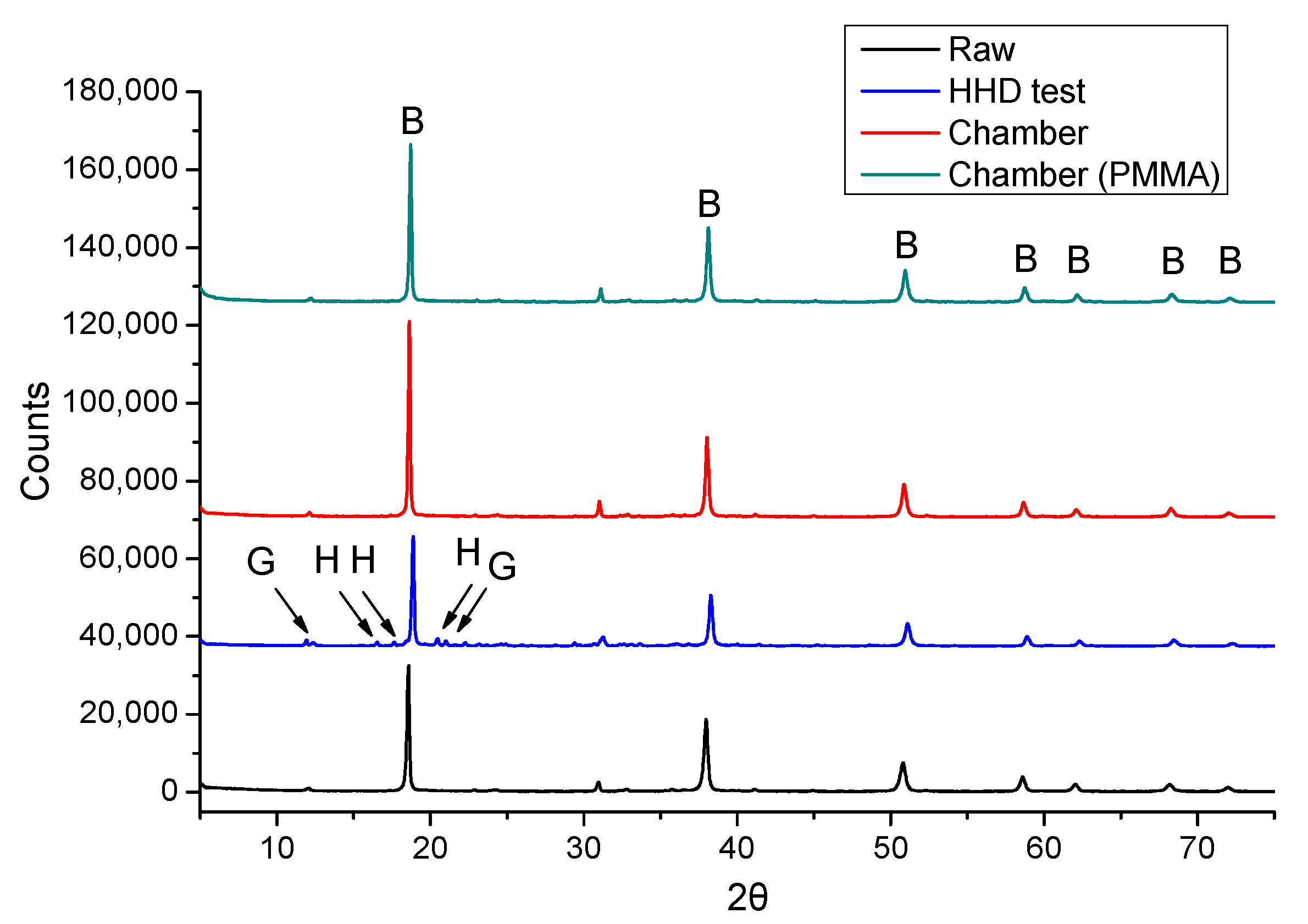
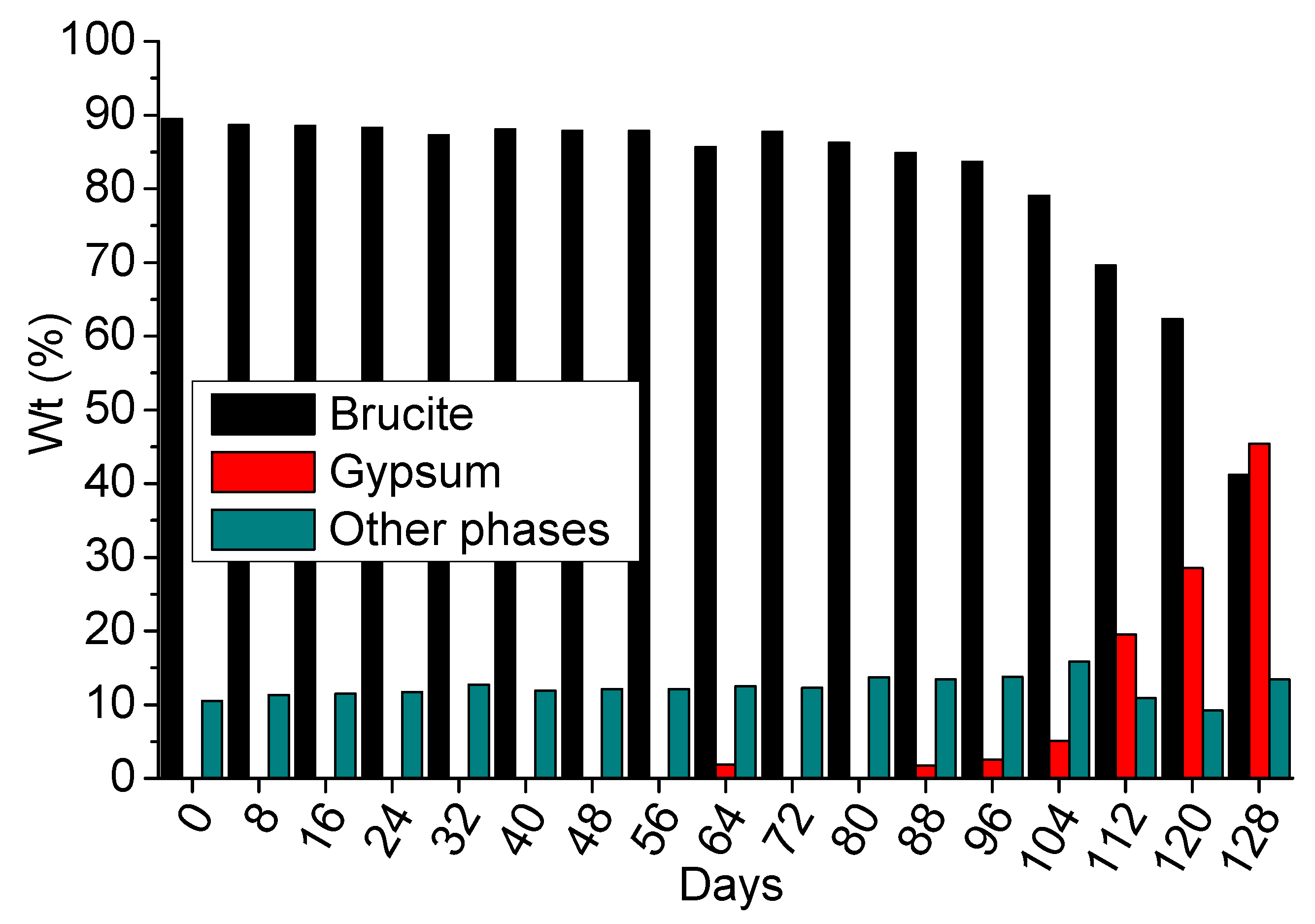
3.4.2. 4-Months Accelerated Acid Spraying Test
3.5. Attenuated Total Reflectance
4. Discussion
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmsted, F.H.; Hamlin, H. Converting Portions of the Los Angeles Outfall Sewer into a Septic Tank. Eng. News 1900, 44, 317–318. [Google Scholar]
- Parker, C. The corrosion of concrete. The isolation of a species of bacterium associated with the corrosion of concrete exposed to atmospheres containing hydrogen sulfide. Aust. J. Exp. Biol. Med. Sci. 1945, 23, 81–90. [Google Scholar] [CrossRef]
- Parker, C. The corrosion of concrete. The function of Thiobacillus Concretivorus in the corrosion of concrete exposed to atmospheres containing hydrogen sulfirde. Aust. J. Exp. Biol. Med. Sci. 1945, 23, 91–98. [Google Scholar] [CrossRef]
- Thistlethwayte, D.K.B. The control of sulphides in sewerage systems. AIChE J. 1972, 19, 173. [Google Scholar] [CrossRef]
- Pomeroy, R.D. Process Design Manual for Sulfide Control in Sanitary Sewerage Systems; US Environmental Protection Agency, Technology Transfer: Washington, DC, USA, 1974.
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, F.; Li, Y.; Song, L.; Jiang, D.; Zeng, R.C.; Tjong, S.C.; Chen, D.C. Corrosion resistance of dodecanethiol-modified magnesium hydroxide coating on AZ31 magnesium alloy. Appl. Phys. A Mater. Sci. Process. 2020, 126, 8. [Google Scholar] [CrossRef]
- Hvitved-Jacobsen, T.; Vollertsen, J.; Nielsen, A.H. Sewer Processes: Microbial and Chemical Process Engineering of Sewer Networks; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439881774. [Google Scholar]
- Okabe, S.; Odagiri, M.; Ito, T.; Satoh, H. Succession of Sulfur-Oxidizing Bacteria in the Microbial Community on Corroding Concrete in Sewer Systems † Downloaded from. Appl. Environ. Microbiol. 2007, 73, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.-R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Ma, Y.; Liu, J. Current status and future prospects of sewer biofilms: Their structure, influencing factors, and substance transformations. Sci. Total Environ. 2019, 695, 133815. [Google Scholar] [CrossRef]
- Wells, T.; Melchers, R.E. Findings of a 4 Year Study of Concrete Sewer Pipe Corrosion; Australasian Corrosion Association: Victoria, Australia, 2014. [Google Scholar]
- Wells, T.; Melchers, R.E.; Bond, P. Factors Involved in the Long Term Corrosion of Concrete Sewers; Australasian Corrosion Association: Victoria, Australia, 2009; Volume 11, pp. 345–356. [Google Scholar]
- Zhang, G.; Xie, Q.; Ma, C.; Zhang, G. Permeable epoxy coating with reactive solvent for anticorrosion of concrete. Prog. Org. Coat. 2018, 117, 29–34. [Google Scholar] [CrossRef]
- Aguiar, J.B.; Camões, A.; Moreira, P.M. Coatings for Concrete Protection against Aggressive Environments. J. Adv. Concr. Technol. 2008, 6, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Roghanian, N.; Banthia, N. Development of a sustainable coating and repair material to prevent bio-corrosion in concrete sewer and waste-water pipes. Cem. Concr. Compos. 2019, 100, 99–107. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M.S.H. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168. [Google Scholar] [CrossRef]
- Herisson, J.; Guéguen-Minerbe, M.; van Hullebusch, E.D.; Chaussadent, T. Influence of the binder on the behaviour of mortars exposed to H2S in sewer networks: A long-term durability study. Mater. Struct. 2017, 50, 8. [Google Scholar] [CrossRef]
- Ganigue, R.; Gutierrez, O.; Rootsey, R.; Yuan, Z. Chemical dosing for sulfide control in Australia: An industry survey. Water Res. 2011, 45, 6564–6574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sharma, K.R.; Ni, B.J.; Fan, L.; Murthy, S.; Tyson, G.Q.; Yuan, Z. Effects of nitrate dosing on sulfidogenic and methanogenic activities in sewer sediment. Water Res. 2015, 74, 155–165. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Kan, L.; Wu, M. Current understanding on microbiologically induced corrosion of concrete in sewer structures: A review of the evaluation methods and mitigation measures. Constr. Build. Mater. 2020, 247, 118539. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Berndt, M.L. Evaluation of coatings, mortars and mix design for protection of concrete against sulphur oxidising bacteria. Constr. Build. Mater. 2011, 25, 3893–3902. [Google Scholar] [CrossRef]
- Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Manganese Phosphatizing Coatings: The Effects of Preparation Conditions on Surface Properties. Materials 2018, 11, 2585. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Guerrero, A.M.; Mejía de Gutiérrez, R. Alkali-activated protective coatings for reinforced concrete exposed to chlorides. Constr. Build. Mater. 2021, 268, 121098. [Google Scholar] [CrossRef]
- Sydney, R.; Esfandi, E.; Surapaneni, S. Control Concrete Sewer Corrosion via the Crown Spray Process. Water Environ. Res. 1996, 68, 338–347. [Google Scholar] [CrossRef]
- James, J. Controlling sewer crown corrosion using the crown spray process with magnesium hydroxide. Proc. Water Environ. Fed. 2003, 2003, 259–268. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.K.; Yiannoulakis, H.; Zouboulis, A. Comparison of Different Magnesium Hydroxide Coatings Applied on Concrete Substrates (Sewer Pipes) for Protection against Bio-Corrosion. Water 2021, 13, 1227. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 1766: 2017 Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Reference; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- European Committee for Standardization. EN 1916: 2002: Concrete Pipes and Fittings, Unreinforced, Steel Fibre and Reinforced; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- Diamanti, M.V.; Brenna, A.; Bolzoni, F.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Fytianos, G.; Papastergiadis, E.; Samaras, P.; Yiannoulakis, H.; Zouboulis, A. Properties and Performance of Novel Mg(OH)2-Based Coatings for Corrosion Mitigation in Concrete Sewer Pipes. Materials 2020, 13, 5291. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standarization. ΕΝ 1542: 1999: Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Measurement of Bond Strength by Pull-Off; European Committee for Standardization: Brussels, Belgium, 1999. [Google Scholar]
- European Committee for Standarization. ΕΝ 13578: 2003: Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Compatibility on Wet Concrete; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- Fockaert, L.I.; Würger, T.; Unbehau, R.; Boelen, B.; Meißner, R.H.; Lamaka, S.V.; Zheludkevich, M.L.; Terryn, H.; Mol, J.M.C. ATR-FTIR in Kretschmann configuration integrated with electrochemical cell as in situ interfacial sensitive tool to study corrosion inhibitors for magnesium substrates. Electrochim. Acta 2020, 345, 136166. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Eghtesad, R.; Sudoi, E.; Vupputuri, S.; Ramsey, J.D.; Ley, M.T. Application of Fourier transform infrared spectroscopy to study concrete degradation induced by biogenic sulfuric acid. Mater. Struct. 2016, 49, 2025–2034. [Google Scholar] [CrossRef]
- Ashrit, S.; Chatti, R.V.; Nair, U.G. An infrared and Raman spectroscopic study of yellow gypsum synthesized from LD slag fines. Mater. Sci. Eng. 2018, 2. [Google Scholar] [CrossRef]





| Nominal Chemical Composition | Mg(OH)2 Content (%) | SSA (m2/g) | PSD (μm) | |||||
|---|---|---|---|---|---|---|---|---|
| MgO (%) | SiO2 (%) | CaO (%) | Fe2O3 (%) | LOI (%) | d50 | d90 | ||
| 62.81 | 4.25 | 2.46 | 0.25 | 30.11 | 89.0 | 7 | 3.8 | 13.1 |
| Test | Measurements |
|---|---|
| 4 days hand-held device test | Surface pH Thickness Mass Mineralogical phases |
| 4 days spraying chamber test (concrete specimens) | Surface pH Mass Mineralogical phases |
| 4 days spraying chamber test (PMMA plates) | Surface pH Mass Mineralogical phases |
| 128 days spraying chamber test | Surface pH Mass Mineralogical phases |
| fh (MPa) | SD (MPa) | Type of Failure | |
|---|---|---|---|
| A/B (%) 1 | B (%) 2 | ||
| 0.30 | 0.025 | 30 | 70 |
| Elements | Weight % | |
|---|---|---|
| Spectrum 1 | Spectrum 2 | |
| Mg | 94.16 | 1.41 |
| Si | 2.31 | 1.24 |
| Ca | 3.54 | 97.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merachtsaki, D.; Tsardaka, E.-C.; Anastasiou, E.; Zouboulis, A. Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests. Materials 2021, 14, 4897. https://doi.org/10.3390/ma14174897
Merachtsaki D, Tsardaka E-C, Anastasiou E, Zouboulis A. Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests. Materials. 2021; 14(17):4897. https://doi.org/10.3390/ma14174897
Chicago/Turabian StyleMerachtsaki, Domna, Eirini-Chrysanthi Tsardaka, Eleftherios Anastasiou, and Anastasios Zouboulis. 2021. "Evaluation of the Protection Ability of a Magnesium Hydroxide Coating against the Bio-Corrosion of Concrete Sewer Pipes, by Using Short and Long Duration Accelerated Acid Spraying Tests" Materials 14, no. 17: 4897. https://doi.org/10.3390/ma14174897









