Ex Situ Examination of Matrix and Inclusions of API-X100 before and after Exposure to Bitumen at Elevated Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bitumen and Organic Solvent
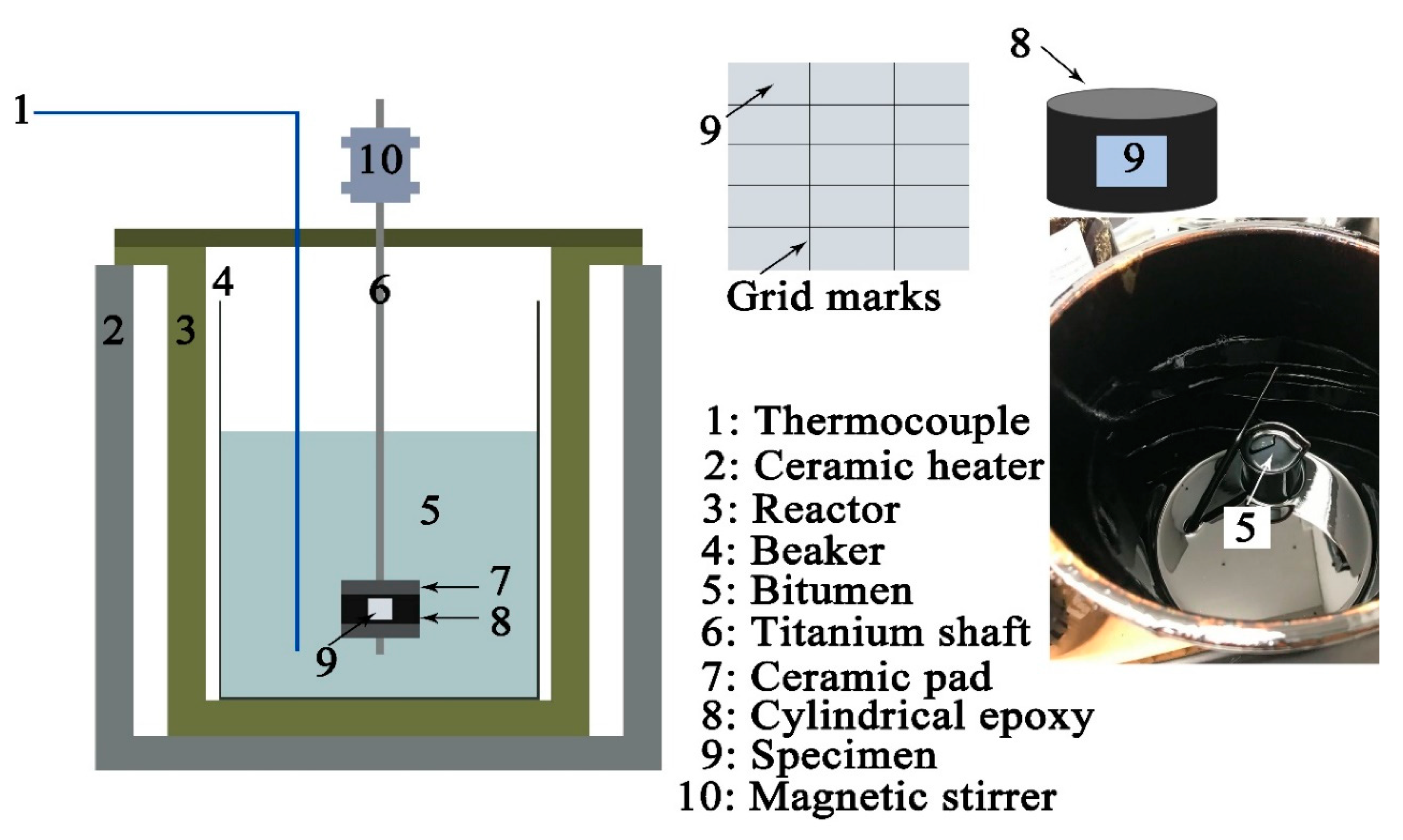
2.3. Autoclave Exposure Experiments
2.4. Surface Morphology
3. Results
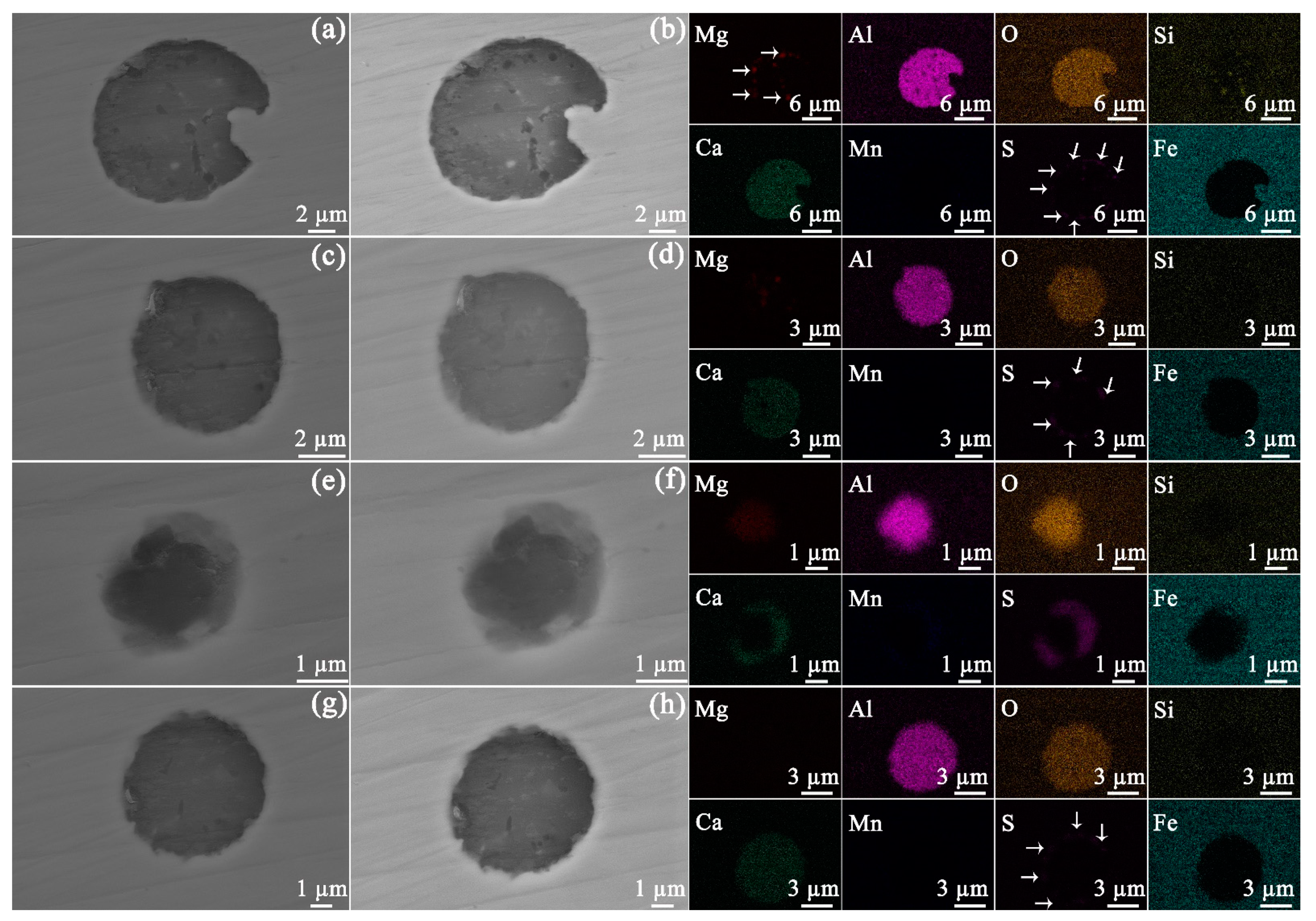
3.1. Inclusions in API-X100 Pipeline Steel
3.2. Evaluation of the Inclusions after Exposure to Bitumen at 60 and 120 °C
4. Discussion
4.1. Inclusions in the Steel
4.2. After Exposure to Bitumen at 60 and 120 °C
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Energy Board. Market Snapshot: Oil Sands Bitumen Production Will Continue to Grow to 2040. 2018. Available online: https://www.nebone.gc.ca/nrg/ntgrtd/mrkt/snpsht/2018/01-05lsndsbtmn-eng.html (accessed on 30 September 2019).
- Rosa, L.; Davis, K.F.; Rulli, M.C.; D’Odorico, P. Environmental consequences of oil production from oil sands. Earth’s Future 2017, 5, 158–170. [Google Scholar] [CrossRef]
- Verma, A.; Nimana, B.; Olateju, B.; Rahman, M.; Radpour, S.; Canter, C.; Subramanyam, V.; Paramashivan, D.; Vaezi, M.; Kumar, A. A techno-economic assessment of bitumen and synthetic crude oil transport (SCO) in the Canadian oil sands industry: Oil via rail or pipeline? Energy 2017, 124, 665–683. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Alcocer, Y.; Bahn, O. An analysis of the impacts of new oil pipeline projects on the Canadian energy sector with a TIMES model for Canada. In Informing Energy and Climate Policies Using Energy Systems Models; Springer: Cham, Swizerland, 2015; pp. 247–260. [Google Scholar]
- Bowles, P.; MacPhail, F. The town that said “No” to the Enbridge Northern Gateway pipeline: The Kitimat plebiscite of 2014. Extr. Ind. Soc. 2017, 4, 15–23. [Google Scholar] [CrossRef]
- Hoberg, G. The Political Economy of Pipelines: The Battle over Oil Sands Access to Tidewater. Can. Public Policy 2013, 39, 371–391. [Google Scholar] [CrossRef]
- Corkal, V. Pipelines or Progress: Government Support for Oil and Gas Pipelines in Canada; International Institute for Sustainable Development: Geneva, Switzerland, 2021. [Google Scholar]
- Dusyk, N.; Axsen, J.; Dullemond, K. Who cares about climate change? The mass media and socio-political acceptance of Canada’s oil sands and Northern Gateway Pipeline. Energy Res. Soc. Sci. 2018, 37, 12–21. [Google Scholar] [CrossRef]
- Bakker, G. The Corrosive Nature of Diluted Bitumen and Crude Oil Literature Review; MR Gordon and Associates, Ltd.: North Vancouver, BC, Canada, 2011. [Google Scholar]
- Place, T.; Holm, M.R.; Cathrea, C.; Ignacz, T. Understanding and Mitigating Under-Deposit Corrosion in Large Diameter Crude Oil Pipelines: A Progress Report. In Proceedings of the 2008 7th International Pipeline Conference, Calgary, AB, Canada, 29 September–3 October; American Society of Mechanical Engineers: New York, NY, USA, 2018; pp. 809–821. [Google Scholar]
- Liang, H.; Schaller, R.F.; Asselin, E. Three phase corrosion of pipeline steel: Size effects of deposited solids under water droplets and an oil diffusion barrier. J. Pipeline Sci. Eng. 2021, 1, 137–147. [Google Scholar] [CrossRef]
- Liang, H.; Schaller, R.F.; Asselin, E. The Effects of Chloride Droplet Properties on the Underoil Corrosion of API X100 Pipeline Steel. Corrosion 2019, 75, 1051–1064. [Google Scholar] [CrossRef]
- Liang, H.; Liu, J.; Alfantazi, A.; Asselin, E. Corrosion behaviour of X100 pipeline steel under a salty droplet covered by simulated diluted bitumen. Mater. Lett. 2018, 222, 196–199. [Google Scholar] [CrossRef]
- Liang, H.; Schaller, R.F.; Asselin, E. Aqueous Corrosion of Deformed Steel Under Simulated Diluted Bitumen. Corrosion 2019, 75, 1194–1206. [Google Scholar] [CrossRef]
- Liang, H.; Liu, J.; Schaller, R.F.; Asselin, E. A New Corrosion Mechanism for X100 Pipeline Steel Under Oil-Covered Chloride Droplets. Corrosion 2018, 74, 947–957. [Google Scholar] [CrossRef]
- Swift, A.; Shope, E.; Casey-Lefkowitz, S.; Club, S. Tar sands Pipelines Safety Risks; Natural Resources Defense Council: New York, NY, USA, 2011. [Google Scholar]
- Warmore, S. Tar Sends Oil and Pipeline Safety: Examining Regulatory Shortcomings. Wayne L. Rev. 2013, 59, 175. [Google Scholar]
- Slavcheva, E.; Shone, B.; Turnbull, A. Review of naphthenic acid corrosion in oilrefining. Br. Corros. J. 1999, 34, 125–131. [Google Scholar] [CrossRef]
- Dilbit Corrosivity. Canadian Energy Pipelines Association. State of the Art Report; Document Number: 12671-RPT-001 REV 1; Penspen Integrity: Newcastle, UK, 2013; pp. 1–39. [Google Scholar]
- McIntyre, D.R.; Achour, M.; Scribner, M.E.; Zimmerman, P.K. Laboratory tests comparing the corrosivity of dilbit and synbit with conventional crudes under pipeline conditions. In Proceedings of the Corrosion 2014, San Antonio, TX, USA, 9–13 March 2014. Paper no. 3824, NACE. [Google Scholar]
- Winter, C.H.; Sillers, R.; Glowach, A.M. High-temp., insulated coating aids construction of Alberta bitumen pipeline. Oil Gas J. 2003, 101, 56. [Google Scholar]
- Atkinson, H.; Shi, G. Characterization of inclusions in clean steels: A review including the statistics of extremes methods. Prog. Mater. Sci. 2003, 48, 457–520. [Google Scholar] [CrossRef]
- Al-Mansour, M.; Alfantazi, A.; El-Boujdaini, M. Sulfide stress cracking resistance of API-X100 high strength low alloy steel. Mater. Des. 2009, 30, 4088–4094. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Li, J.; Hu, Q.; Peng, C. Evolution of nonmetallic inclusions in pipeline steel during LF and VD refining process. High Temp. Mater. Process. 2020, 39, 424–432. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Li, X.; Cheng, Y. Effects of hydrogen-charging on the susceptibility of X100 pipeline steel to hydrogen-induced cracking. Int. J. Hydrog. Energy 2009, 34, 9879–9884. [Google Scholar] [CrossRef]
- Jin, T.; Cheng, Y. In situ characterization by localized electrochemical impedance spectroscopy of the electrochemical activity of microscopic inclusions in an X100 steel. Corros. Sci. 2011, 53, 850–853. [Google Scholar] [CrossRef]
- Peng, X.Y.; Liang, G.C.; Jin, T.Y.; Cheng, Y.F. Correlation of initiation of corrosion pits and metallurgical features of X100 pipeline steel. Can. Met. Q. 2013, 52, 484–487. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Ex situ characterization of metallurgical inclusions in X100 pipeline steel before and after immersion in a neutral pH bicarbonate solution. J. Alloys Compd. 2016, 673, 28–37. [Google Scholar] [CrossRef]
- Arafin, M.; Szpunar, J. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Yang, Z.; Kan, B.; Li, J.; Su, Y.; Qiao, L.; Volinsky, A.A. Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments. Materials 2017, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Derungs, W.A. Naphthenic Acid Corrosion—An Old Enemy of the Petroleum Industry. Corrosion 1956, 12, 41–46. [Google Scholar] [CrossRef]
- Ayello, F.; Robbins, W.; Richter, S.; Nešić, S. Crude oil chemistry effects on inhibition of corrosion and phase wetting. In Proceedings of the CORROSION 2011, Houston, TX, USA, 13–17 March 2011. Paper no. 11060, NACE. [Google Scholar]
- Ramirez, M.; Perdomo, J.; Morales, J.L.; Viloria, A. Effect of crude oil contaminants on the internal corrosion in gas pipelines. In Proceedings of the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000. Paper no. 40, NACE. [Google Scholar]



| Element | C | Cu | Mn | V | Cr | Nb | Ni | Al | Mo | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| API-X100 (wt.%) | 0.1 | 0.25 | 1.66 | 0.003 | 0.016 | 0.043 | 0.13 | 0.02 | 0.19 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Asselin, E. Ex Situ Examination of Matrix and Inclusions of API-X100 before and after Exposure to Bitumen at Elevated Temperature. Materials 2021, 14, 5007. https://doi.org/10.3390/ma14175007
Liang H, Asselin E. Ex Situ Examination of Matrix and Inclusions of API-X100 before and after Exposure to Bitumen at Elevated Temperature. Materials. 2021; 14(17):5007. https://doi.org/10.3390/ma14175007
Chicago/Turabian StyleLiang, Hongxing, and Edouard Asselin. 2021. "Ex Situ Examination of Matrix and Inclusions of API-X100 before and after Exposure to Bitumen at Elevated Temperature" Materials 14, no. 17: 5007. https://doi.org/10.3390/ma14175007
APA StyleLiang, H., & Asselin, E. (2021). Ex Situ Examination of Matrix and Inclusions of API-X100 before and after Exposure to Bitumen at Elevated Temperature. Materials, 14(17), 5007. https://doi.org/10.3390/ma14175007






