Experimental Study on Ultra-Precision Polishing of Ti-6Al-4V by Ultraviolet-Induced Nanoparticle Colloid Jet Machining
Abstract
:1. Introduction
2. Materials and Methods
3. Results
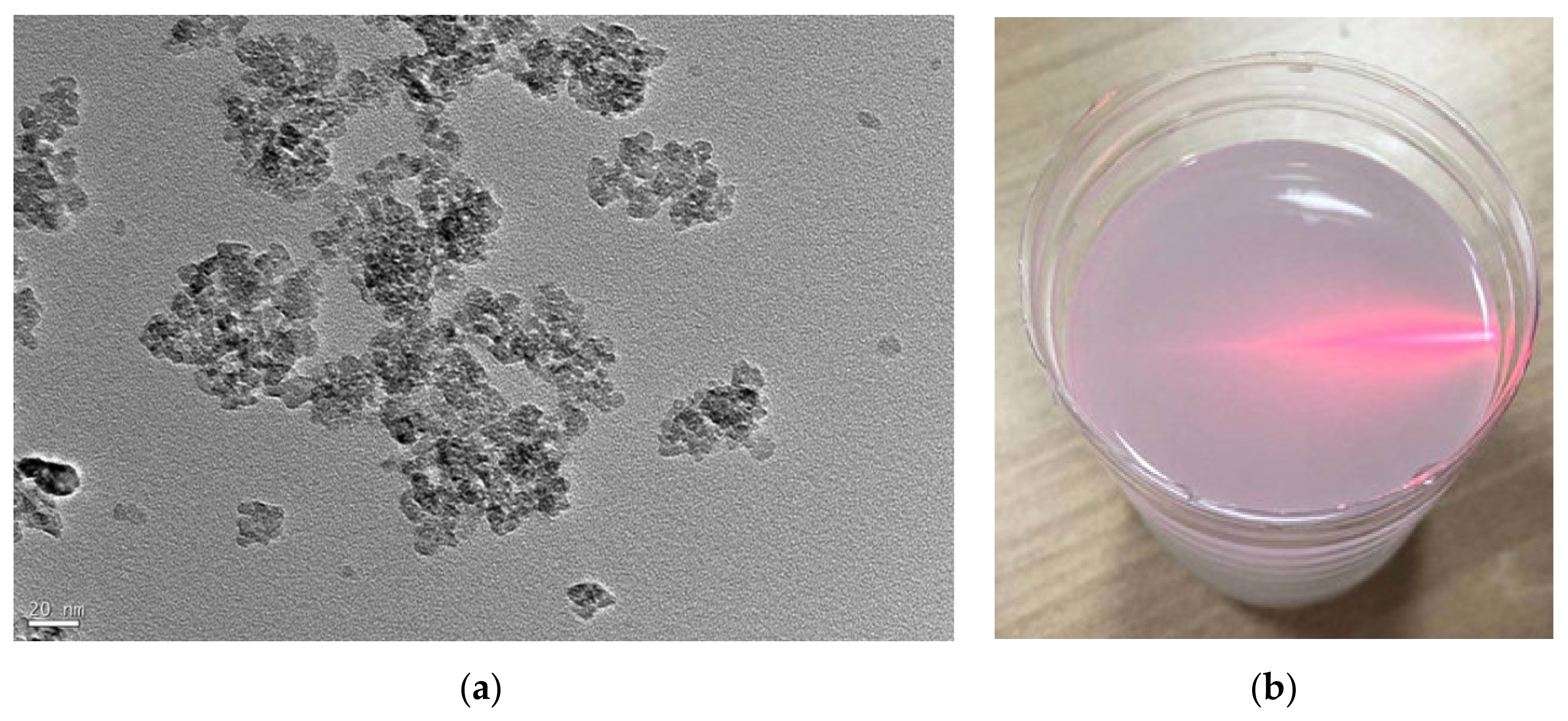
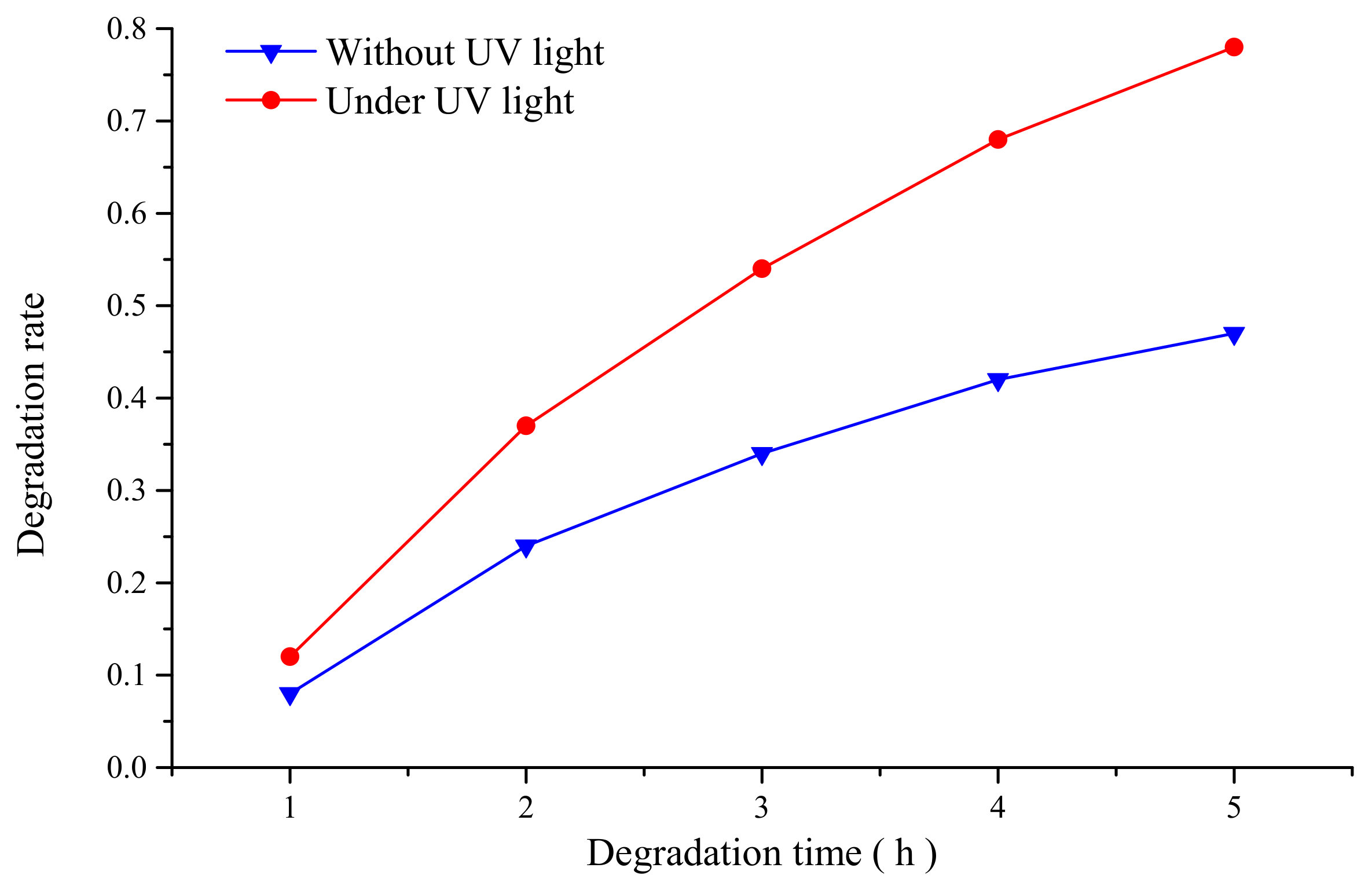
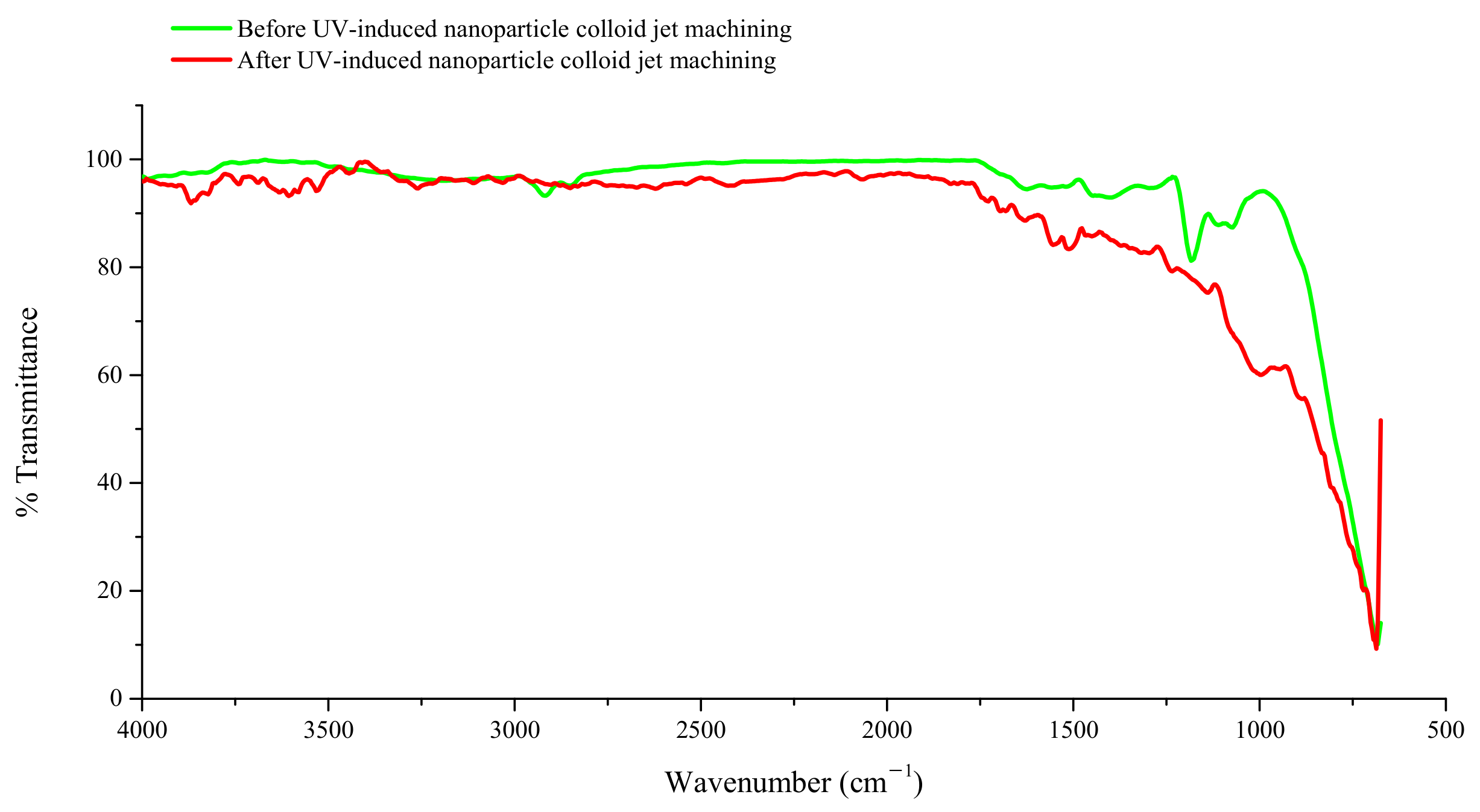
3.1. Interaction between TiO2 Nanoparticles and Ti-6Al-4V Workpiece
3.2. Surface Quality of Ti-6Al-4V Polished by UV-Induced Nanoparticle Colloid Jet Machining
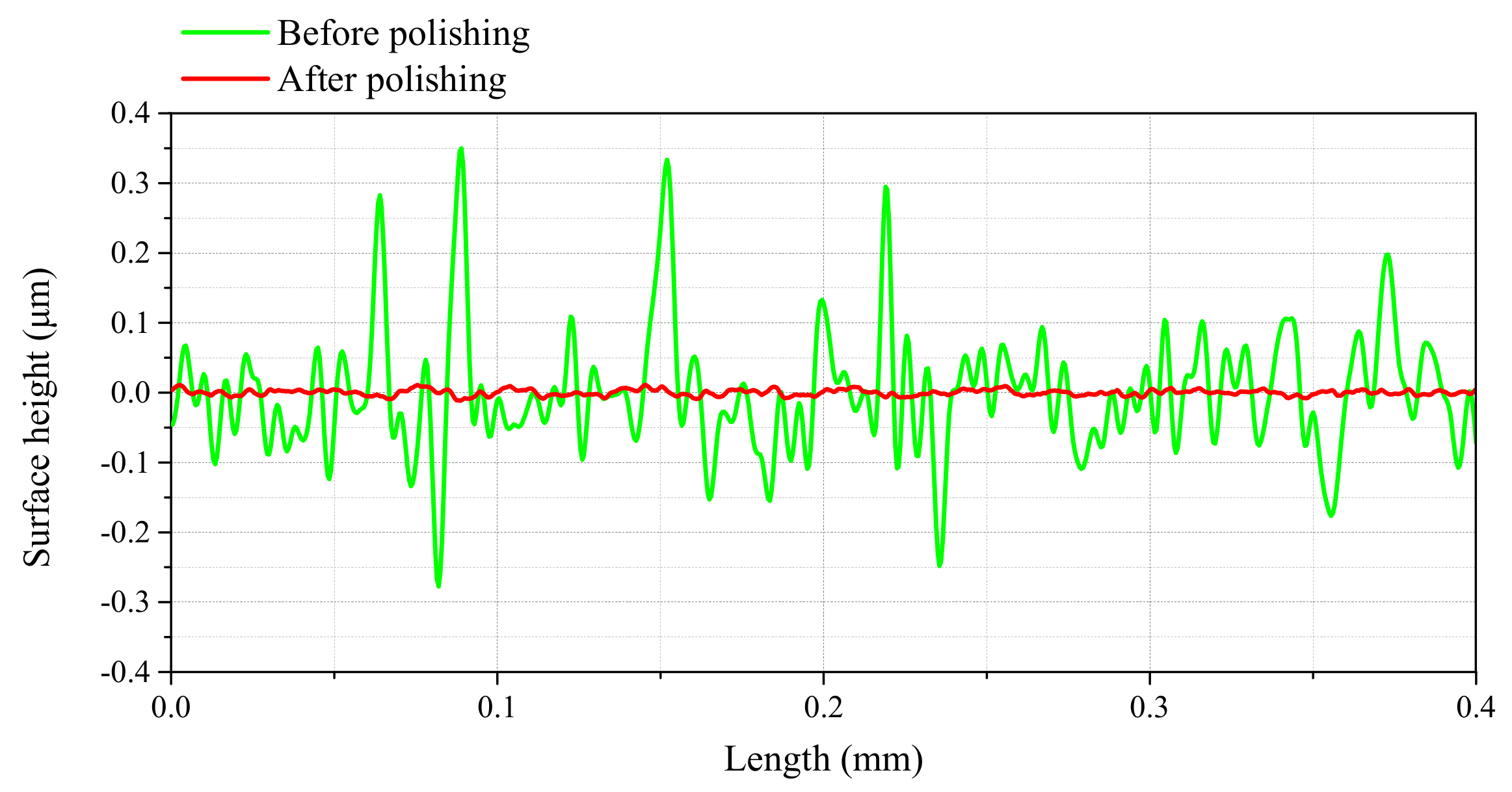
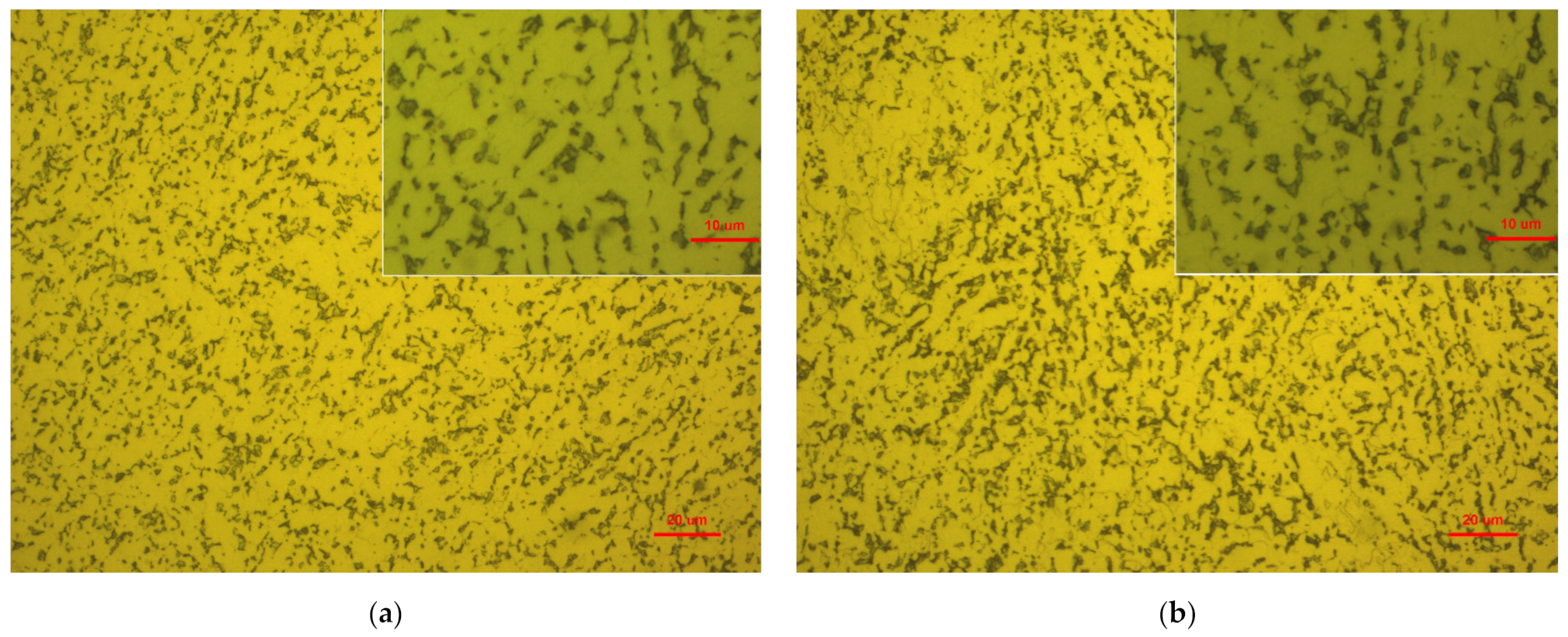
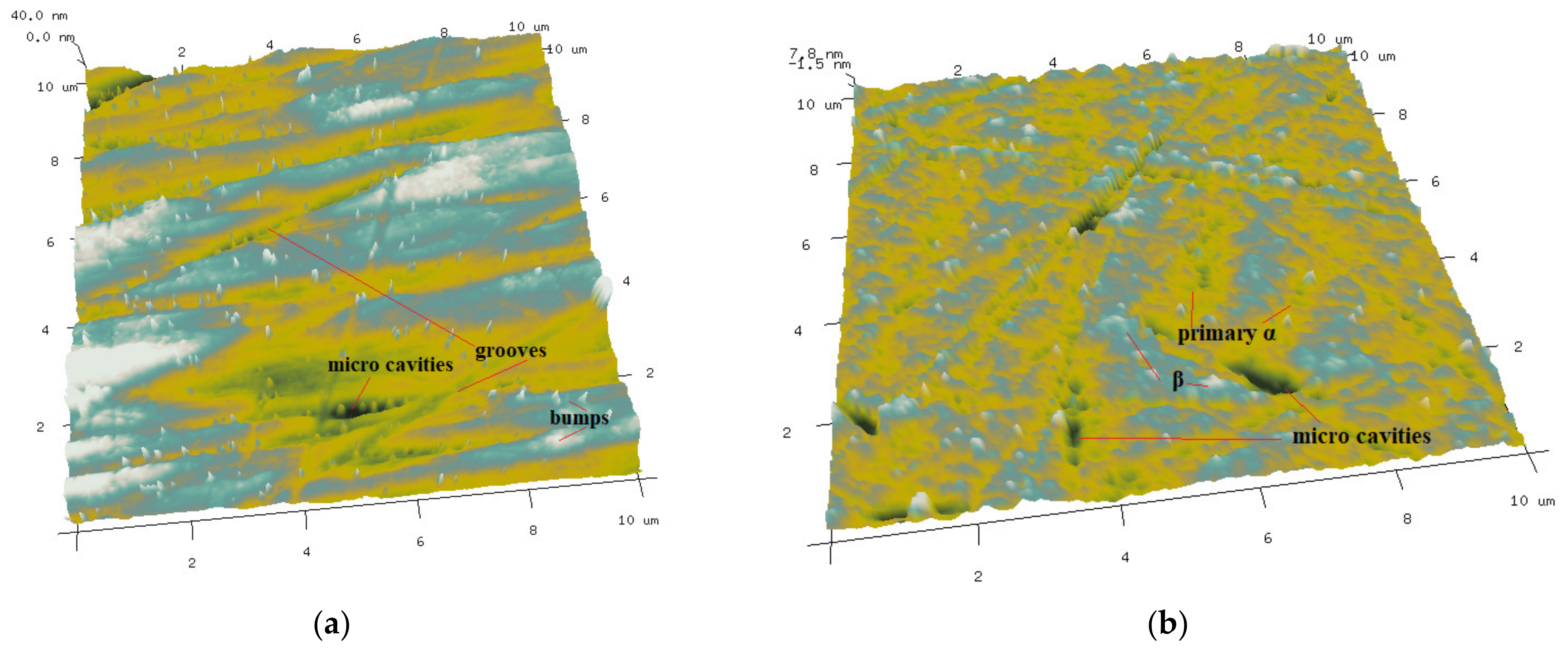
3.2.1. Surface Roughness and Micro Surface Morphology of Ti-6Al-4V
3.2.2. Surface Residual Stress Investigation of Ti-6Al-4V Workpiece
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ASTM. Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate; ASTM B265-20; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Boyer, R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Hu, N.; Gao, N.; Starink, M.J. The influence of surface roughness and high pressure torsion on the growth of anodic titania nanotubes on pure titanium. Appl. Surf. Sci. 2016, 387, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, J.; Wang, X.; Wang, X.; Yue, Z. Corrosion fatigue performance of TC4 plates with holes in aviation kerosene. Aerosp. Sci. Technol. 2015, 47, 420–424. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Effect of thermal oxidation on corrosion and corrosion wear behavior of a Ti-6Al-4V alloy. Biomaterials 2004, 25, 3325–3333. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.-H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Schmutz, P.; Quach-Vu, N.-C.; Gerber, I. Metallic Medical Implants: Electrochemical Characterization of Corrosion Processes. Electrochem. Soc. Interface 2008, 17, 35–40. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Schwartz, Z.; Kster, G.; Jahn, U.; Boyan, B.D. Phagocytsis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials 2000, 21, 551–561. [Google Scholar] [CrossRef]
- Cotterell, M.; Byrne, G. Dynamics of chip formation during orthogonal cutting of titanium alloy Ti–6Al–4V. Cirp J. Manuf. Sci. Technol. 2009, 57, 93–96. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, H.K.; Lee, B.S.; Choi, J.; Seo, B.; Kim, H.K.; Kim, G.H.; Kim, H.G. Study on surface shape control of pure Ti fabricated by electron beam melting using electrolytic polishing. Surf. Coat. Technol. 2017, 324, 106–110. [Google Scholar] [CrossRef]
- Magdalena, J.; Joanna, K.K.; Marian, J.; Sulka, G.D. Effect of Different Polishing Methods on Anodic Titanium Dioxide Formation. J. Nanomater. 2015, 2015, 295126. [Google Scholar]
- Ma, C.P.; Guan, Y.C.; Zhou, W. Laser polishing of additive manufactured Ti alloys. Opt. Lasers Eng. 2017, 93, 171–177. [Google Scholar] [CrossRef]
- Song, X.; Gong, J. Properties of ultraviolet-visible beam propagation in TiO2 nanoparticle colloid. High Power Laser Part. Beams 2015, 27, 024110. [Google Scholar] [CrossRef]
- Song, X.; Yao, T. Effect of nozzle cavity on polishing ability of light coupled colloid jet. Opt. Precis. Eng. 2019, 27, 2011–2019. [Google Scholar] [CrossRef]
- Song, X.Z.; Gao, G.; Zhou, Y.X.; Wang, H.G.; Gong, J. Adsorption of TiO2 nanoparticles on monocrystalline silicon surface. Opt. Precis. Eng. 2016, 24, 1694–1702. [Google Scholar] [CrossRef]
- Song, X.; Ge, S.; Wang, X.; Liu, S. Experimental Investigation on the Effects of Photocatalysis in Ultraviolet-Induced Nanoparticle Colloid Jet Machining. Materials 2021, 14, 1070. [Google Scholar] [CrossRef]
- Song, X.; Gao, G. Removal Mechanism Investigation of Ultraviolet Induced Nanoparticle Colloid Jet Machining. Molecules 2020, 26, 68. [Google Scholar] [CrossRef]
- Lin, J.; Ma, N.; Lei, Y.; Murakawa, H. Measurement of residual stress in arc welded lap joints by cosα X-ray diffraction method. J. Mater. Process. Technol. 2017, 243, 387–394. [Google Scholar] [CrossRef]
- Vosough, M.; Liu, P.; Svenningsson, I. Depth Profile of Titanium Alloy (Ti-6Al-4V) and Residual Stress Measured by Using X-Ray Diffraction after Metal Cutting Assisted by High-Pressured Jet Cooling Evaluation of Etching Methods: ION Beam (EDOS) and Electro-Chemical Etching. Mater. Sci. Forum 2005, 490–491, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Augugliaro, V.; Yurdakal, S.; Loddo, V.; Palmisano, G.; Palmisano, L. Determination of Photoadsorption Capacity of Polychrystalline TiO2 Catalyst in Irradiated Slurry. Adv. Chem. Eng. 2009, 36, 1–35. [Google Scholar]
- Farnoush, H.; Mohandesi, J.A.; Fatmehsari, D.H.; Moztarzadeh, F. Modification of electrophoretically deposited nano-hydroxyapatite coatings by wire brushing on Ti–6Al–4V substrates. Ceram. Int. 2012, 38, 4885–4893. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Park, Y.-B.; Park, D.-W. Kinetic and mechanistic study on the chemical vapor deposition of titanium dioxide thin films by in situ FT-IR using TTIP. Surf. Coat. Technol. 2003, 171, 198–204. [Google Scholar] [CrossRef]
- Jensen, H.; Soloviev, A.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of the surface properties of different prepared titania nano-powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Basim, B. Effect of chemical mechanical polishing on surface nature of titanium implants FT-IR and wettability data of titanium implants surface after chemical mechanical polishing implementation. Data Brief 2017, 10, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Q.; Xi, L.; Zhang, C. Investigation of the states of water and OH groups on the surface of silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 112–115. [Google Scholar] [CrossRef]
- Su, B.; Zhang, W.G.; Yu, X.D.; Wang, C.T. Surface and interface analysis of HAP/TiO2 composite films on Ti6Al4V. J. China Nonferr. Metals Soc. 2004, 14, 193–198. [Google Scholar]
- Wang, J.; Lyu, B.; Jiang, L.; Shao, Q.; Deng, C.; Zhou, Y.; Wang, J.; Yuan, J. Chemistry enhanced shear thickening polishing of Ti–6Al–4V. Precis. Eng. 2021, 72, 59–68. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003; pp. 10–26. [Google Scholar]
- Jhong, Y.-T.; Chao, C.-Y.; Hung, W.-C.; Du, J.-K. Effects of Various Polishing Techniques on the Surface Characteristics of the Ti-6Al-4V Alloy and on Bacterial Adhesion. Coatings 2020, 10, 1057. [Google Scholar] [CrossRef]






| Experimental Conditions | Value |
|---|---|
| Workpiece material | Ti-6Al-4V |
| Workpiece size | 25 mm × 25 mm × 1.5 mm |
| Nanoparticles material | TiO2 |
| Diameter of nanoparticles | 20–30 nm |
| Concentration of colloid | 10% (volume percentage) |
| pH of colloid | 5 |
| UV light intensity | 70 mW/cm2 |
| Intensity of pressure | 3 Mpa |
| Polishing time | 180 min |
| Injection distance | 3 mm |
| Surface Roughness | Ra (nm) | Rq (nm) | Rz (nm) |
|---|---|---|---|
| Before polishing | 66 | 93 | 662 |
| After polishing | 3 | 4 | 18 |
| Surface Roughness | Sa (nm) | Sq (nm) | Sz (nm) |
|---|---|---|---|
| Before polishing | 76.7 | 104 | 1180 |
| After polishing | 2.87 | 3.61 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Wang, X.; Wang, S.; Liu, S.; Ge, S. Experimental Study on Ultra-Precision Polishing of Ti-6Al-4V by Ultraviolet-Induced Nanoparticle Colloid Jet Machining. Materials 2021, 14, 5014. https://doi.org/10.3390/ma14175014
Song X, Wang X, Wang S, Liu S, Ge S. Experimental Study on Ultra-Precision Polishing of Ti-6Al-4V by Ultraviolet-Induced Nanoparticle Colloid Jet Machining. Materials. 2021; 14(17):5014. https://doi.org/10.3390/ma14175014
Chicago/Turabian StyleSong, Xiaozong, Xiaorong Wang, Shun Wang, Shengkai Liu, and Shundong Ge. 2021. "Experimental Study on Ultra-Precision Polishing of Ti-6Al-4V by Ultraviolet-Induced Nanoparticle Colloid Jet Machining" Materials 14, no. 17: 5014. https://doi.org/10.3390/ma14175014
APA StyleSong, X., Wang, X., Wang, S., Liu, S., & Ge, S. (2021). Experimental Study on Ultra-Precision Polishing of Ti-6Al-4V by Ultraviolet-Induced Nanoparticle Colloid Jet Machining. Materials, 14(17), 5014. https://doi.org/10.3390/ma14175014






