Corrosion Behavior of Zn, Fe and Fe-Zn Powder Materials Prepared via Uniaxial Compression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fe, Zn and Fe-Zn Pellet Preparation
2.2. Surface Morphology, X-ray and EDX Analysis
2.3. Corrosion Measurements
2.3.1. Electrochemical Tests
2.3.2. PH and Ions Concentration Determination
3. Results
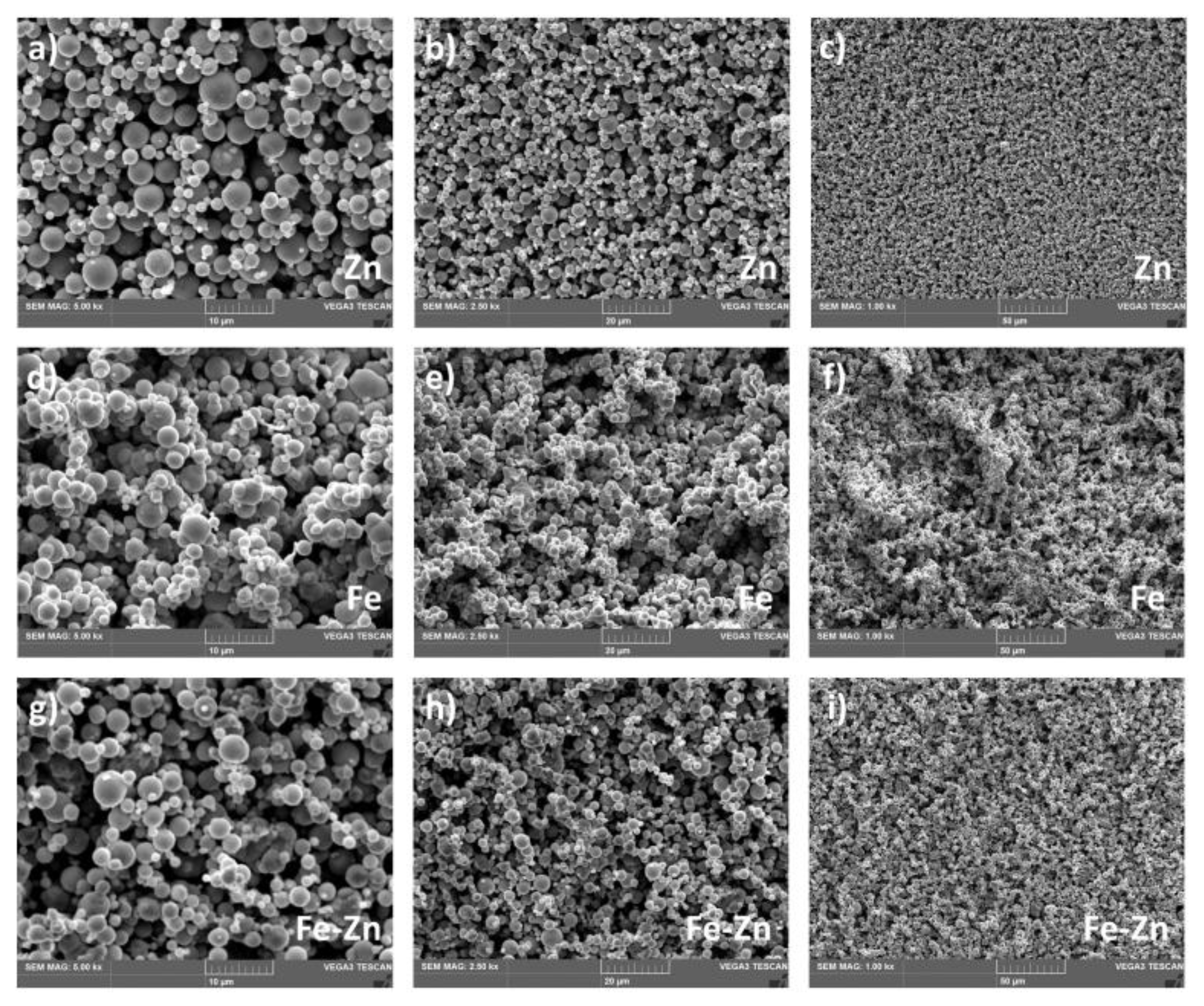
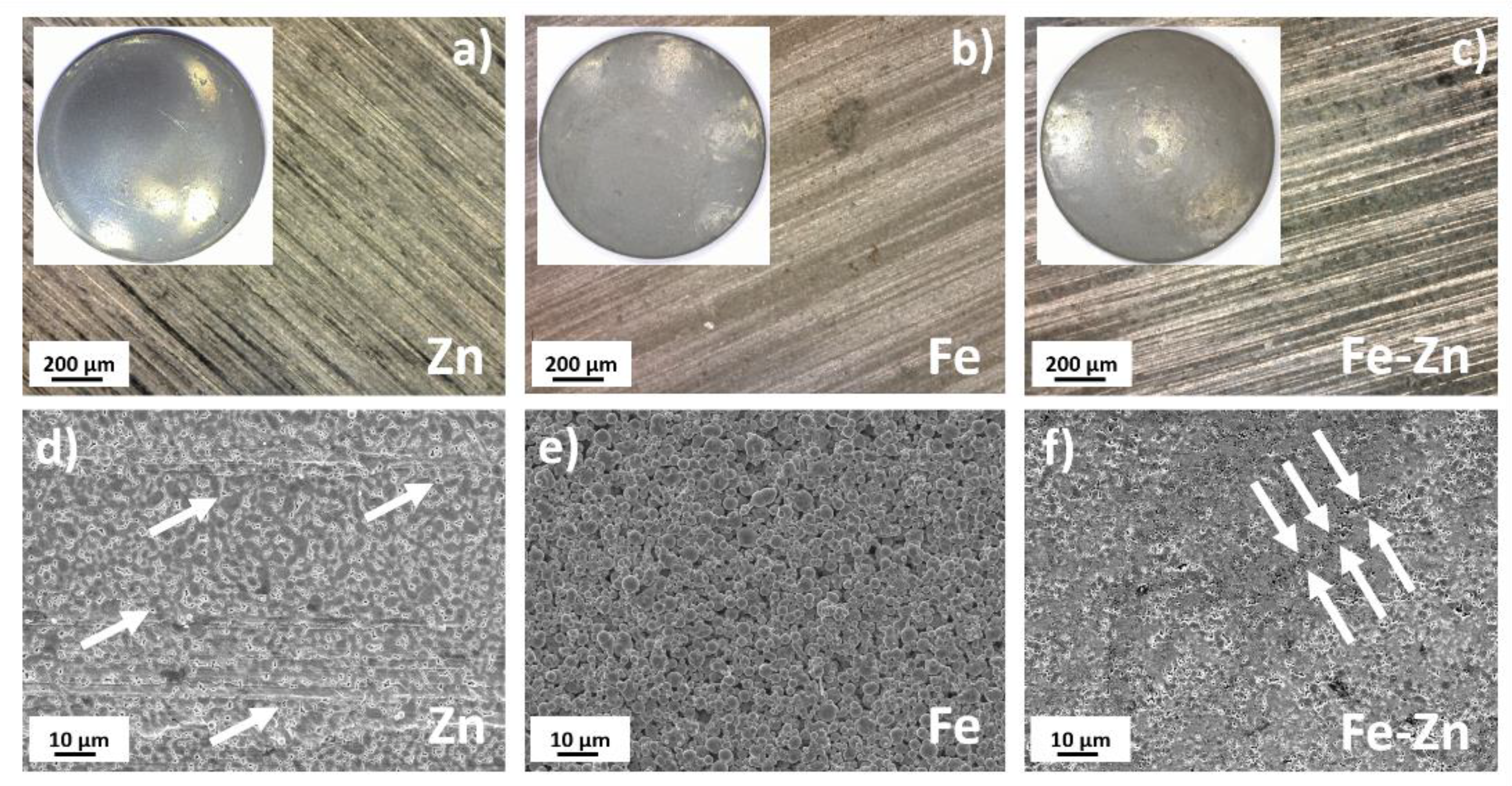
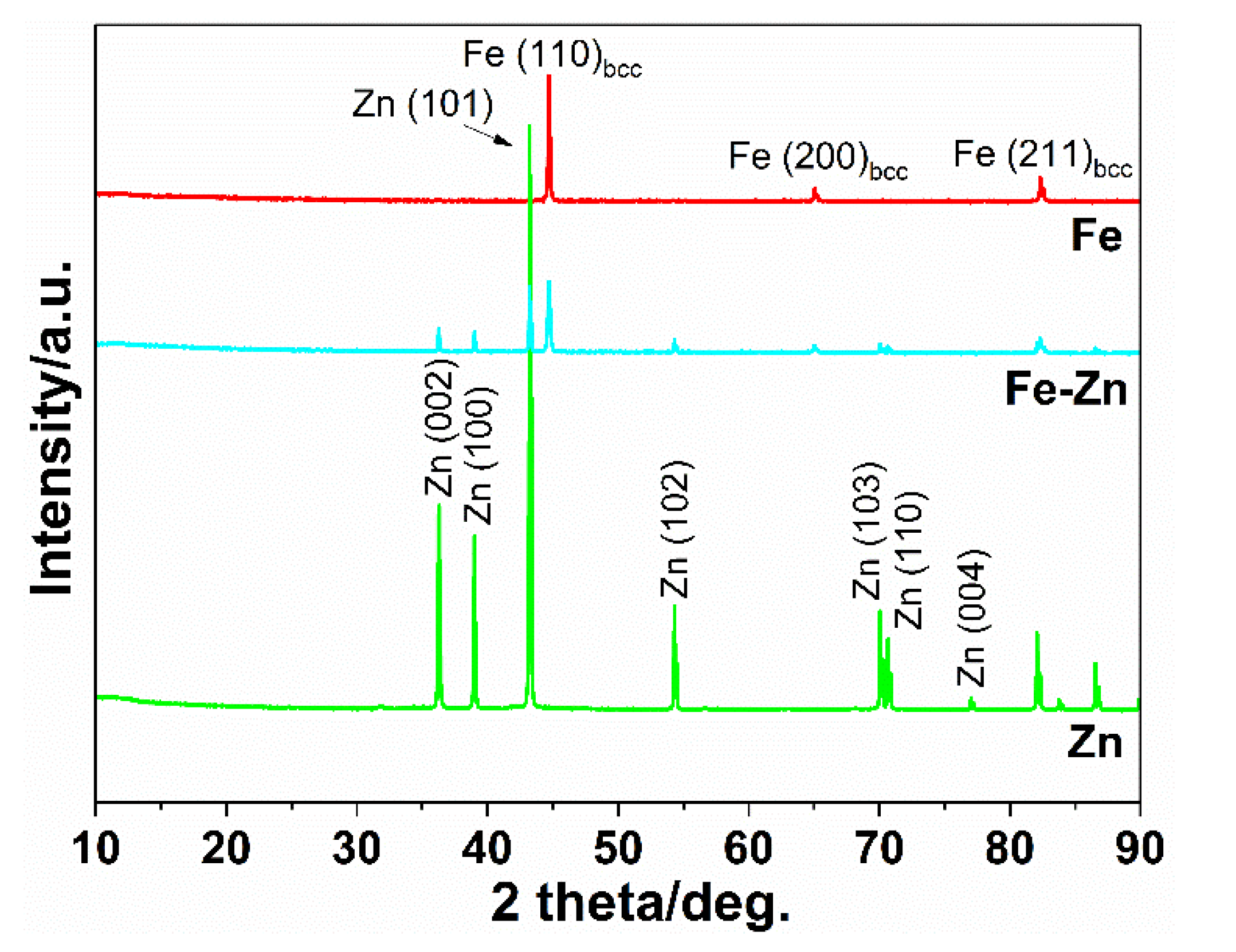
3.1. Fe, Zn and Fe-Zn Powders and Compressed Samples Characterization
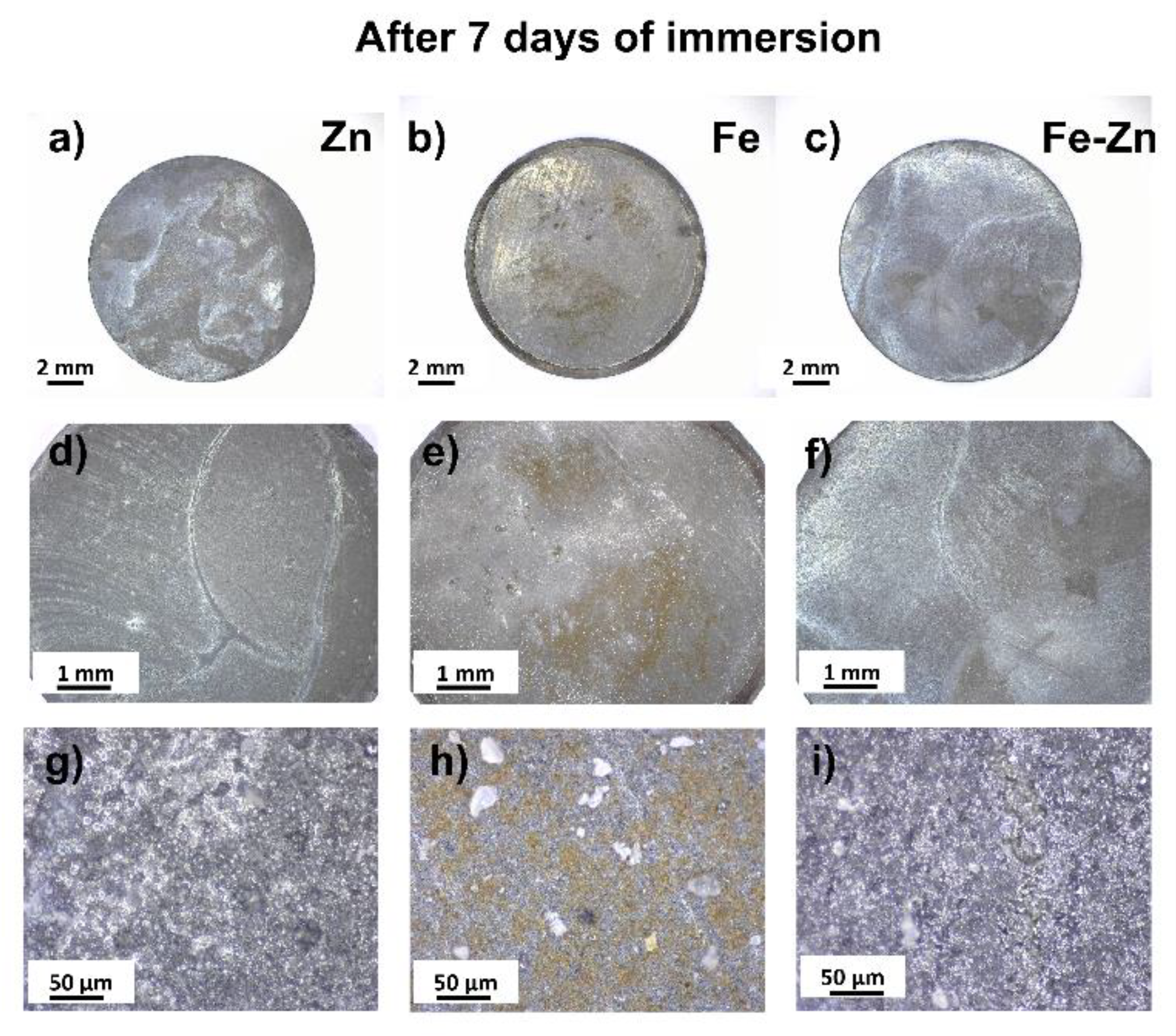
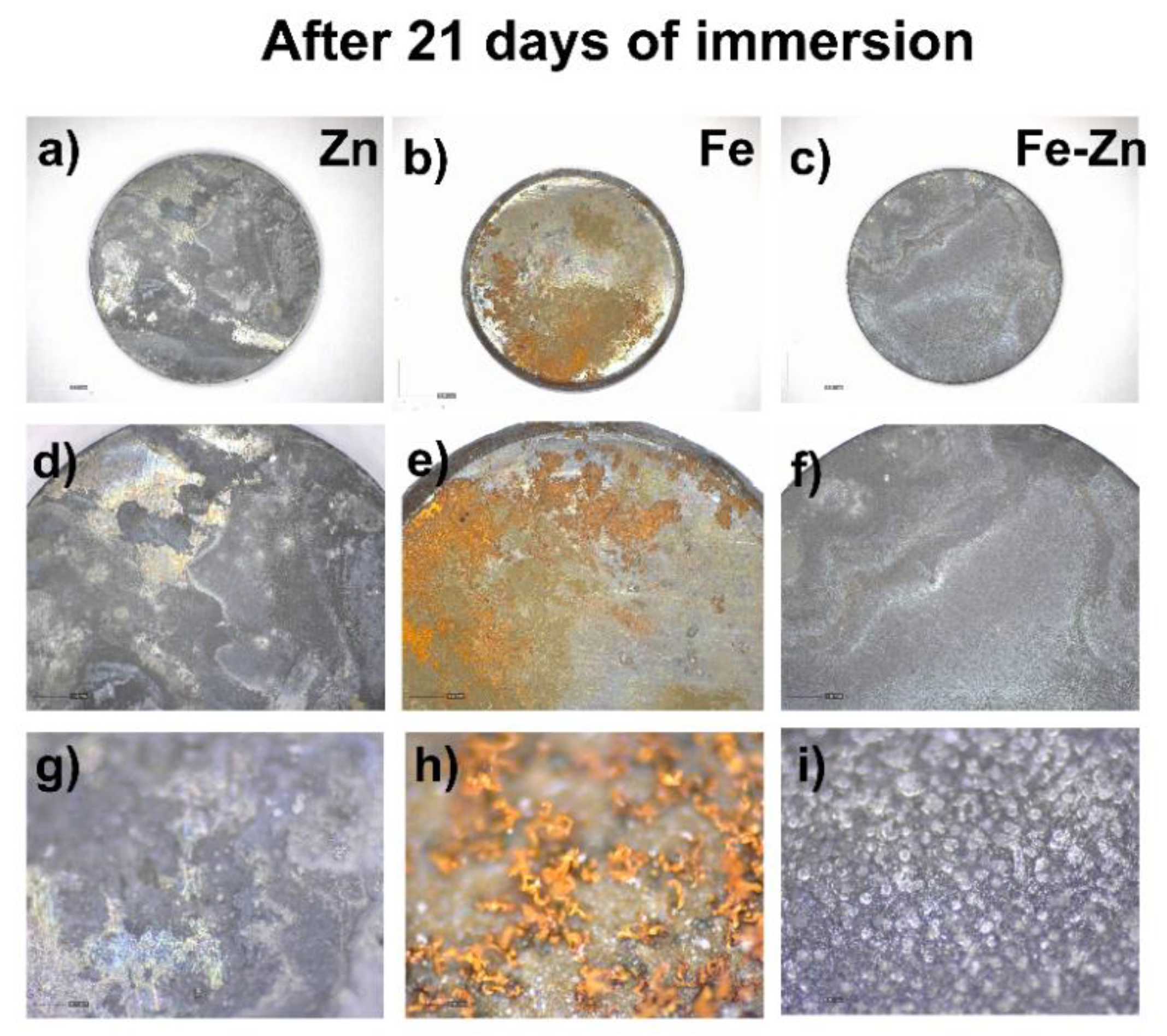
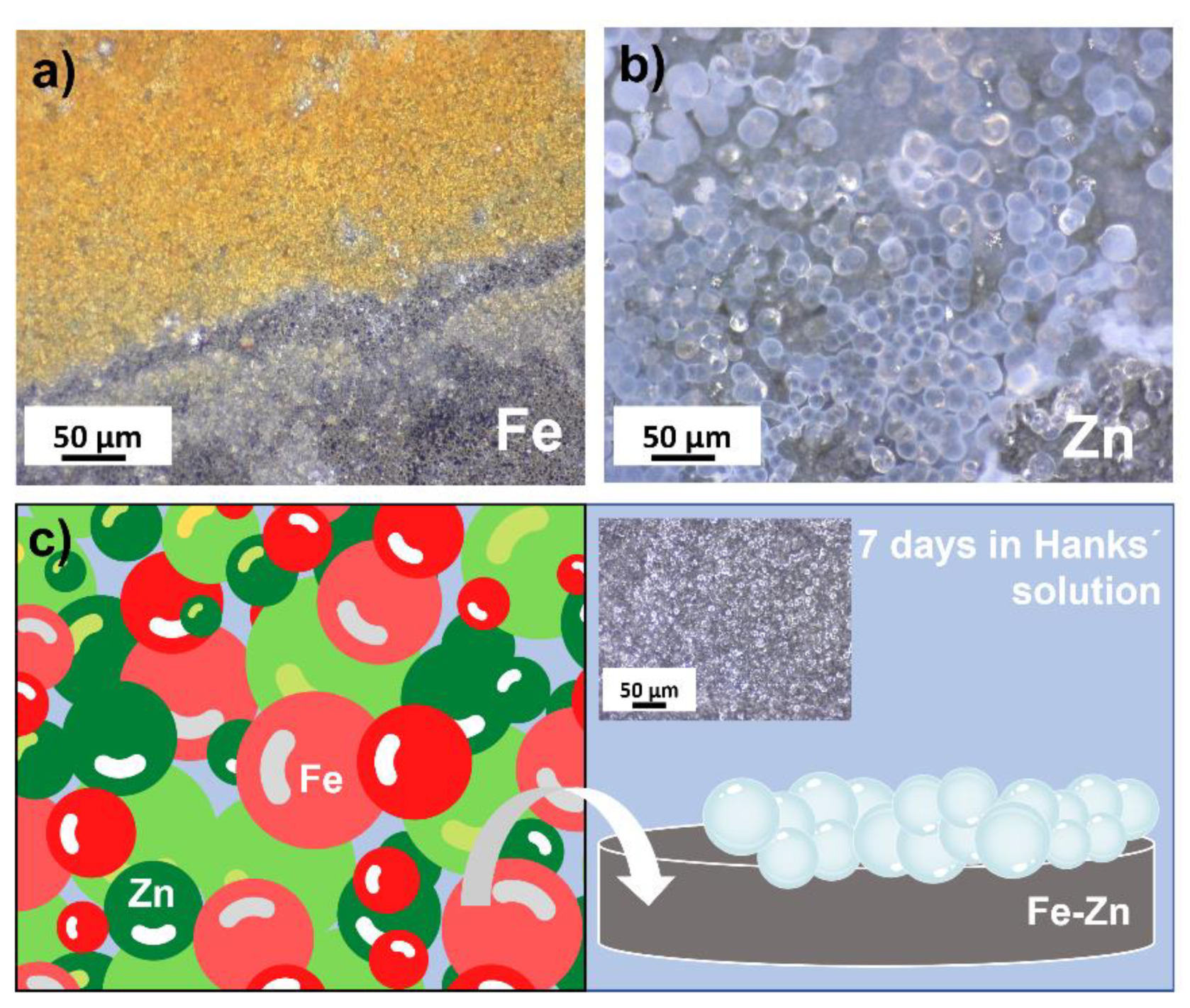
3.2. Degradation Behavior of Compressed Fe, Zn and Fe-Zn Powders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 85–98. [Google Scholar] [CrossRef]
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Binyamin, G.; Shafi, B.M.; Mery, C.M. Biomaterials: A primer for surgeons. Semin. Pediatric Surg. 2006, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Turitto, V.; Slack, S.M. Blood and Related Fluids; Springer: New York, NY, USA, 2016; ISBN 9781493933051. [Google Scholar]
- Thouas, G.A.; Chen, Q. Metallic Implant Materials. In Materials Science and Engineering: R: Reports; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–57. [Google Scholar]
- Cohn, M.R.; Unnanuntana, A.; Pannu, T.J.; Warner, S.J.; Lane, J.M. 7.16 Materials in fracture fixation. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 278–297. ISBN 9780081006924. [Google Scholar]
- Pellicer, E.; Lorenzetti, M.; Fornell, J.; Baró, M.D.; Novak, S.; Sort, J. Progress Beyond the State-of-the-Art in the Field of Metallic Materials for Bioimplant Applications. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 25. ISBN 978-3-319-68025-5. [Google Scholar]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe-Mn alloys for stents. J. Biomed. Mater. Res. Part A 2010, 93, 1–11. [Google Scholar] [CrossRef]
- Oriňaková, R.; Gorejová, R.; Petráková, M.; Králová, Z.O.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Podobová, M.; Baláž, M.; Smith, R.M. Degradation performance of open-cell biomaterials from phosphated carbonyl iron powder with PEG coating. Materials 2020, 13, 4134. [Google Scholar] [CrossRef]
- Popescu, I.N.; Vidu, R.; Bratu, V. Porous Metallic Biomaterials Processing (Review) Part 1: Compaction, Sintering Behavior, Properties and Medical Applications. Sci. Bull. Valahia Univ. Mater. Mech. 2017, 15, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Li, P.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal-Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl. Mater. Interfaces 2018, 10, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Vojtěch, D.; Kubásek, J.; Čapek, J.; Pospíšilová, I. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, Y.F. In vitro study on newly designed biodegradable Fe-X composites (X = W, CNT) prepared by spark plasma sintering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 485–497. [Google Scholar] [CrossRef]
- Chou, D.T.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Stehlíková, K.; Michalcová, A.; Msallamová, Š.; Vojtěch, D. Microstructure, mechanical and corrosion properties of biodegradable powder metallurgical Fe-2 wt% X (X = Pd, Ag and C) alloys. Mater. Chem. Phys. 2016, 181, 501–511. [Google Scholar] [CrossRef]
- Králová, Z.O.; Gorejová, R.; Oriňaková, R.; Petráková, M.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Sopčák, T.; Baláž, M.; Maskaľová, I.; et al. Biodegradable zinc-iron alloys: Complex study of corrosion behavior, mechanical properties and hemocompatibility. Prog. Nat. Sci. Mater. Int. 2021, 31, 279–287. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. The Effects of 4%Fe on the Performance of Pure Zinc as Biodegradable Implant Material. Ann. Biomed. Eng. 2019, 47, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn–Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Wegener, B.; Sichler, A.; Milz, S.; Sprecher, C.; Pieper, K.; Hermanns, W.; Jansson, V.; Nies, B.; Kieback, B.; Müller, P.E.; et al. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3–22. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Oriňaková, R.; Gorejová, R.; Králová, Z.O.; Petráková, M.; Oriňak, A. Novel trends and recent progress on preparation methods of biodegradable metallic foams for biomedicine: A review. J. Mater. Sci. 2021, 13925–13963. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Casales-Diaz, M.; Rivera-Grau, L.M.; Ortega-Toledo, D.M.; Ascencio-Gutierrez, J.A.; Martinez-Gomez, L. Effect of the diesel, inhibitor, and CO2 additions on the corrosion performance of 1018 carbon steel in 3% NaCl solution. J. Chem. 2014, 2014, 940579. [Google Scholar] [CrossRef] [Green Version]
- Gandha, K.; Tsai, P.; Chaubey, G.; Poudyal, N.; Elkins, K.; Cui, J.; Liu, J.P. Synthesis and characterization of FeCo nanowires with high coercivity. Nanotechnology 2015, 26, 075601. [Google Scholar] [CrossRef]
- Singh, S.; Basu, S.; Gupta, M.; Vedpathakz, M.; Kodama, R.H. Investigation of interface magnetic moment of FeGe multilayer: A neutron reflectivity study. J. Appl. Phys. 2007, 101, 033913. [Google Scholar] [CrossRef] [Green Version]
- Hirano, Y.; Kasai, Y.; Sagata, K.; Kita, Y. Unique approach for transforming glucose to C3 platform chemicals using metallic iron and a Pd/C catalyst in water. Bull. Chem. Soc. Jpn. 2016, 89, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Vourlias, G. Application of X-rays diffraction for identifying thin oxide surface layers on zinc coatings. Coatings 2020, 10, 1005. [Google Scholar] [CrossRef]
- Ullah, S.; Badshah, A.; Ahmed, F.; Raza, R.; Altaf, A.A.; Hussain, R. Electrodeposited zinc electrodes for high current Zn/AgO bipolar batteries. Int. J. Electrochem. Sci. 2011, 6, 3801–3811. [Google Scholar]
- Qasim, I.; Mumtaz, M.; Nadeem, K.; Abbas, S.Q. Zinc Nanoparticles at Intercrystallite Sites of (Cu0.5Tl0.5)Ba2Ca3Cu4O12-δ Superconductor. J. Nanomater. 2016, 2016, 9781790. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Virtanen, S. Influence of bovine serum albumin on biodegradation behavior of pure Zn. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Liu, Y.; Xiong, P.; Guo, H.; Huang, H.H.; Zheng, Y. Comparative Studies on Degradation Behavior of Pure Zinc in Various Simulated Body Fluids. JOM 2019, 71, 1414–1425. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Li, X.; Xiao, K.; Dong, C.; Liu, Z.; Wang, L. Corrosion behavior of field-exposed zinc in a tropical marine atmosphere. Corrosion 2014, 70, 731–748. [Google Scholar] [CrossRef]
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef]
- Shen, D.; Qi, H.; Lin, W.; Zhang, W.; Bian, D.; Shi, X.; Qin, L.; Zhang, G.; Fu, W.; Dou, K.; et al. PDLLA-Zn-nitrided Fe bioresorbable scaffold with 53-μm-thick metallic struts and tunable multistage biodegradation function. Sci. Adv. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Huang, T.; Zheng, Y.; Han, Y. Accelerating degradation rate of pure iron by zinc ion implantation. Regen. Biomater. 2016, 3, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubásek, J.; Dvorský, D.; Čapek, J.; Pinc, J.; Vojtěch, D. Zn-Mg biodegradable composite: Novel material with tailored mechanical and corrosion properties. Materials 2019, 12, 3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative invitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Obayi, C.S.; Tolouei, R.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Cassar, G.; Buhagiar, J.; Mantovani, D. Influence of cross-rolling on the micro-texture and biodegradation of pure iron as biodegradable material for medical implants. Acta Biomater. 2015, 17, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bohnes, H.; Franke, G. Galvanic (Sacrificial) Anodes. Handb. Cathodic Corros. Prot. 1997, 179–206. [Google Scholar] [CrossRef]

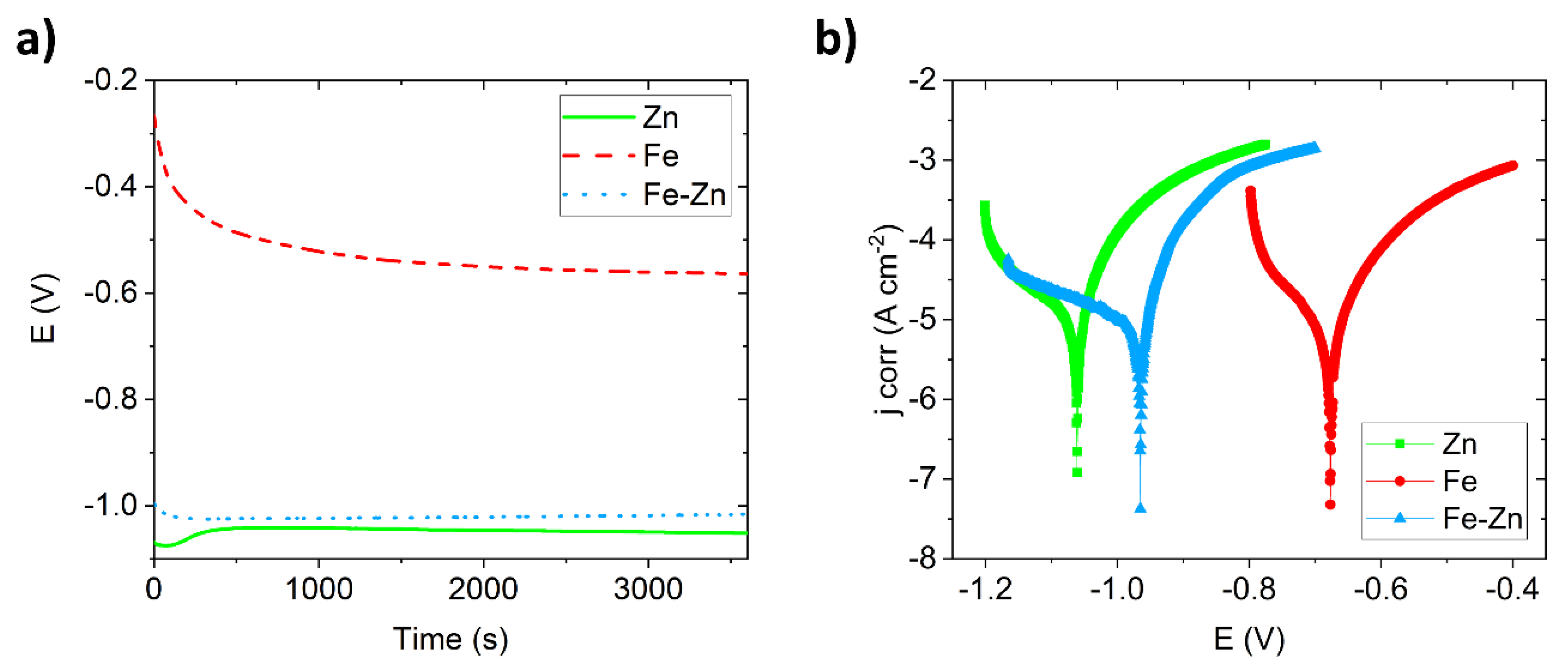
| Sample | Ecorr (V) | jcorr (µA·cm−²) | Corrosion Rate (mm·year−1) | Polarization Resistance (Ω) |
|---|---|---|---|---|
| Zn | −1.05 ± 0.10 | 37 ± 2.9 | 0.549 ± 0.07 | 140 ± 32 |
| Fe | −0.68 ± 0.10 | 18 ± 4.1 | 0.209 ± 0.11 | 339 ± 58 |
| Fe-Zn | −0.97 ± 0.04 | 43 ± 2.9 | 0.491 ± 0.04 | 164 ± 28 |
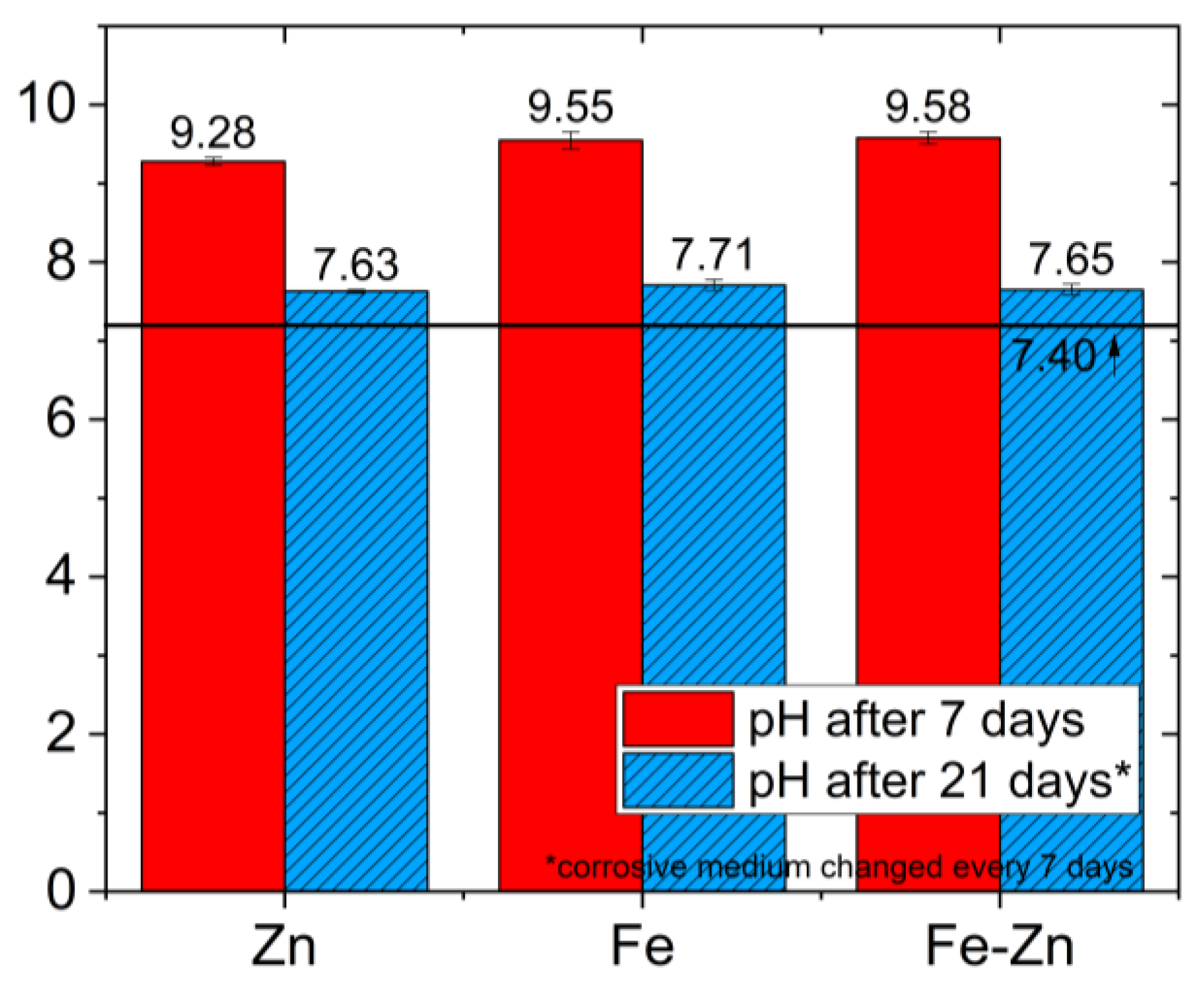
| Sample | pH after 7 Days | ΔpH after 7 Days | Ions Concentration (mg·L−1) after 7 Days | pH after 21 Days | ΔpH after 21 Days | Ions Concentration (mg·L−1) after 21 Days | ||
|---|---|---|---|---|---|---|---|---|
| Fe | Zn | Fe | Zn | |||||
| Zn | 9.28 ± 0.05 | +1.88 | - | 6.04 ± 0.7 | 7.63 ± 0.02 | +0.23 | - | 7.42 ± 0.8 |
| Fe | 9.55 ± 0.11 | +2.15 | 0.94 ± 0.5 | - | 7.71 ± 0.07 | +0.31 | 0.26 ± 0.2 | - |
| Fe-Zn | 9.58 ± 0.08 | +2.18 | Non-detectable | 6.14 ± 0.6 | 7.65 ± 0.07 | +0.25 | Non-detectable | 6.04 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorejová, R.; Šišoláková, I.; Cipa, P.; Džunda, R.; Sopčák, T.; Oriňak, A.; Oriňaková, R. Corrosion Behavior of Zn, Fe and Fe-Zn Powder Materials Prepared via Uniaxial Compression. Materials 2021, 14, 4983. https://doi.org/10.3390/ma14174983
Gorejová R, Šišoláková I, Cipa P, Džunda R, Sopčák T, Oriňak A, Oriňaková R. Corrosion Behavior of Zn, Fe and Fe-Zn Powder Materials Prepared via Uniaxial Compression. Materials. 2021; 14(17):4983. https://doi.org/10.3390/ma14174983
Chicago/Turabian StyleGorejová, Radka, Ivana Šišoláková, Pavol Cipa, Róbert Džunda, Tibor Sopčák, Andrej Oriňak, and Renáta Oriňaková. 2021. "Corrosion Behavior of Zn, Fe and Fe-Zn Powder Materials Prepared via Uniaxial Compression" Materials 14, no. 17: 4983. https://doi.org/10.3390/ma14174983







