Evaluation of the Potential of Modified Calcium Carbonate as a Carrier for Unsaturated Fatty Acids in Oxygen Scavenging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Loading of MCC with UFAs
2.2. Characterisation of MCC Loaded with UFAs
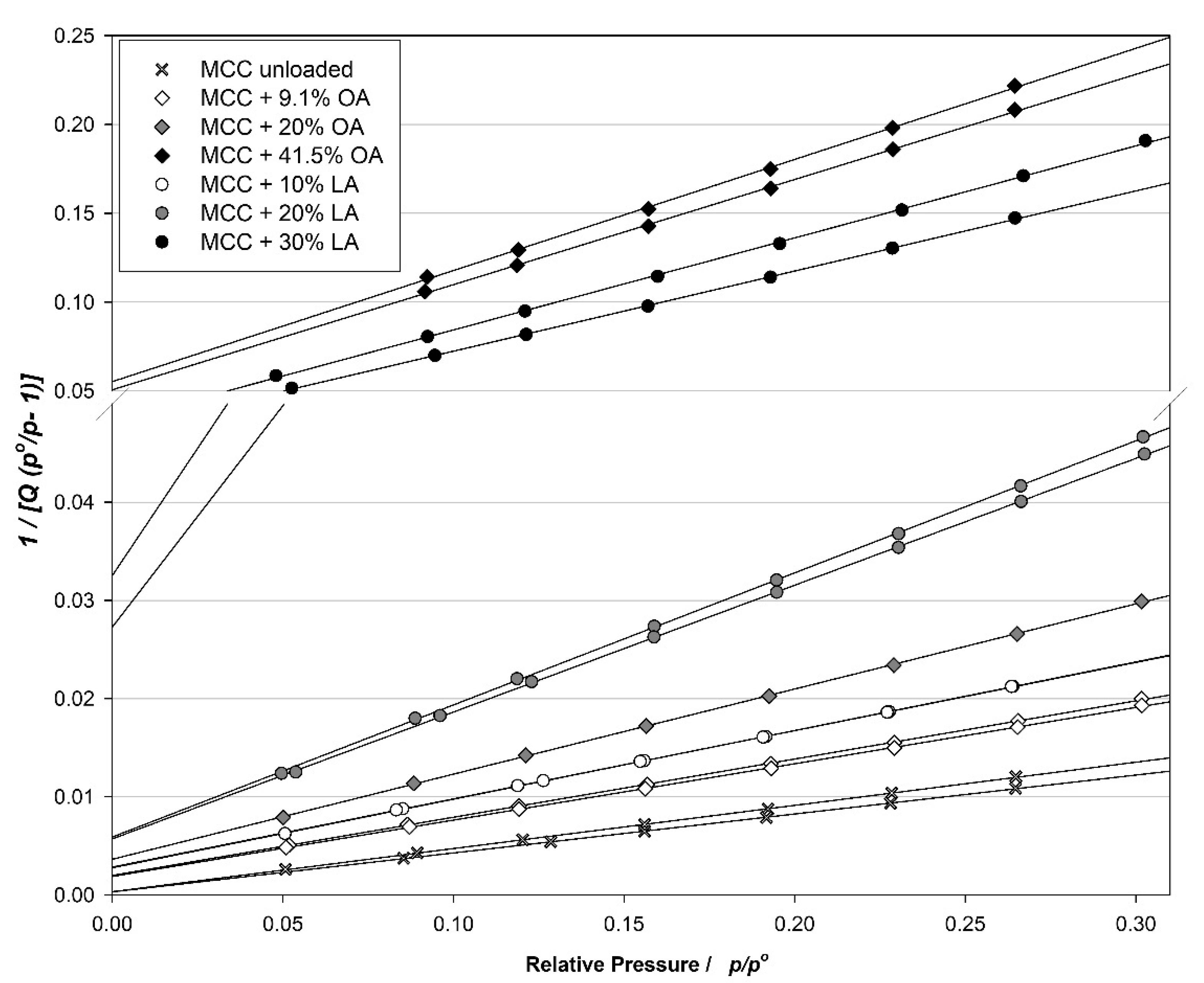
2.2.1. Mercury Intrusion Porosimetry (MIP)
2.2.2. Brunauer–Emmett–Teller (BET) Analysis of the Specific Surface Area
2.2.3. Field Emission Scanning Electron Microscopy (SEM)
2.3. Sample Preparation for Evaluation of Oxygen Scavenging Activity
2.4. Setting of Controlled in-Package Humidity
2.5. Packaging Process and Measurement of Oxygen Scavenging Activity
3. Results
3.1. Structural Properties of MCC Loaded with UFAs
3.2. Oxygen Scavenging Activity
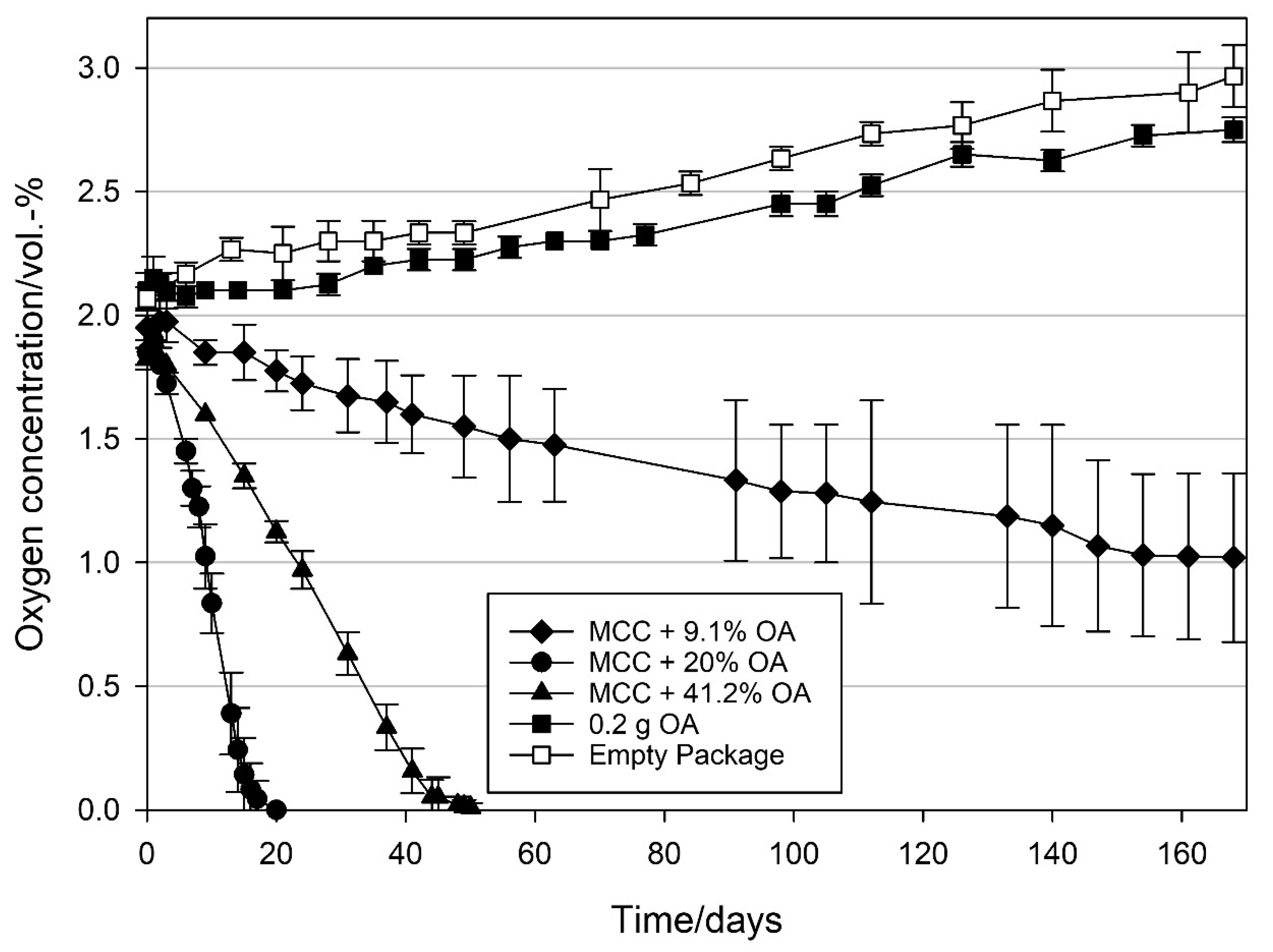
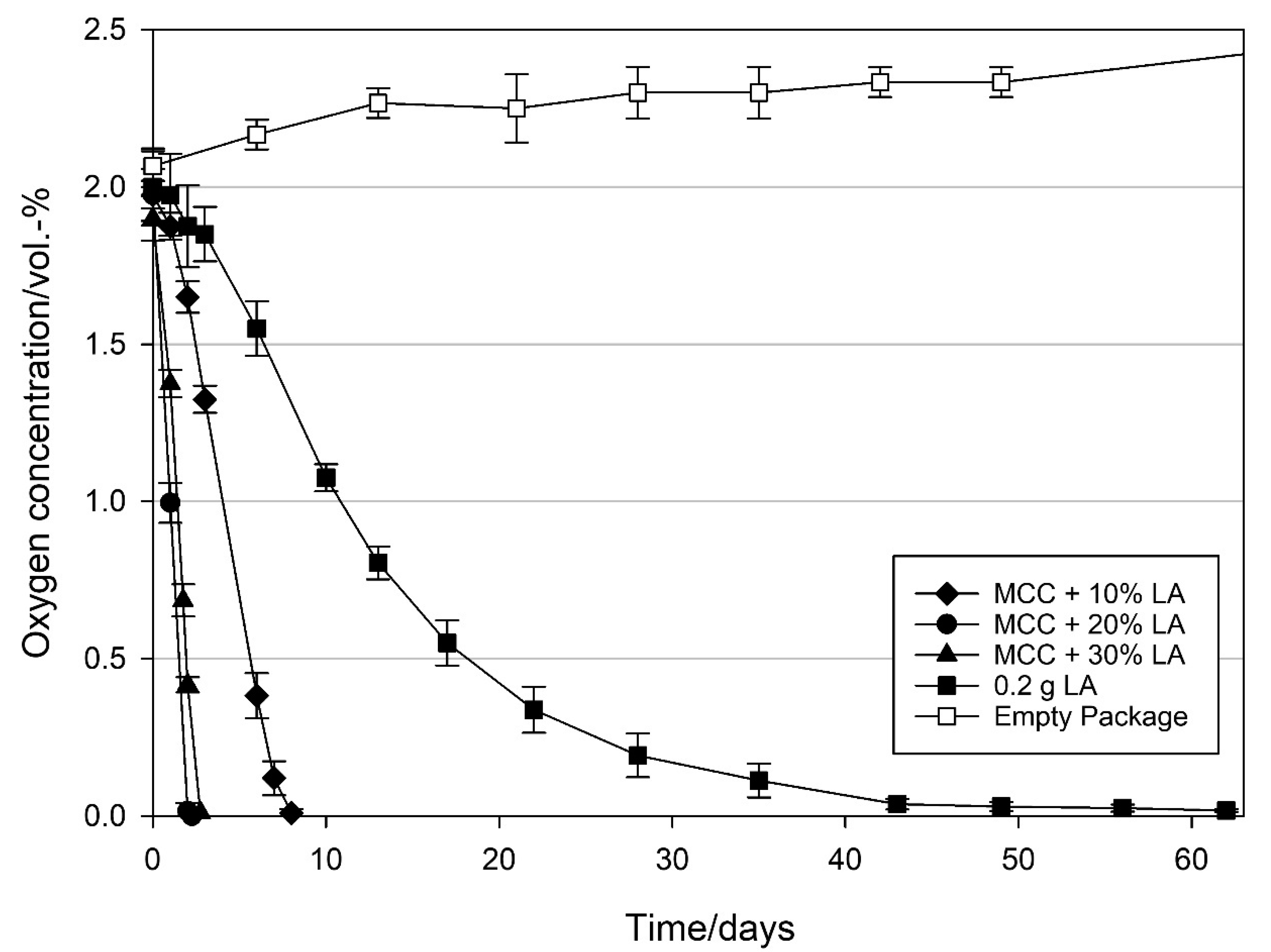
3.2.1. Effect of Loading Amount on the Oxygen Scavenging Activity of MCC Loaded with UFAs
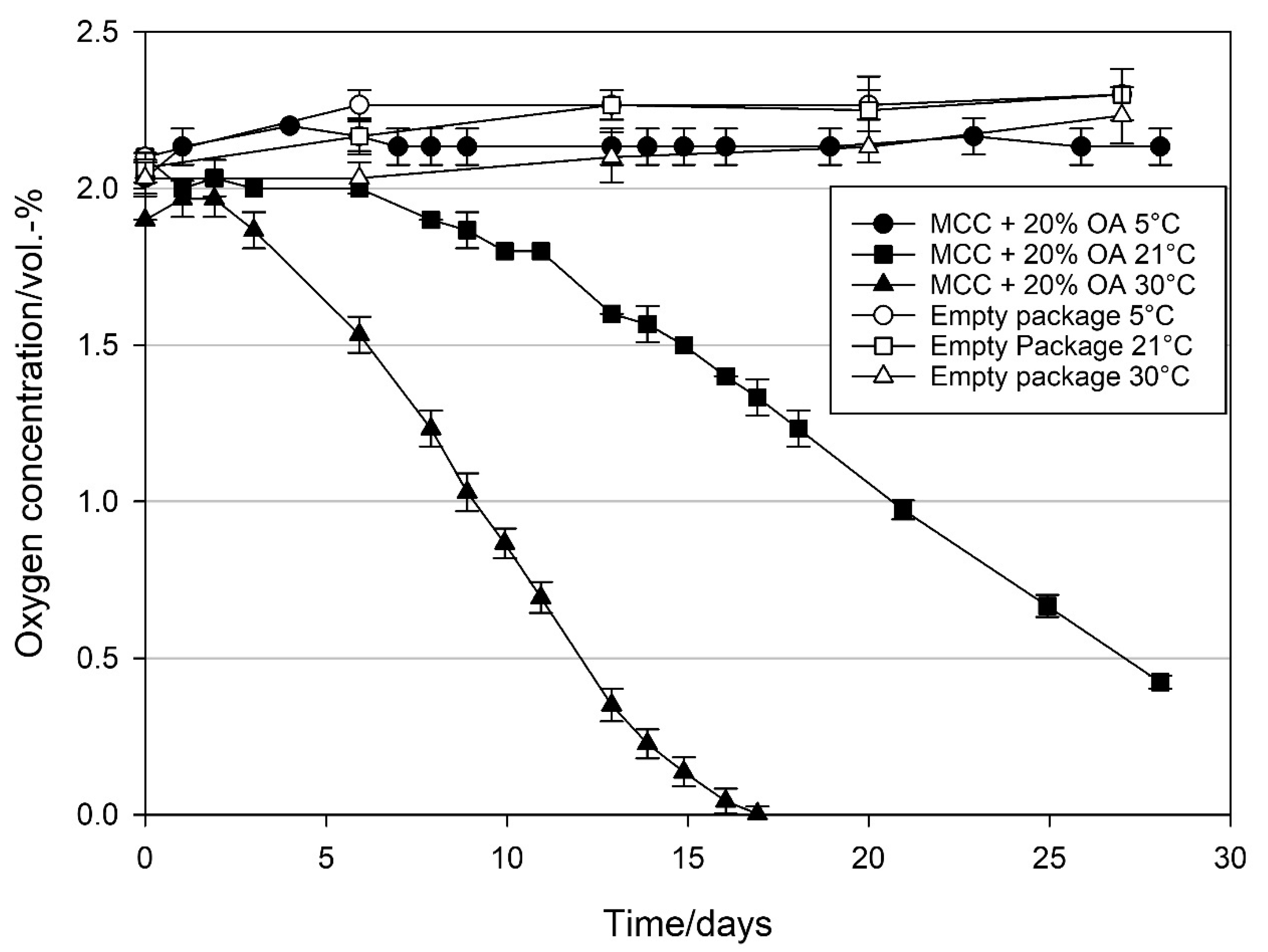
3.2.2. Effect of Temperature on the Oxygen Scavenging Activity of MCC Loaded with UFAs
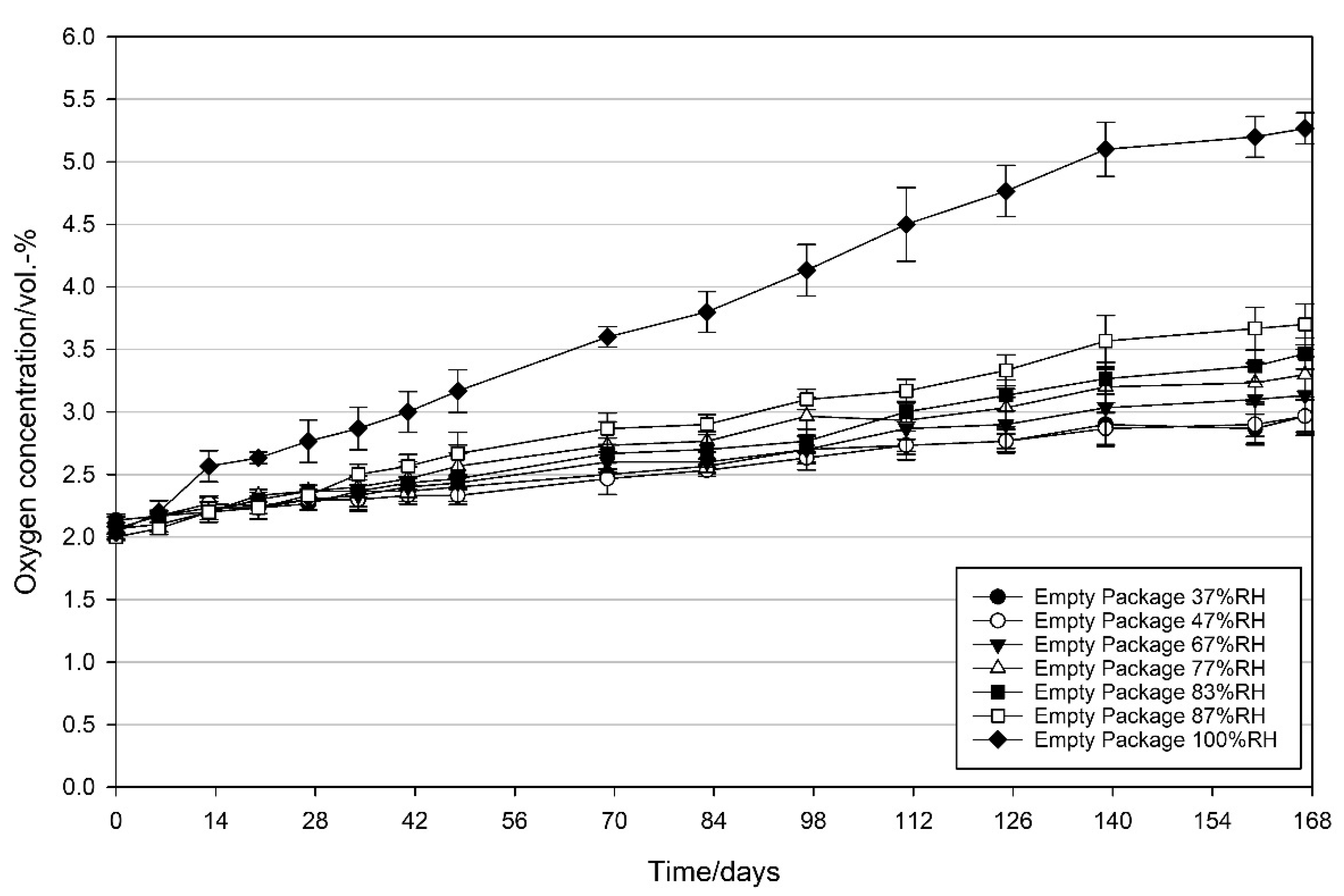
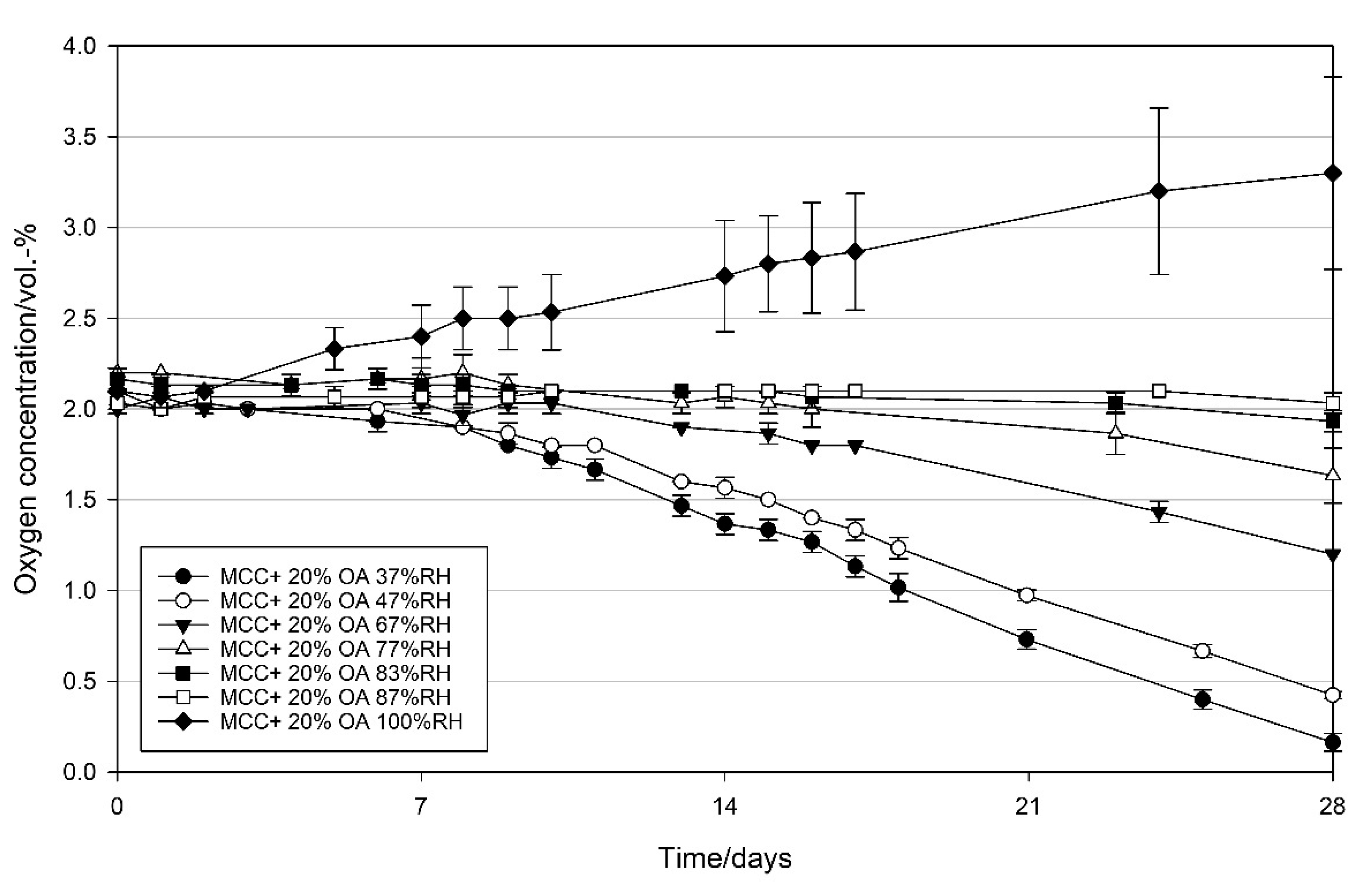
3.2.3. Effect of Relative Humidity on the Oxygen Scavenging Activity of MCC Loaded with UFAs
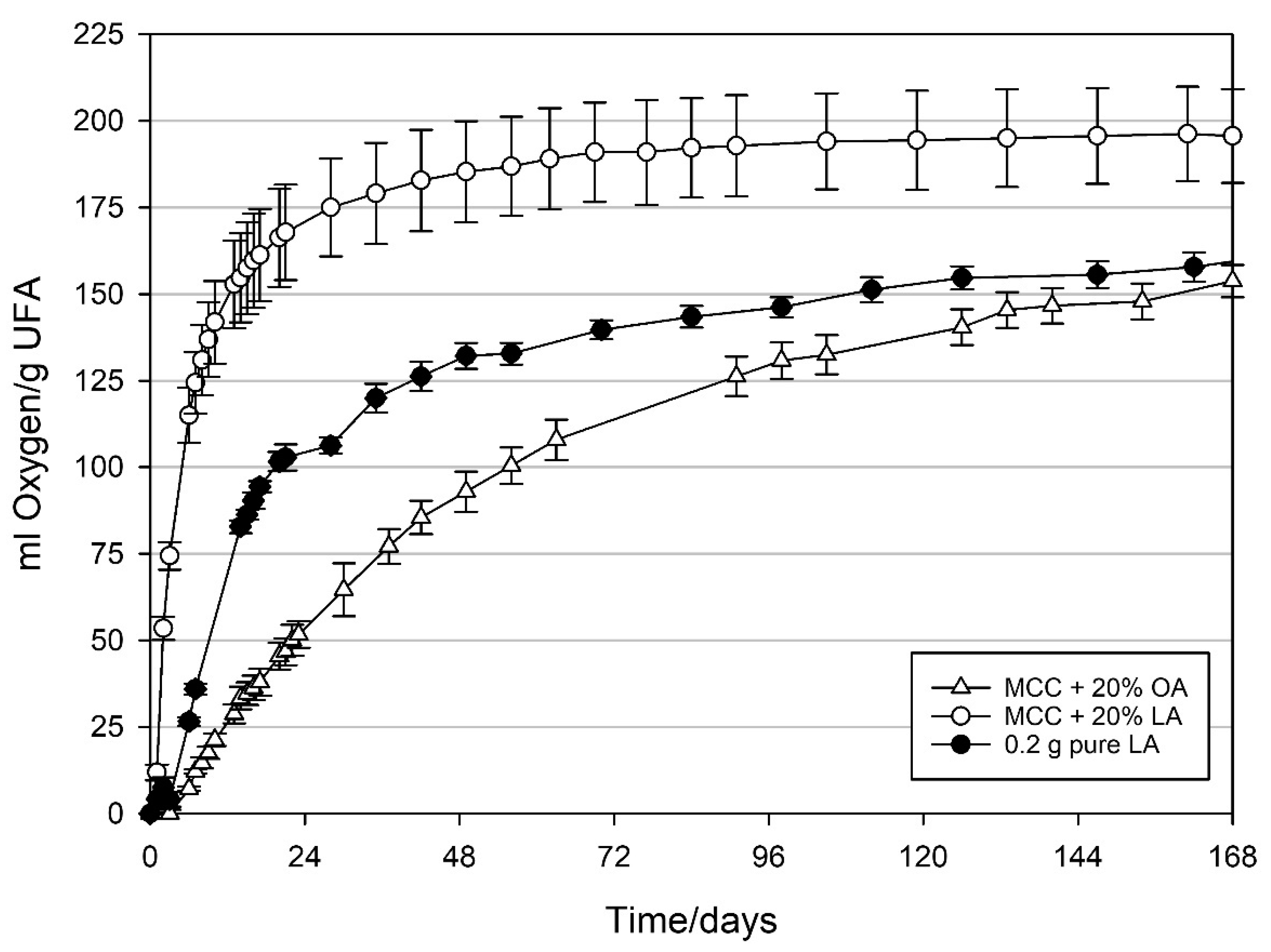
3.3. Oxygen Absorption Capacity of MCC Loaded with UFAs
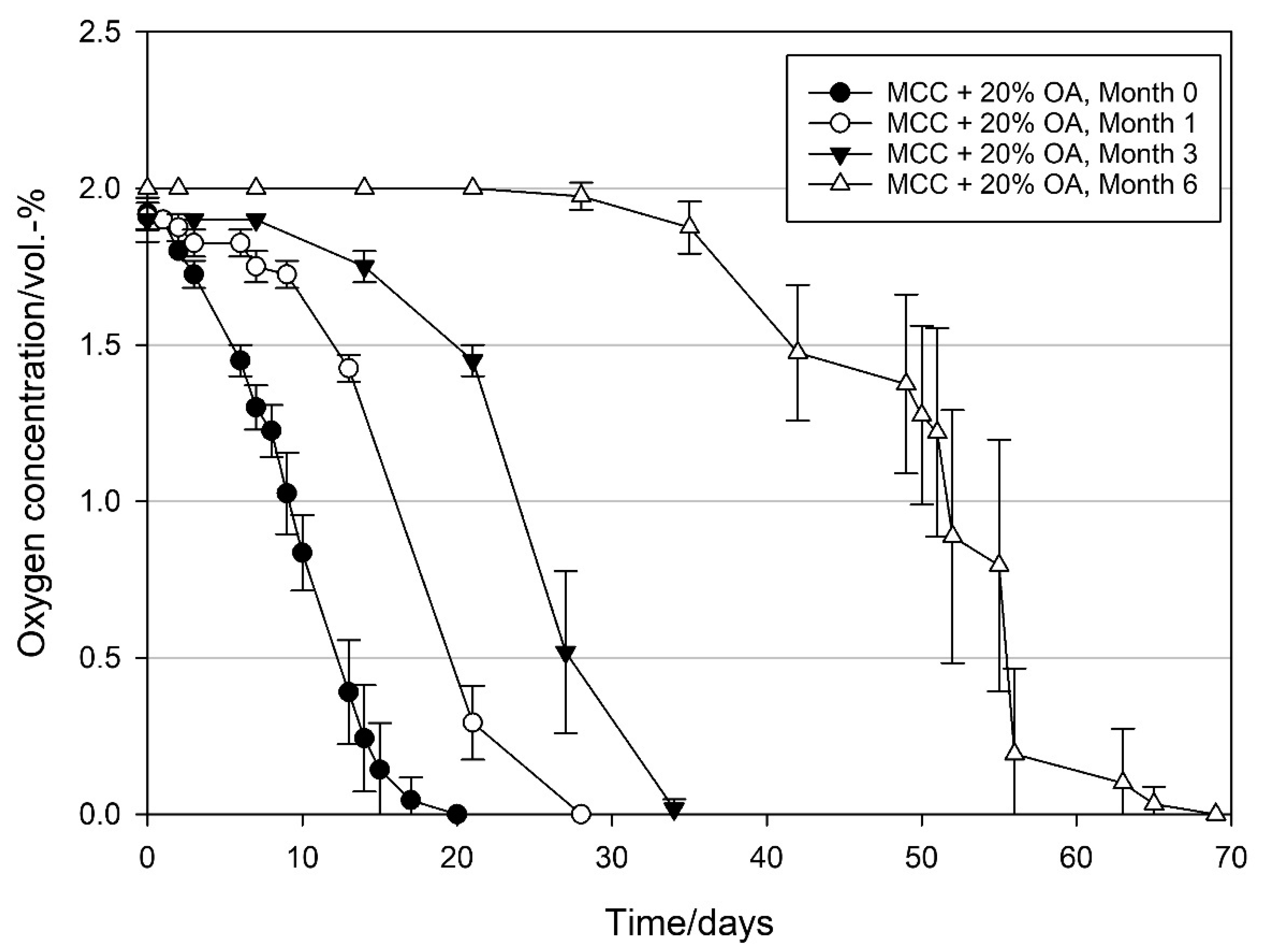
3.4. Storage Stability of MCC Loaded with UFAs
4. Discussion
4.1. Effect of Loading Amount on the Oxygen Scavenging Activity of MCC Loaded with UFAs
4.2. Effect of Temperature on the Oxygen Scavenging Activity of MCC Loaded with UFAs
4.3. Effect of Relative Humidity on the Oxygen Scavenging Activity of MCC Loaded with UFAs
4.4. Oxygen Absorption Capacity of MCC Loaded with UFAs
4.5. Storage Stability of MCC Loaded with UFAs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Velasco, J.; Andersen, M.L.; Skibsted, L.H. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 2004, 85, 623–632. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Oikonomou, G. Active and intelligent packaging. In Modified Atmosphere and Active Packaging Technologies; Arvanitoyannis, I.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 628–654. [Google Scholar]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Tech. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Van Wezemael, L.; Ueland, Ø.; Verbeke, W. European consumer response to packaging technologies for improved beef safety. Meat Sci. 2011, 89, 45–51. [Google Scholar] [CrossRef]
- Wilson, C.T.; Harte, J.; Almenar, E. Effects of sachet presence on consumer product perception and active packaging acceptability—A study of fresh-cut cantaloupe. LWT Food Sci. Technol. 2018, 92, 531–539. [Google Scholar] [CrossRef]
- Mikkola, V.; Lähteenmäki, L.; Hurme, E.; Heiniö, R.-L.; Järvi-Kääriäinen, T.; Ahvenainen, R. Consumer Attitudes towards Oxygen Absorbers in Food Packages; VTT Technical Research Centre of Finland: Espoo, Finland, 1997; pp. 1–34. Available online: http://www.vtt.fi/inf/pdf/tiedotteet/1997/T1858.pdf (accessed on 6 July 2021).
- O’Callaghan, K.A.M.; Kerry, J.P. Consumer attitudes towards the application of smart packaging technologies to cheese products. Food Packag. Shelf Life 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Beckwith, S.; Edwards, F.B.; Rivett, J.; Ebner, C.L.; Kennedy, T.; Mcdowell, R.; Speer, D.V. Multilayer Film Having Active Oxygen Barrier Layer and Iron-Based Oxygen Scavenging Layer. AU Patent 2008293811A1, 5 March 2009. [Google Scholar]
- Foltynowicz, Z.; Kozak, W.; Stoinska, J.; Urbanska, M.; Muc, K.; Dominiak, A.; Kublicka, K. Nanoiron-Based Oxygen Scavengers. U.S. Patent 20140004232, 2 January 2014. [Google Scholar]
- Chau, C.-C. Methods of Making Oxygen Scavenging Articles Containing Moisture. U.S. Patent 20130075655A1, 28 March 2013. [Google Scholar]
- Yildirim, S.; Röcker, B.; Rüegg, N.; Lohwasser, W. Development of Palladium-based Oxygen Scavenger: Optimization of Substrate and Palladium Layer Thickness. Packag. Technol. Sci. 2015, 28, 710–718. [Google Scholar] [CrossRef]
- Lohwasser, W.; Wanner, T. Composite System, Associated Use and Method for the Oxygen-Free Packaging of Items Susceptible to Oxidation. CA Patent CA2628733A1, 31 May 2007. [Google Scholar]
- Yildirim, S.; Jammet, J.C.; Lohwasser, W. Multi-Layer Film. European Patent 2,236,284, 6 October 2010. [Google Scholar]
- Sängerlaub, S.; Glas, C.E.; Schlemmer, D.; Müller, K. Influence of multiple extrusions of blends made of polyethylene terephthalate and an oxygen scavenger on processing and packaging-related properties. J. Plast. Film Sheeting 2020, 36, 260–284. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Loukidi, A.; Chatzimichailidou, S.; Salmas, C.E.; Giannakas, A.E.; Achilias, D.S. Effect of Na- and Organo-Modified Montmorillonite/Essential Oil Nanohybrids on the Kinetics of the In Situ Radical Polymerization of Styrene. Nanomaterials 2021, 11, 474. [Google Scholar] [CrossRef]
- Johnson, M.L.; Noreland, D.; Gane, P.; Schoelkopf, J.; Ridgway, C.; Millqvist-Fureby, A. Porous calcium carbonate as a carrier material to increase the dissolution rate of poorly soluble flavouring compounds. Food Funct. 2017, 8, 1627–1640. [Google Scholar] [CrossRef]
- Stirnimann, T.; Di Maiuta, N.; Gerard, D.E.; Alles, R.; Huwyler, J.; Puchkov, M. Functionalized Calcium Carbonate as a novel pharmaceutical excipient for the preparation of orally dispersible tablets. J. Pharm. Res. 2013, 30, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Eberle, V.A. Floating Gastroretentive Drug Delivery Systems Based on Functionalized Calcium Carbonate. Ph.D. Thesis, University of Basel, Basel, Switzerland, 23 June 2015. [Google Scholar] [CrossRef]
- Rüegg, N.; Beck, B.M.; Monnard, F.W.; Hilty, F.M.; Wicht, A.; Schoelkopf, J.; Yildirim, S. Evaluation of the potential of functionalised calcium carbonate as carrier for essential oils with regard to antimicrobial packaging applications. Packag. Technol. Sci. 2020, 33, 333–343. [Google Scholar] [CrossRef]
- Gantenbein, D.; Schoelkopf, J.; Matthews, G.P.; Gane, P.A.C. The use of porous high surface area calcium carbonate for the adsorption of dissolved and colloidal substances from thermo mechanical pulp filtrates. Nord. Pulp Pap. Res. J. 2012, 27, 631–638. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Budi Santosa, F.X.; Padua, G.W. Tensile properties and water absorption of zein sheets plasticized with Oleic and Linoleic acids. J. Agric. Food Chem. 1999, 47, 2070–2074. [Google Scholar] [CrossRef]
- Sander, M.M.; Nicolau, A.; Guzatto, R.; Samios, D. Plasticiser effect of oleic acid polyester on polyethylene and polypropylene. Polym. Test. 2012, 31, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Altenhofen da Silva, M.; Adeodato Vieira, M.G.; Gomes Maçumoto, A.C.; Beppu, M.M. Polyvinylchloride (PVC) and natural rubber films plasticized with a natural polymeric plasticizer obtained through polyesterification of rice fatty acid. Polym. Test. 2011, 30, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.S.; Camilloto, G.P.; dos Santos Pires, A.C. Oxygen scavengers: An approach on food preservation. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef]
- Solovyov, S.E. Oxygen scavengers. In The Wiley Encyclopedia of Packaging Technology, 3rd ed.; Yam, K.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 841–850. [Google Scholar]
- Miranda, M.A. Bio Based Active Barrier Materials and Package Development. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, 30 December 2016. [Google Scholar]
- Miranda, M.A.; Jabarin, S.A.; Coleman, M. Modification of poly(ethylene terephthalate) (PET) using linoleic acid for oxygen barrier improvement: Impact of processing methods. J. Appl. Polym. Sci. 2017, 134, 45023. [Google Scholar] [CrossRef]
- ISO 9277-2010. Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method; International Standard Organisation: London, UK, 2010. [Google Scholar]
- Gane, P.A.; Kettle, J.P.; Matthews, G.P.; Ridgway, C.J. Void space structure of compressible polymer spheres and consolidated Calcium carbonate paper-coating formulations. Ind. Eng. Chem. Res. 1996, 35, 1753–1764. [Google Scholar] [CrossRef]
- O’Brien, F.E.M. The control of humidity by saturated salt solutions. J. Sci. Instrum. 1948, 25, 73. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Giancoli, D.C. Physik: Lehr- und Übungsbuch, 3rd ed.; Pearson Studium: München, Germany, 2010. [Google Scholar]
- Zhang, Z.B.; Britt, I.J.; Tung, M.A. Permeation of oxygen and water vapor through EVOH films as influenced by relative humidity. J. Appl. Polym. Sci. 2001, 82, 1866–1872. [Google Scholar] [CrossRef]
- Schulz, H. Beta oxidation of fatty acids. BBA Lipid Lipid Met. 1991, 1081, 109–120. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Lehrbuch der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Ternes, W. Naturwissenschaftliche Grundlagen der Lebensmittelzubereitung, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibino, M.; Sumi, A.; Hatta, I. Molecular arrangements of fatty-acids and Cholesterol at liquid Graphite interface observed by Scanning-Tunneling-Microscopy. Jpn. J. Appl. Phys. 1995, 34, 3354–3359. [Google Scholar] [CrossRef]
- Pfaller, P. Enrichment of Calcium Carbonate with Omega-3 Fatty Acids for Food Fortification. Master’s Thesis, (Unpublished, Confidential). ETH Zürich, Zürich, Switzerland, 2016. [Google Scholar]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Evolution of the oxidation process in olive oil triacylglycerol under accelerated storage conditions (40–60 °C). J. Am. Oil Chem. Soc. 2004, 81, 177–184. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Fregapane, G.; Desamparados Salvador, M. Kinetic study for the development of an accelerated oxidative stability test to estimate virgin olive oil potential shelf life. Eur. J. Lipid Sci. Technol. 2008, 110, 969–976. [Google Scholar] [CrossRef]
- Crapiste, G.H.; Brevedan, M.I.V.; Carelli, A.A. Oxidation of sunflower oil during storage. J. Am. Oil Chem. Soc. 1999, 76, 1437. [Google Scholar] [CrossRef]
- Braga, L.R.; Sarantópoulos, C.I.G.L.; Peres, L.; Braga, J.W.B. Evaluation of absorption kinetics of oxygen scavenger sachets using response surface methodology. Packag. Technol. Sci. 2010, 23, 351–361. [Google Scholar] [CrossRef]
- Barden, L.M. Understanding Lipid Oxidation in Low-Moisture Food. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, February 2014. [Google Scholar] [CrossRef]
- Roos, Y.H. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process. Pres. 1993, 16, 433–447. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H.; Reid, D.S. Beyond Water Activity—Recent Advances Based on an Alternative Approach to the Assessment of Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.A.; Labuza, T.P. Relationship between water and lipid oxidation rates. In Lipid Oxidation in Food; St. Angelo, A.J., Ed.; American Chemical Society: Washington, DC, USA, 1992; Volume 500, pp. 93–103. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]





| Sample | Total Intra Particle Intruded Specific Pore Volume (0.004–0.34 μm) cm−3 g−1 | Specific Surface Area m2 g−1 |
|---|---|---|
| 1.019 | 103 | |
 MCC + oleic acid MCC + oleic acid | ||
| 9.1 wt% loading | 0.802 | 72 |
| 20 wt% loading | 0.648 | 49 |
| 41.5 wt% loading | 0.163 | 6.6 |
| MCC + linoleic acid | ||
| 10 wt% loading | 0.766 | 60 |
| 20 wt% loading | 0.527 | 31.6 |
| 30 wt% loading | 0.352 | 8.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röcker, B.; Mäder, G.; Monnard, F.W.; Jancikova, M.; Welker, M.; Schoelkopf, J.; Yildirim, S. Evaluation of the Potential of Modified Calcium Carbonate as a Carrier for Unsaturated Fatty Acids in Oxygen Scavenging Applications. Materials 2021, 14, 5000. https://doi.org/10.3390/ma14175000
Röcker B, Mäder G, Monnard FW, Jancikova M, Welker M, Schoelkopf J, Yildirim S. Evaluation of the Potential of Modified Calcium Carbonate as a Carrier for Unsaturated Fatty Acids in Oxygen Scavenging Applications. Materials. 2021; 14(17):5000. https://doi.org/10.3390/ma14175000
Chicago/Turabian StyleRöcker, Bettina, Gabriel Mäder, Fabien Wilhelm Monnard, Magdalena Jancikova, Matthias Welker, Joachim Schoelkopf, and Selçuk Yildirim. 2021. "Evaluation of the Potential of Modified Calcium Carbonate as a Carrier for Unsaturated Fatty Acids in Oxygen Scavenging Applications" Materials 14, no. 17: 5000. https://doi.org/10.3390/ma14175000







