Hydrothermal Synthesis and Characterization of Zeolite A from Corn (Zea Mays) Stover Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Raw Materials
2.2. Methods
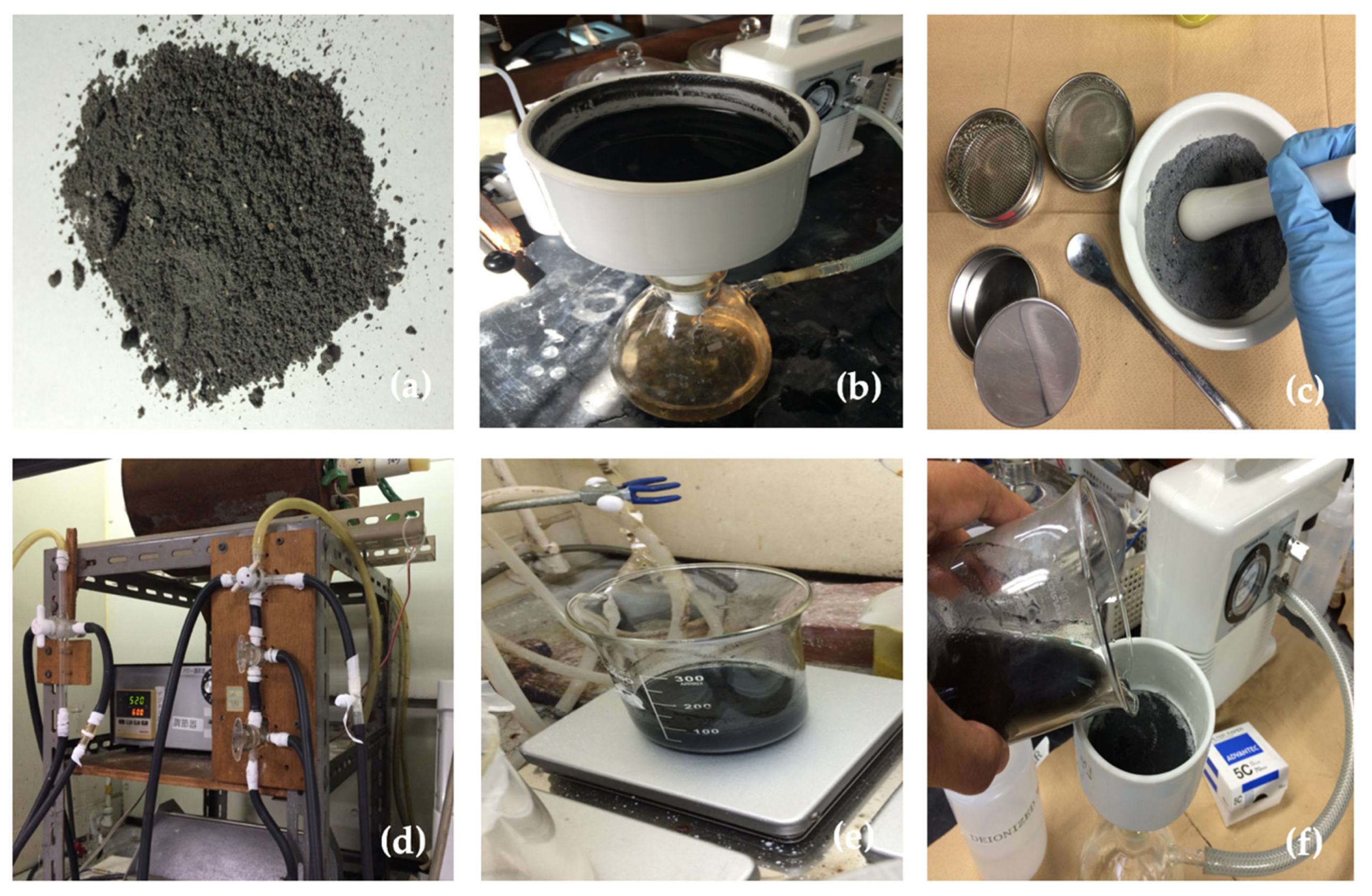
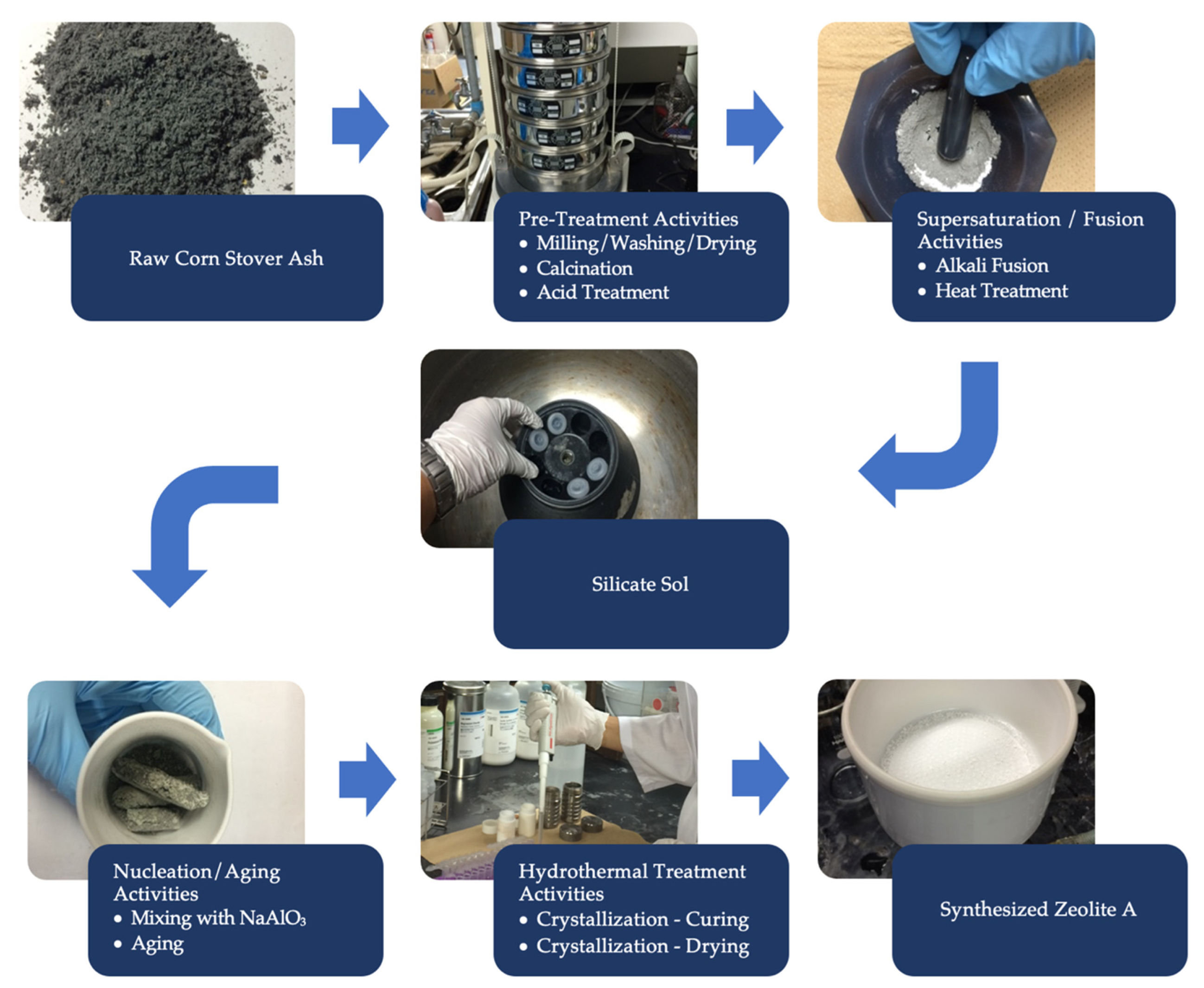
2.2.1. Silica Extraction from Corn Stover Ash
2.2.2. Zeolite A Preparation
2.3. Characterizations and Cation Exchange Capacity (CEC)
3. Results and Discussion
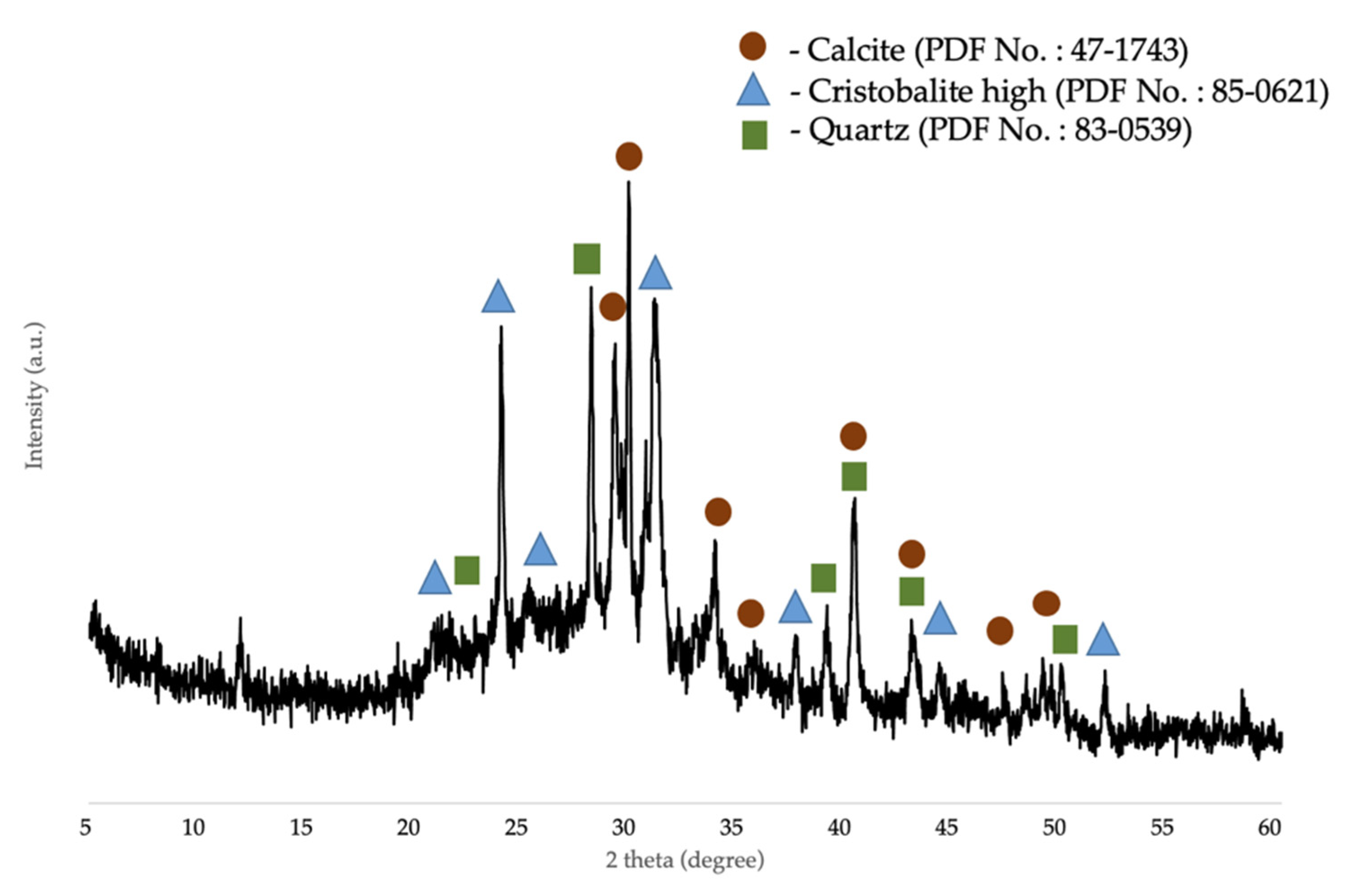
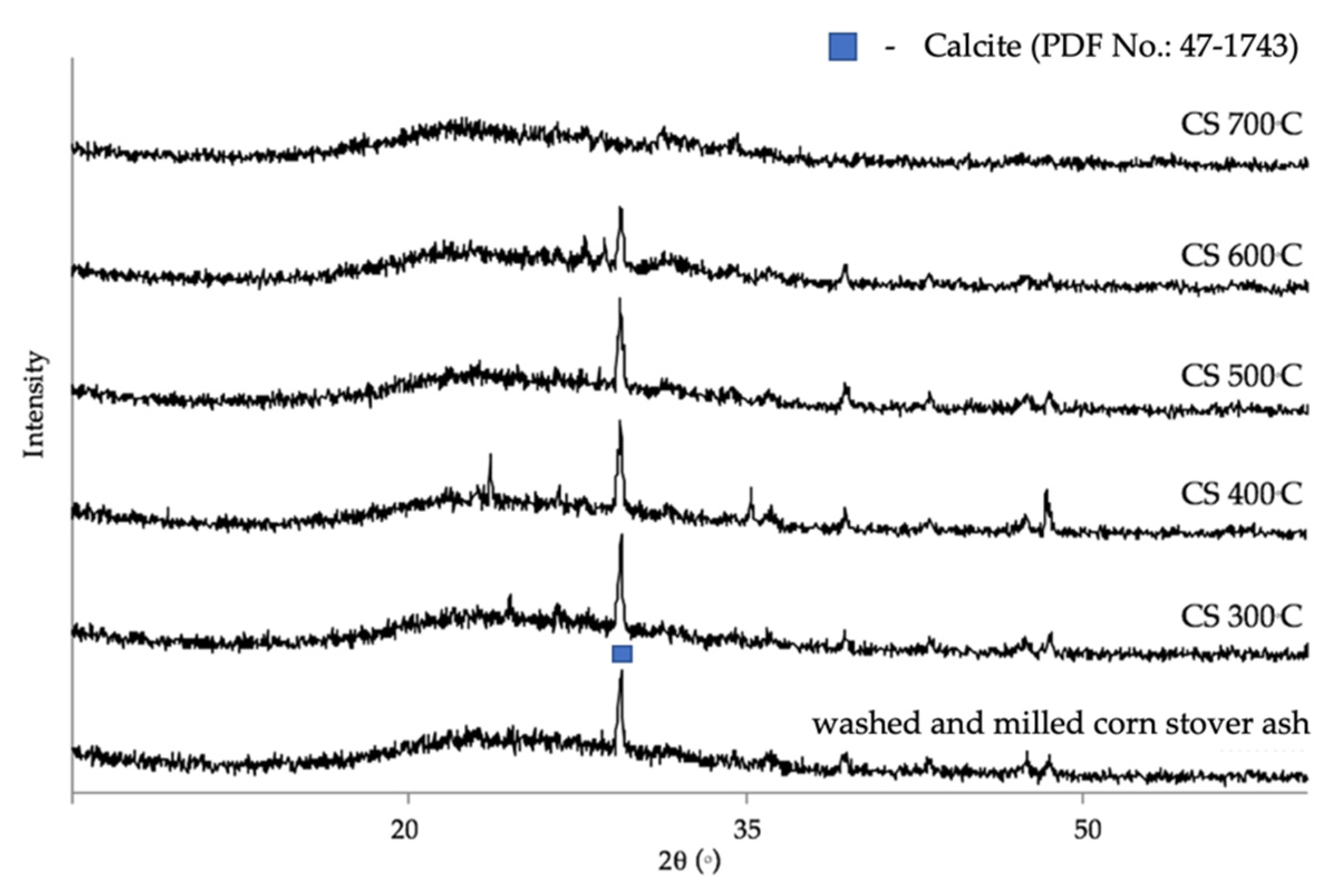
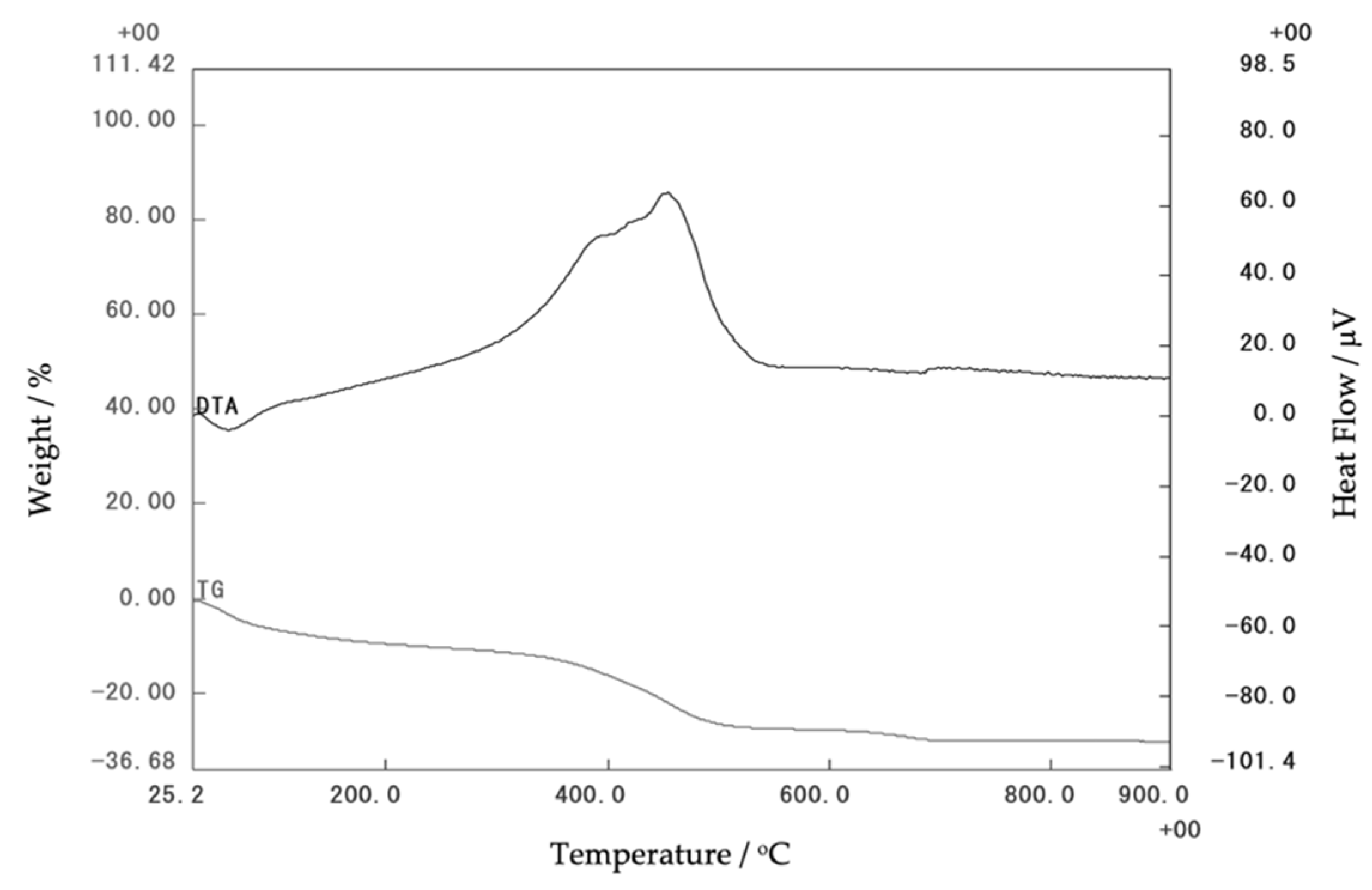
3.1. Characterization of Corn Stover Ash
3.2. The Effect of Calcination Temperature
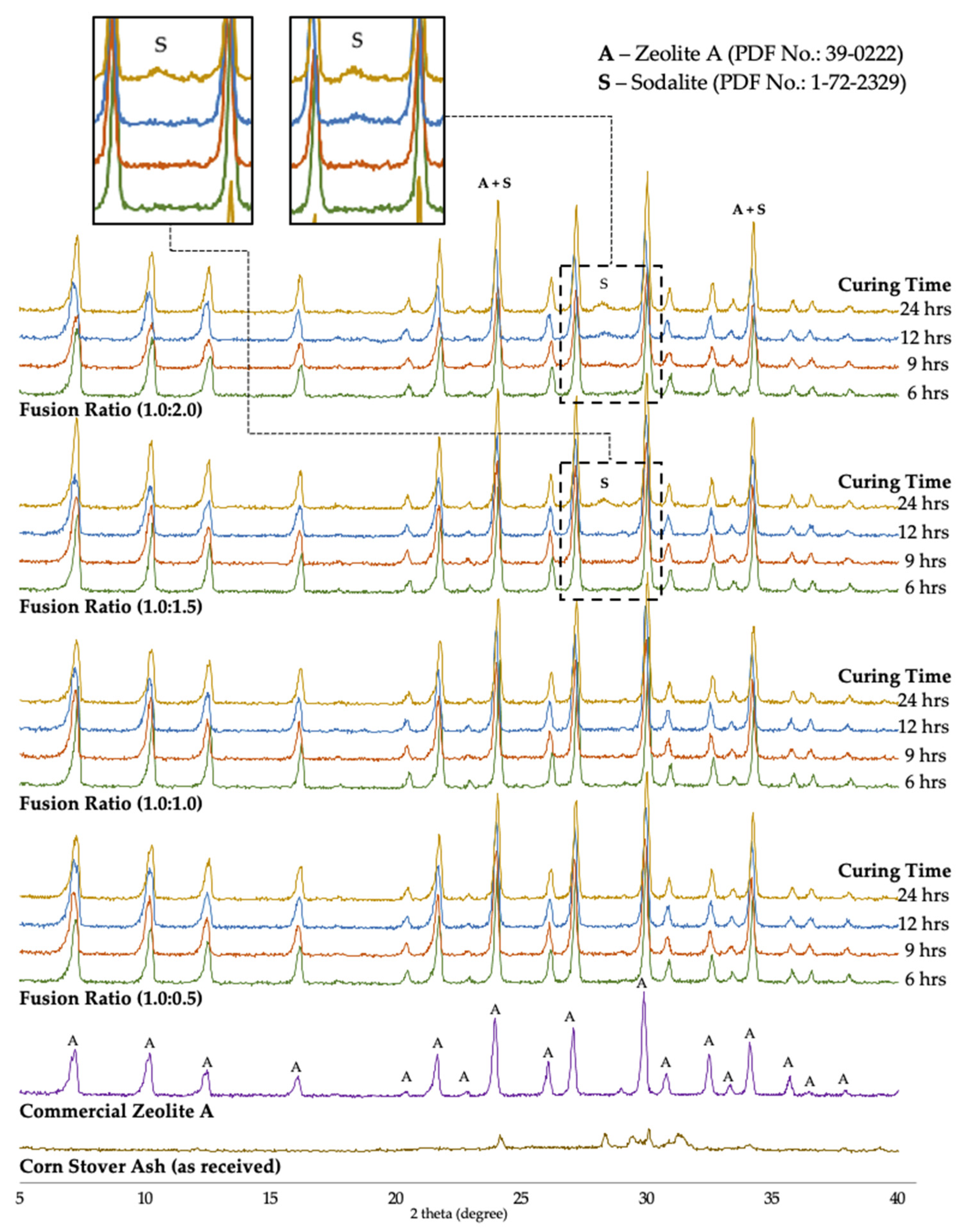
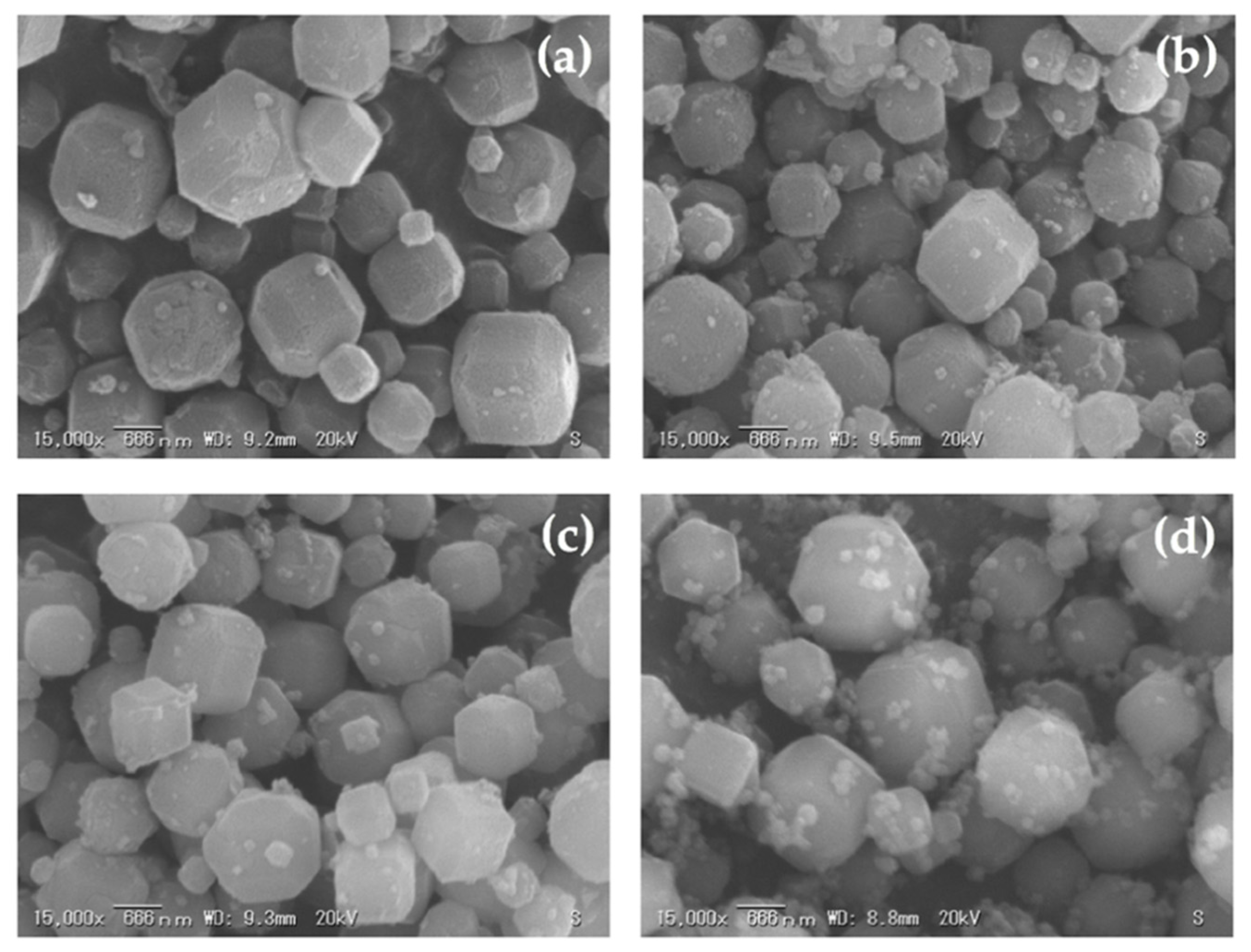
3.3. The Effect of Alkalinity
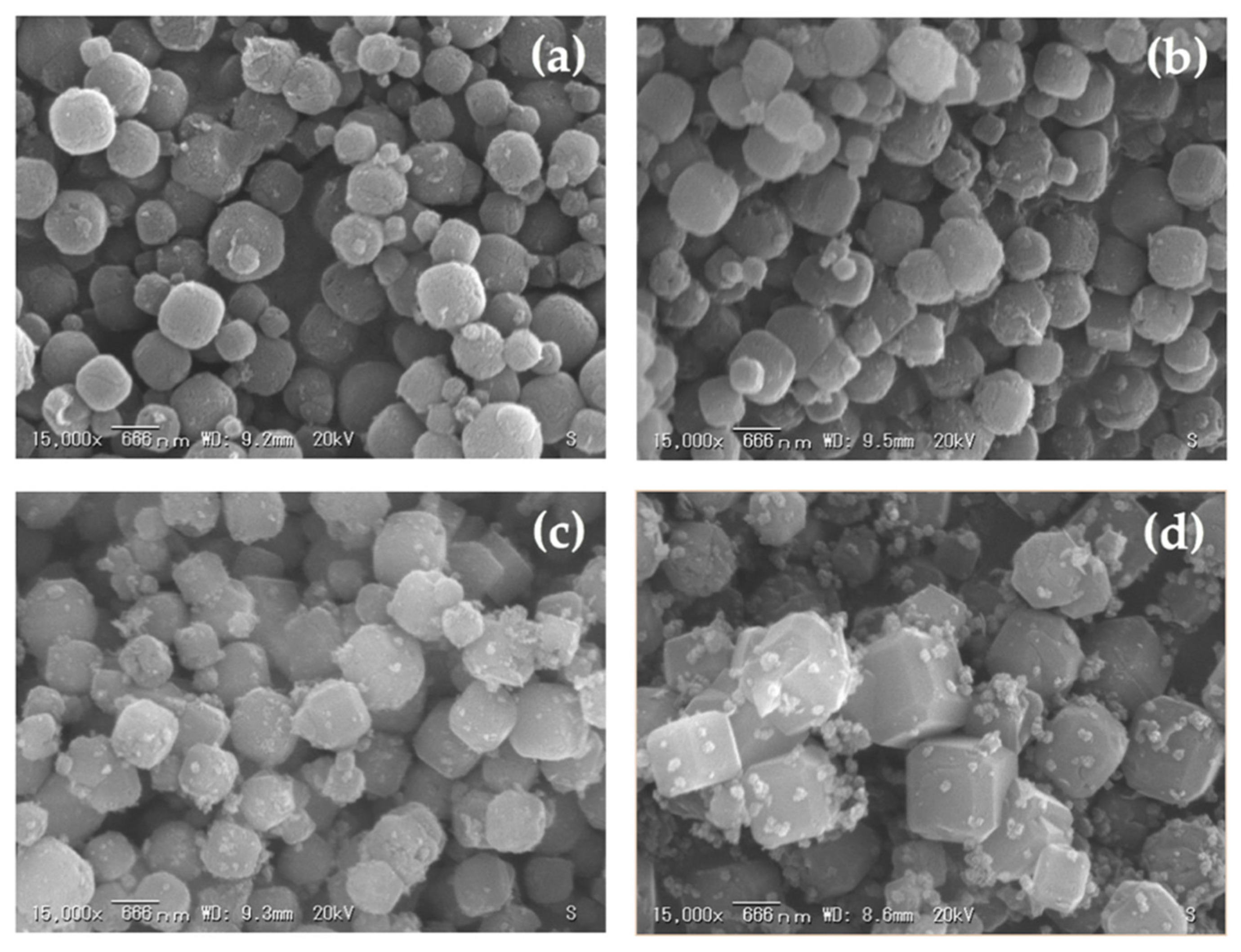
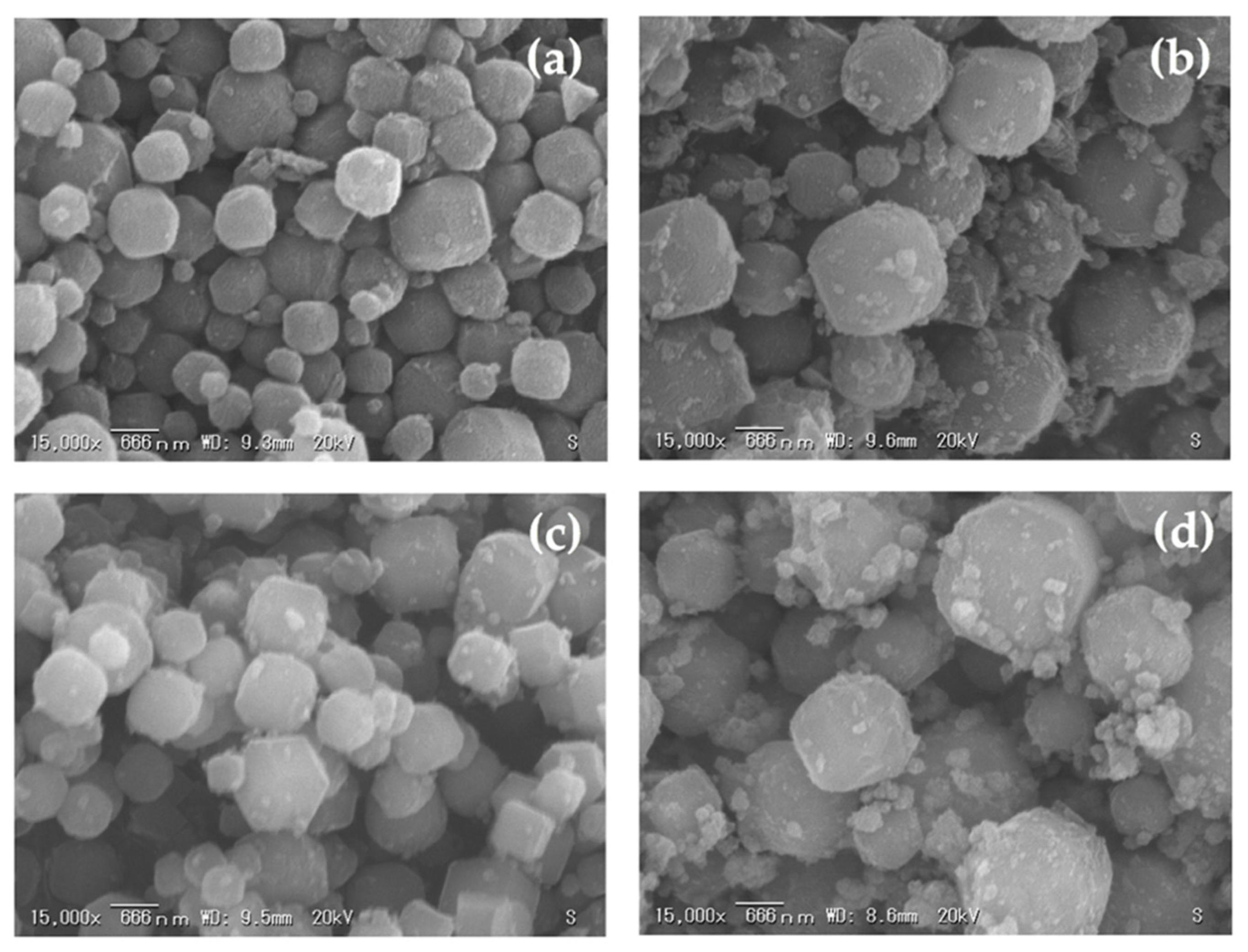
3.4. The Effect of Curing Time
3.5. Cation Exchange Capacity (CEC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippine Statistics Authority. Rice and Corn Situation and Outlook; Philippine Statistics Authority: Manila, Philippines, October 2018; pp. 1–7. [Google Scholar]
- Habito, C.F.; Briones, R.M. Philippine agriculture over the years: Performance, policies and pitfalls. Agriculture 2005, 38. [Google Scholar]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gerpacio, R.V.; Labios, J.D.; Labios, R.V.; Diangkinay, E.I. Maize in the Philippines: Production Systems, Constraints, and Research Priorities; Maize Production Systems Papers; CIMMYT International Maize and Wheat Improvement Center: Los Baños, Philippines, 2004. [Google Scholar]
- Tanchuling, H. Issues and Prospects of the Philippine Corn Industry; Jessica Reyes-Cantos, J.S., Ed.; Rice Watch and Action Network: Quezon City, Philippines, 2007. [Google Scholar]
- Kriz, A.L.; Larkins, B.A. Molecular Genetic Approaches to Maize; Kriz, A.L., Larkins, B.A., Eds.; Springer Science & Business Media: New Delhi, India, 2008; ISBN 9783540689225. [Google Scholar]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, N.; Sucahya, T.N.; Dani Nandiyanto, A.B. Review: Agricultural Wastes as a Source of Silica Material. Indones. J. Sci. Technol. 2016, 1, 82. [Google Scholar] [CrossRef]
- Adam, F.; Appaturi, J.N.; Iqbal, A. The utilization of rice husk silica as a catalyst: Review and recent progress. Catal. Today 2012, 190, 2–14. [Google Scholar] [CrossRef]
- Nakashima, H.; Omae, K.; Takebayashi, T.; Ishizuka, C.; Uemura, T. Toxicity of silicon compounds in semiconductor industries. J. Occup. Health 1998, 40, 270–275. [Google Scholar] [CrossRef]
- Petrov, I.; Michalev, T. Synthesis of Zeolite A: A Review. 2012, pp. 30–35. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1049.109&rep=rep1&type=pdf (accessed on 27 August 2021).
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X. Preparation and characteristics of anion exchanger from corn stalks. Desalination 2011, 274, 113–119. [Google Scholar] [CrossRef]
- Bohra, S.; Kundu, D.; Naskar, M.K. One-pot synthesis of NaA and NaP zeolite powders using agro-waste material and other low cost organic-free precursors. Ceram. Int. 2014, 40, 1229–1234. [Google Scholar] [CrossRef]
- Farías, T.; de Ménorval, L.C.; Picazo, O.; Jordán, R. Ultrasonic and conventional synthesis of NaA zeolite from rice husk ash. J. Phys. Conf. Ser. 2017, 792, 12032. [Google Scholar] [CrossRef]
- Petkowicz, D.I.; Rigo, R.T.; Radtke, C.; Pergher, S.B.; dos Santos, J.H.Z. Zeolite NaA from Brazilian chrysotile and rice husk. Microporous Mesoporous Mater. 2008, 116, 548–554. [Google Scholar] [CrossRef]
- Santasnachok, C.; Kurniawan, W.; Hinode, H. The use of synthesized zeolites from power plant rice husk ash obtained from Thailand as adsorbent for cadmium contamination removal from zinc mining. J. Environ. Chem. Eng. 2015, 3, 2115–2126. [Google Scholar] [CrossRef]
- Panpa, W.; Jinawath, S. Synthesis of ZSM-5 zeolite and silicalite from rice husk ash. Appl. Catal. B Environ. 2009, 90, 389–394. [Google Scholar] [CrossRef]
- Saada, M.A.; Soulard, M.; Patarin, J.; Regis, R.C. Synthesis of zeolite materials from asbestos wastes: An economical approach. Microporous Mesoporous Mater. 2009, 122, 275–282. [Google Scholar] [CrossRef]
- Mukherjee, S.; Barman, S.; Halder, G. Fluoride uptake by zeolite NaA synthesized from rice husk: Isotherm, kinetics, thermodynamics and cost estimation. Groundw. Sustain. Dev. 2018, 7, 39–47. [Google Scholar] [CrossRef]
- Wang, Y.; Du, T.; Jia, H.; Qiu, Z.; Song, Y. Synthesis, characterization and CO2 adsorption of NaA, NaX and NaZSM-5 from rice husk ash. Solid State Sci. 2018, 86, 24–33. [Google Scholar] [CrossRef]
- Maghfirah, A.; Ilmi, M.M.; Fajar, A.T.N.; Kadja, G.T.M. A review on the green synthesis of hierarchically porous zeolite. Mater. Today Chem. 2020, 17. [Google Scholar] [CrossRef]
- Othman Ali, I.; Hassan, A.M.; Shaaban, S.M.; Soliman, K.S. Synthesis and characterization of ZSM-5 zeolite from rice husk ash and their adsorption of Pb2+ onto unmodified and surfactant-modified zeolite. Sep. Purif. Technol. 2011, 83, 38–44. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, M.; Li, J.; Su, X.; Pan, S.; Jiao, C.; Feng, M. Synthesis of MCM-22 zeolite using rice husk as a silica source under varying-temperature conditions. J. Colloid Interface Sci. 2012, 369, 388–394. [Google Scholar] [CrossRef]
- Katsuki, H.; Komarneni, S. Synthesis of Na-A and/or Na-X zeolite/porous carbon composites from carbonized rice husk. J. Solid State Chem. 2009, 182, 1749–1753. [Google Scholar] [CrossRef]
- Moisés, M.P.; Da Silva, C.T.P.; Meneguin, J.G.; Girotto, E.M.; Radovanovic, E. Synthesis of zeolite NaA from sugarcane bagasse ash. Mater. Lett. 2013, 108, 243–246. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na-X and Na-A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Esmaeili, A.; Saremnia, B. Synthesis and characterization of NaA zeolite nanoparticles from Hordeum vulgare L. husk for the separation of total petroleum hydrocarbon by an adsorption process. J. Taiwan Inst. Chem. Eng. 2016, 61, 276–286. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, A. Effect of stirring on the dissolution of coal fly ash and synthesis of pure-form Na-A and -X zeolites by two-step process. Adv. Powder Technol. 2009, 20, 473–479. [Google Scholar] [CrossRef]
- Fernandes Machado, N.R.C.; Miotto, D.M.M. Synthesis of Na-A and -X zeolites from oil shale ash. Fuel 2005, 84, 2289–2294. [Google Scholar] [CrossRef]
- Ríos R., C.A.; Williams, C.D.; Roberts, C.L. A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites. Fuel 2009, 88, 1403–1416. [Google Scholar] [CrossRef]
- Izidoro, J.C.; Kim, M.C.; Bellelli, V.F.; Pane, M.C.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Synthesis of zeolite A using the waste of iron mine tailings dam and its application for industrial effluent treatment. J. Sustain. Min. 2019, 18, 277–286. [Google Scholar] [CrossRef]
- Hamadi, A.; Nabih, K. Synthesis of Zeolites Materials Using Fly Ash and Oil Shale Ash and Their Applications in Removing Heavy Metals from Aqueous Solutions. J. Chem. 2018, 2018, 6207910. [Google Scholar] [CrossRef] [Green Version]
- Łach, M.; Grela, A.; Komar, N.; Mikuła, J.; Hebda, M. Calcined post-production waste as materials suitable for the hydrothermal synthesis of zeolites. Materials 2019, 12, 2742. [Google Scholar] [CrossRef] [Green Version]
- Ginting, S.B.; Yulia, Y.; Wardono, H.; Darmansyah; Hanif, M.; Iryani, D.A. Synthesis and Characterization of Zeolite Lynde Type A (LTA): Effect of Aging Time. J. Phys. Conf. Ser. 2019, 1376. [Google Scholar] [CrossRef] [Green Version]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Gougazeh, M.; Buhl, J.C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Díaz, I. Conventional versus alkali fusion synthesis of zeolite A from low grade kaolin. Appl. Clay Sci. 2016, 132–133, 485–490. [Google Scholar] [CrossRef]
- Maia, A.Á.B.; Dias, R.N.; Angélica, R.S.; Neves, R.F. Influence of an aging step on the synthesis of zeolite NaA from Brazilian Amazon kaolin waste. J. Mater. Res. Technol. 2019, 8, 2924–2929. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Díaz, I. Synthesis of zeolite A from Ethiopian kaolin. Microporous Mesoporous Mater. 2015, 215, 29–36. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally synthesized zeolites based on kaolinite: A review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Anuwattana, R.; Khummongkol, P. Conventional hydrothermal synthesis of Na-A zeolite from cupola slag and aluminum sludge. J. Hazard. Mater. 2009, 166, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Park, J.W.; Kam, S.K.; Lee, C.H. Synthesis of Na-A zeolite from Jeju Island scoria using fusion/hydrothermal method. Chemosphere 2018, 207, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Optimisation of zeolite LTA synthesis from alum sludge and the influence of the sludge source. J. Environ. Sci. (China) 2021, 99, 130–142. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P.K. Zeolites: A Primer. ChemInform 2004, 35. [Google Scholar] [CrossRef]
- Flanigen, E.M. Chapter 2 Zeolites and Molecular Sieves an Historical Perspective. Stud. Surf. Sci. Catal. 1991, 58, 13–34. [Google Scholar] [CrossRef]
- Breck, D.W.; Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; A Wiley-Interscience Publication; Wiley: Hoboken, NJ, USA, 1973; ISBN 9780471099857. [Google Scholar]
- Chester, A.W.; Derouane, E.G. Zeolite Characterization and Catalysis, 5th ed.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2009; ISBN 9781402096778. [Google Scholar]
- Mackinnon, I.D.R.; Millar, G.J.; Stolz, W. Hydrothermal syntheses of zeolite N from kaolin. Appl. Clay Sci. 2012, 58, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Geng, H.; Li, G.; Liu, D.; Liu, C. Rapid and efficient synthesis of CHA-type zeolite by interzeolite conversion of LTA-type zeolite in the presence of N, N, N-trimethyladamantammonium hydroxide. J. Solid State Chem. 2018, 265, 193–199. [Google Scholar] [CrossRef]
- Mayoral, A.; Carey, T.; Anderson, P.A.; Diaz, I. Atomic resolution analysis of porous solids: A detailed study of silver ion-exchanged zeolite A. Microporous Mesoporous Mater. 2013, 166, 117–122. [Google Scholar] [CrossRef]
- Benaliouche, F.; Hidous, N.; Guerza, M.; Zouad, Y.; Boucheffa, Y. Characterization and water adsorption properties of Ag- and Zn-exchanged A zeolites. Microporous Mesoporous Mater. 2015, 209, 184–188. [Google Scholar] [CrossRef]
- Ryu, T.; Kim, H.; Hong, S.B. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13. Appl. Catal. B Environ. 2019, 245, 513–521. [Google Scholar] [CrossRef]
- Xu, J.; Harold, M.P.; Balakotaiah, V. Modeling the effects of Pt loading on NOx storage on Pt/BaO/Al2O3 catalysts. Appl. Catal. B Environ. 2011, 104, 305–315. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Mohan, N.; Cindrella, L. Template-free synthesis of Pt-MOx (M = Ni, Co & Ce) supported on cubic zeolite-A and their catalytic role in methanol oxidation and oxygen reduction reactions characterized by the hydrodynamic study. Int. J. Hydrogen Energy 2017, 42, 21719–21731. [Google Scholar] [CrossRef]
- Bin Lim, J.; Cha, S.H.; Hong, S.B. Direct N2O decomposition over iron-substituted small-pore zeolites with different pore topologies. Appl. Catal. B Environ. 2019, 243, 750–759. [Google Scholar] [CrossRef]
- Cay-Durgun, P.; Fink, S.G.; Shabilla, A.; Yin, H.; Sasaki, K.A.; Lind, M.L. Analysis of the Water Permeability of Linde Type A Zeolites in Reverse Osmosis. Sep. Sci. Technol. 2014, 49, 2824–2833. [Google Scholar] [CrossRef]
- Shirazian, S.; Ashrafizadeh, S.N. Synthesis of substrate-modified LTA zeolite membranes for dehydration of natural gas. Fuel 2015, 148, 112–119. [Google Scholar] [CrossRef]
- Tokmachev, M.G.; Tikhonov, N.A.; Khamizov, R.K. Investigation of cyclic self-sustaining ion exchange process for softening water solutions on the basis of mathematical modeling. React. Funct. Polym. 2008, 68, 1245–1252. [Google Scholar] [CrossRef]
- Bessa, R.; da Costa, L.S.; Oliveira, C.P.; Bohn, F.; Nascimento, R.; Sasaki, J.M.; Loiola, A.R. Kaolin-based magnetic zeolites A and P as water softeners. Microporous Mesoporous Mater. 2017, 245, 64–72. [Google Scholar] [CrossRef]
- Millar, G.J.; Winnett, A.; Thompson, T.; Couperthwaite, S.J. Equilibrium studies of ammonium exchange with Australian natural zeolites. J. Water Process Eng. 2016, 9, 47–57. [Google Scholar] [CrossRef]
- Djamel, N.; Samira, A. Mechanism of Cu2+ ions uptake process by synthetic NaA zeolite from aqueous solution: Characterization, Kinetic, intra-crystalline diffusion and thermodynamic studies. J. Mol. Liq. 2021, 323, 114642. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Abdelrahman, E.A. Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq. 2018, 253, 72–82. [Google Scholar] [CrossRef]
- Mahmodi, G.; Dangwal, S.; Zarrintaj, P.; Zhu, M.; Mao, Y.; Mcllroy, D.N.; Reza Saeb, M.; Vatanpour, V.; Ramsey, J.D.; Kim, S.J. NaA zeolite-coated meshes with tunable hydrophilicity for oil-water separation. Sep. Purif. Technol. 2020, 240, 116630. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Barakat, M.A. Rice husk ash as a renewable source for the production of zeolite NaY and its characterization. Arab. J. Chem. 2015, 8, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.W.; Yang, H.C.; Roselin, L.S.; Kuo, W.Y. Ethanol dehydrogenation over copper catalysts on rice husk ash prepared by ion exchange. Appl. Catal. A Gen. 2006, 304, 30–39. [Google Scholar] [CrossRef]
- Sannino, F.; Ruocco, S.; Marocco, A.; Esposito, S.; Pansini, M. Cyclic process of simazine removal from waters by adsorption on zeolite H-Y and its regeneration by thermal treatment. J. Hazard. Mater. 2012, 229–230, 354–360. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brooks/Cole Cengage Learning: Belmont, CA, USA, 2010; ISBN 9780495114789. [Google Scholar]
- Evi, M.; Sari, F.; Prasetyoko, D. Direct Synthesis of Sodalite from Kaolin: The Influence of Alkalinity. Indones. J. Chem. 2018, 18, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Zunino, F.; Boehm-Courjault, E.; Scrivener, K. The impact of calcite impurities in clays containing kaolinite on their reactivity in cement after calcination. Mater. Struct. Constr. 2020, 53. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Zeolite Science and Technology; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203911167. [Google Scholar]
- Krznarić, I.; Antonić, T.; Subotić, B.; Babić-Ivančić, V. Results of thermal and hydrothermal treatment of the aluminosilicate gels prepared at different batch concentrations. Thermochim. Acta 1998, 317, 73–84. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, Y.P.; Cui, X.M.; He, Y.; Mao, J. Influence of synthesis parameters on NaA zeolite crystals. Powder Technol. 2013, 243, 184–193. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, D.; Jia, W.; Tang, D.; Li, X.; Yang, R. Studies on room-temperature synthesis of zeolite NaA. Mater. Res. Bull. 2014, 52, 96–102. [Google Scholar] [CrossRef]
- Bronić, J.; Palčić, A.; Subotić, B.; Itani, L.; Valtchev, V. Influence of alkalinity of the starting system on size and morphology of the zeolite A crystals. Mater. Chem. Phys. 2012, 132, 973–976. [Google Scholar] [CrossRef]
- Andrades, R.C.; Neves, R.F.; Diaz, F.R.V.; Júnior, A.H.M. Influence of alkalinity on the synthesis of zeolite A and hydroxysodalite from metakaolin. J. Nano Res. 2020, 61, 51–60. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of zeolites at low temperatures in fly ash-kaolinite mixtures. Microporous Mesoporous Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Youssef, H.F.; Materials, A. Utilization of Egyptian kaolin for Zeolite-A Preparation and Performance Evaluation. Int. Conf. Environ. Sci. Technol. 2011, 6, 43–48. [Google Scholar]
- Dali Youcef, L.; López-Galindo, A.; Verdugo-Escamilla, C.; Belaroui, L.S. Synthesis and characterization of zeolite LTA by hydrothermal transformation of a natural Algerian palygorskite. Appl. Clay Sci. 2020, 193, 105690. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Jafari, M.; Kazemimoghadam, M.; Mohammadi, T. Template free crystallization of zeolite Rho via Hydrothermal synthesis: Effects of synthesis time, synthesis temperature, water content and alkalinity. Ceram. Int. 2013, 39, 7149–7158. [Google Scholar] [CrossRef]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 2017, 28, 1827–1840. [Google Scholar] [CrossRef]
- Bayati, B.; Babaluo, A.A.; Karimi, R. Hydrothermal synthesis of nanostructure NaA zeolite: The effect of synthesis parameters on zeolite seed size and crystallinity. J. Eur. Ceram. Soc. 2008, 28, 2653–2657. [Google Scholar] [CrossRef]
- Loiola, A.R.; Andrade, J.C.R.A.; Sasaki, J.M.; da Silva, L.R.D. Structural analysis of zeolite NaA synthesized by a cost-effective hydrothermal method using kaolin and its use as water softener. J. Colloid Interface Sci. 2012, 367, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Munthali, M.W.; Kabwadza-Corner, P.; Johan, E.; Matsue, N. Decrease in Cation Exchange Capacity of Zeolites at Neutral pH: Examples and Proposals of a Determination Method. J. Mater. Sci. Chem. Eng. 2014, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Jain, S. History, introduction, and kinetics of ion exchange materials. J. Chem. 2013, 2013, 957647. [Google Scholar] [CrossRef]
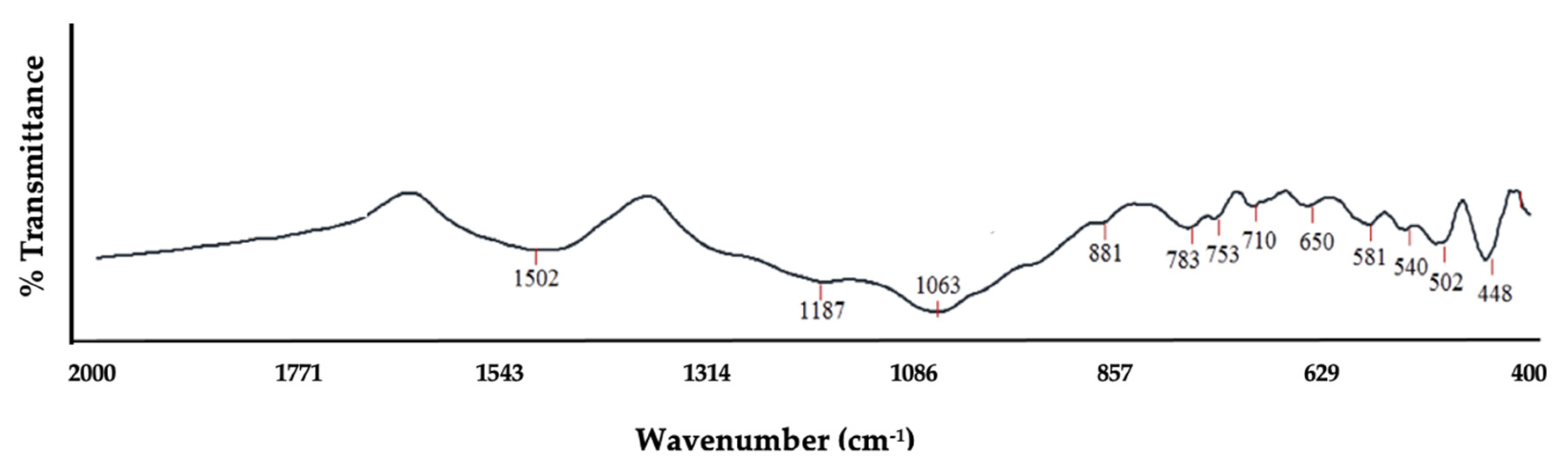


| Vibrations | Chemical Shift | Functionality | Absorption Bands (cm−1) |
|---|---|---|---|
| bending vibrations –O–T–O (deformation vibration of Si–O) | 448, 502, 540 | ||
| coordinated Al octahedral | C–H Bend (medium) | Alkene | 581 |
| symmetric stretching vibrations of bridge bonds –vs T–O–T (vs Si–O–Si) | Si–O | Silanol/Siloxane | 650, 710, 753, 783 |
| Si translation | C–H Bend (strong) | Aromatic | 881 |
| Bending vibration O–H | OH | 1028 | |
| asymmetric stretching vibrations of bridge bonds –vas T–O(T) (Si–O stretching) | C–O Stretch (strong) | Ether/Alcohol | 1063, 1187 |
| Curing Time (h) | Fusion Ratios (Corn Stover Ash:NaOH) meq Na+/g | |||
|---|---|---|---|---|
| 0.5:1.0 | 1.0:1.0 | 1.0:1.5 | 1.0:2.0 | |
| 6.0 | 49.52% | 52.38% | 56.64% | 51.26% |
| 9.0 | 55.08% | 55.97% | 58.18% | 52.30% |
| 12.0 | 51.80% | 52.73% | 55.01% | 50.50% |
| 24.0 | 53.37% | 55.16% | 55.14% | 53.07% |
| Curing Time (h) | Fusion Ratios (Corn Stover Ash:NaOH) meq Na+/g | |||
|---|---|---|---|---|
| 0.5:1.0 | 1.0:1.0 | 1.0:1.5 | 1.0:2.0 | |
| 6.0 | 1.789 | 1.852 | 1.925 | 1.849 |
| 9.0 | 1.827 | 2.142 | 2.439 | 2.275 |
| 12.0 | 1.861 | 2.139 | 2.428 | 2.158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pangan, N.; Gallardo, S.; Gaspillo, P.-a.; Kurniawan, W.; Hinode, H.; Promentilla, M. Hydrothermal Synthesis and Characterization of Zeolite A from Corn (Zea Mays) Stover Ash. Materials 2021, 14, 4915. https://doi.org/10.3390/ma14174915
Pangan N, Gallardo S, Gaspillo P-a, Kurniawan W, Hinode H, Promentilla M. Hydrothermal Synthesis and Characterization of Zeolite A from Corn (Zea Mays) Stover Ash. Materials. 2021; 14(17):4915. https://doi.org/10.3390/ma14174915
Chicago/Turabian StylePangan, Norway, Susan Gallardo, Pag-asa Gaspillo, Winarto Kurniawan, Hirofumi Hinode, and Michael Promentilla. 2021. "Hydrothermal Synthesis and Characterization of Zeolite A from Corn (Zea Mays) Stover Ash" Materials 14, no. 17: 4915. https://doi.org/10.3390/ma14174915
APA StylePangan, N., Gallardo, S., Gaspillo, P.-a., Kurniawan, W., Hinode, H., & Promentilla, M. (2021). Hydrothermal Synthesis and Characterization of Zeolite A from Corn (Zea Mays) Stover Ash. Materials, 14(17), 4915. https://doi.org/10.3390/ma14174915







