Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
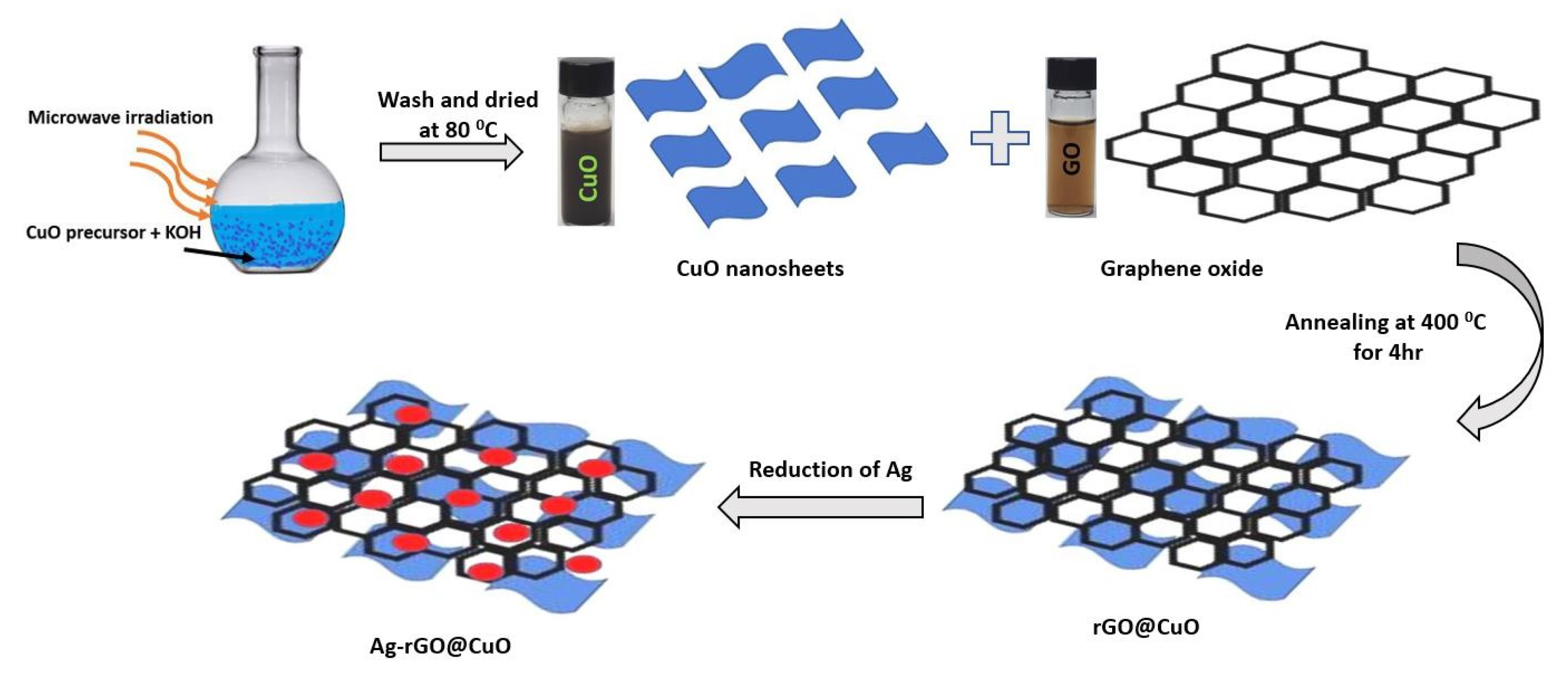
2.2. Synthesis of Electrode Materials
2.3. Characterization Techniques
2.4. Fabrication of Electrodes and Electrochemical Measurements
3. Results and Discussion
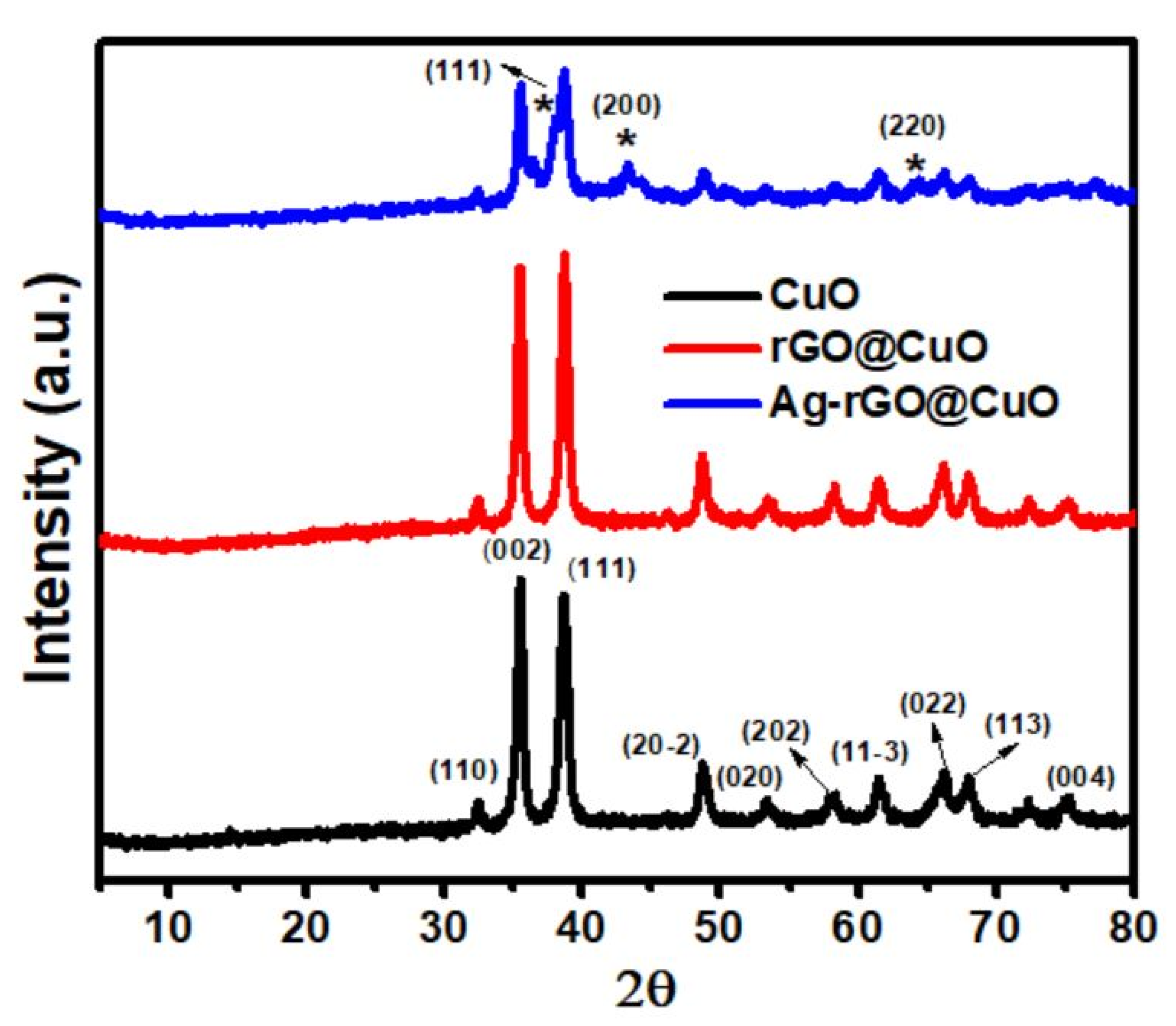
3.1. X-ray Diffraction Studies
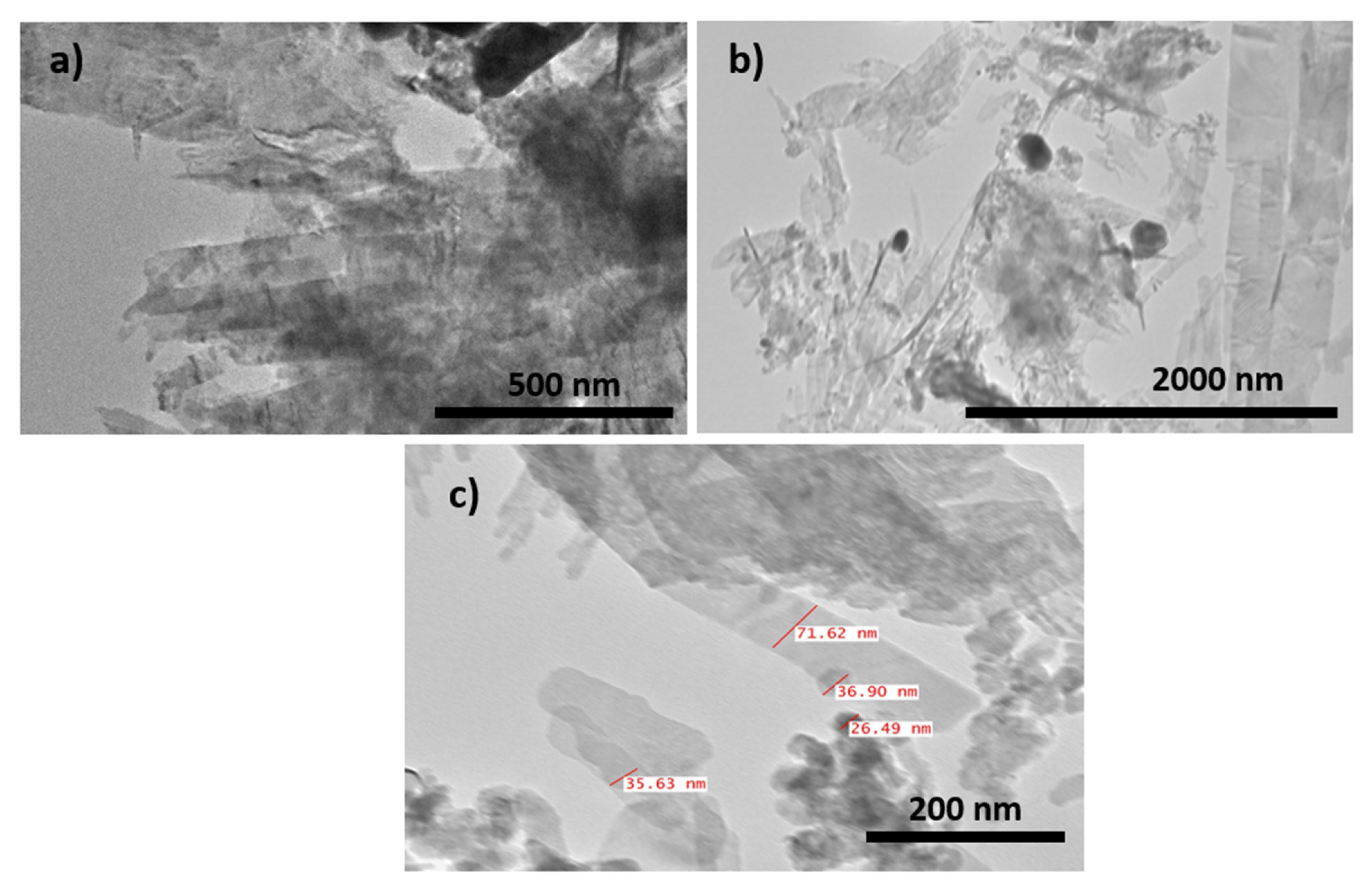
3.2. SEM, EDX and TEM Characterization
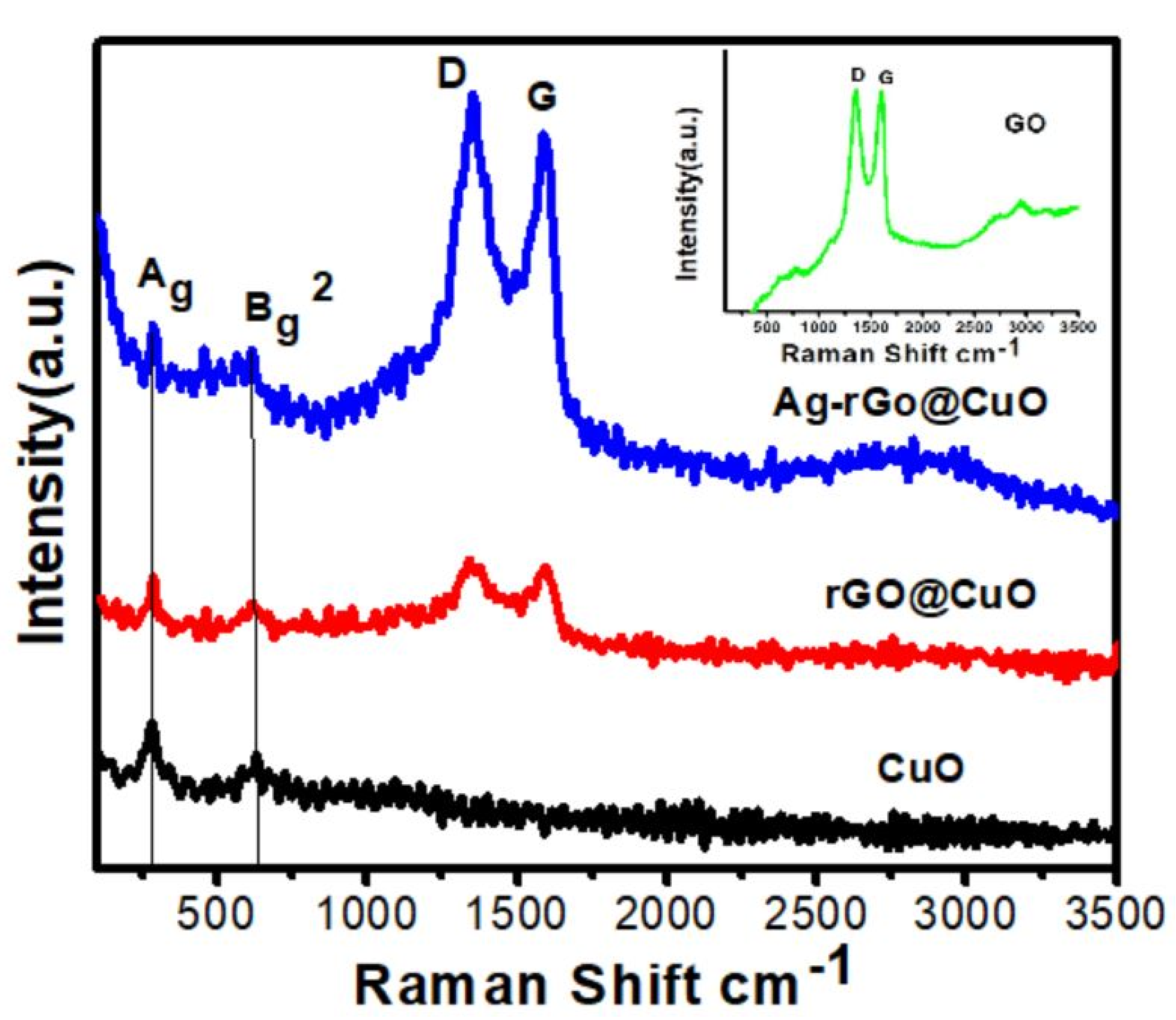
3.3. Raman Spectrum
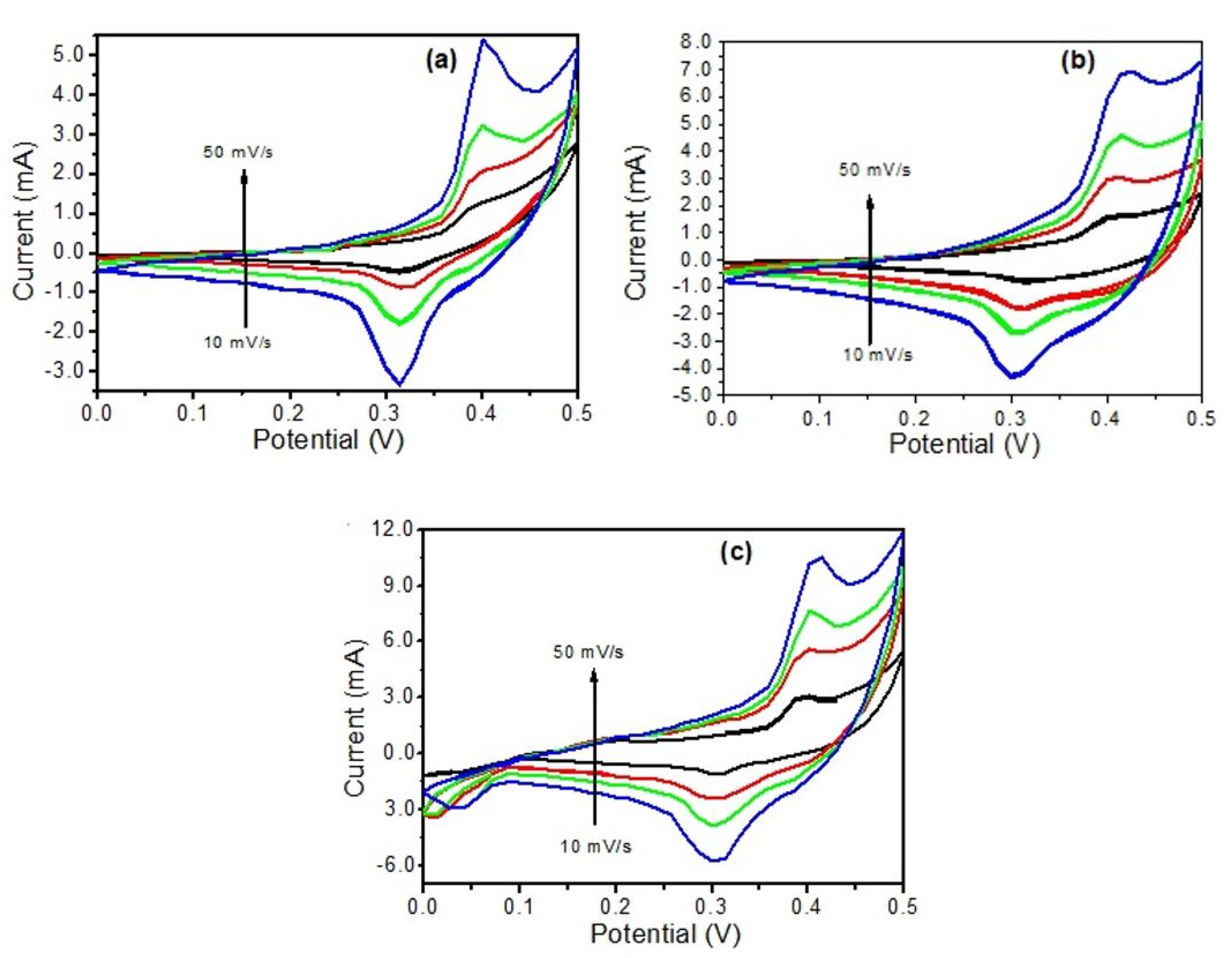
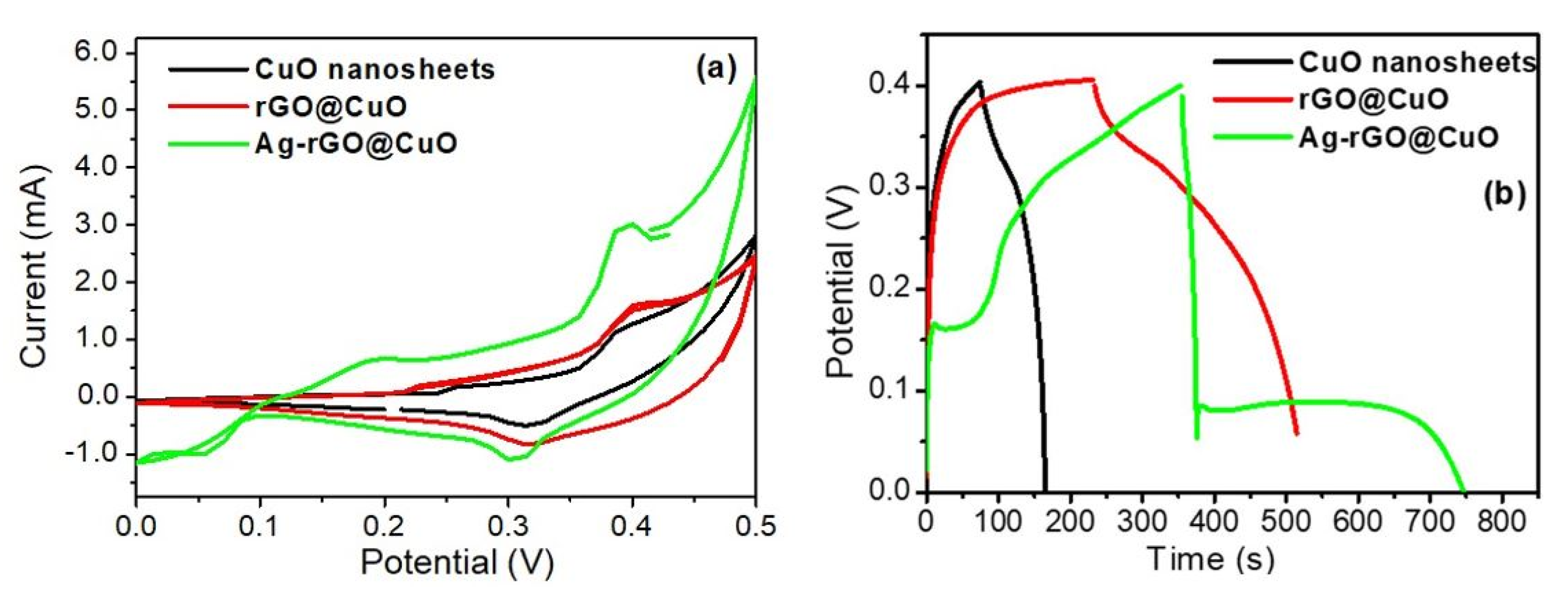
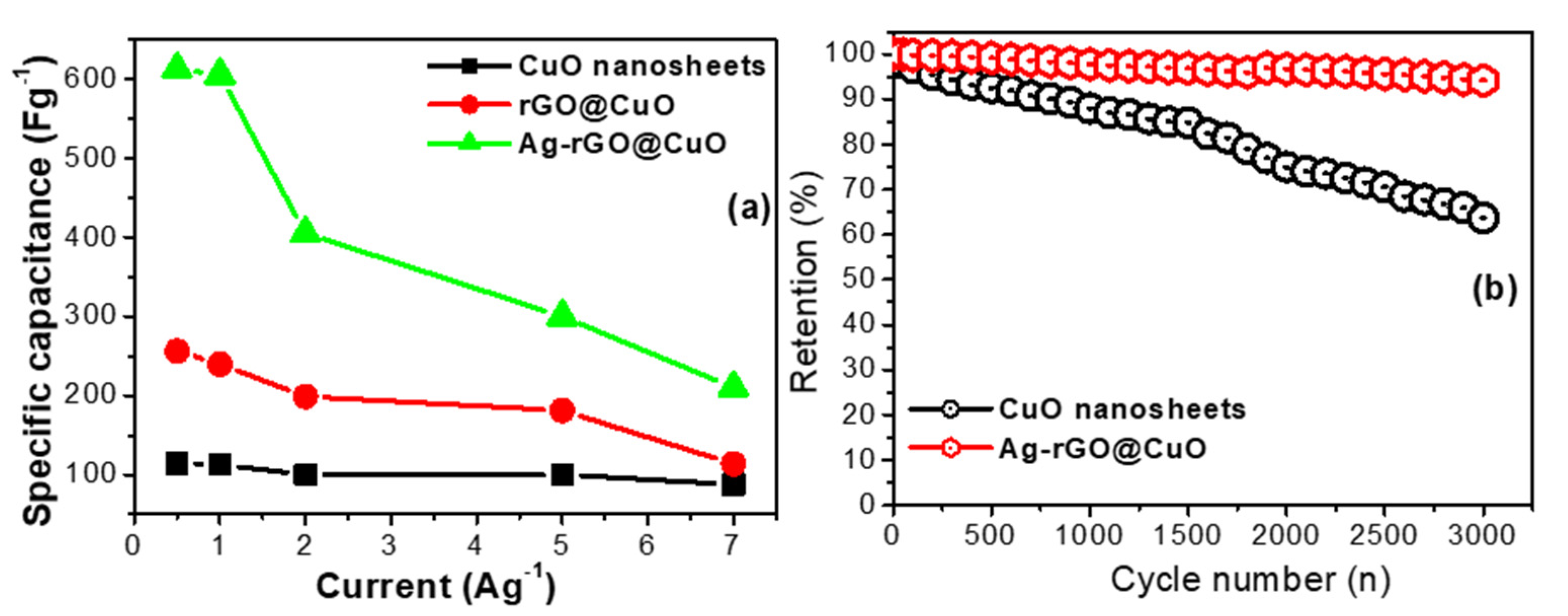
3.4. Electrochemical Capacitive Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, I.T.; Oladipo, A.O.; Adedokun, O.; Dhlamini, S.M. Recent advances on the preparation and electrochemical analysis of MoS2 based materials for supercapacitor applications. A Mini-Rev. Mater. Today Commun. 2020, 14, 101664. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, A.; Numan, A.; Alzaid, M.; Iqbal, J. Facile sonochemical synthesis of strontium phosphate based materials for potential application in supercapattery devices. Int. J. Hydrog. Energy 2020, 45, 32331–32342. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, C.; Li, M.; Nie, S.; Dang, W.; Zhao, W.; Dai, L.; Ni, Y. Non-carbonized porous lignin-free wood as an effective scaffold to fabricate lignin-free Wood@ Polyaniline supercapacitor material for renewable energy storage application. J. Power Sources 2020, 471, 228448. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D.; Gopal Khan, G.; Mandal, K. Designing one dimensional Co-Ni/Co3O4-NiO core/shell nano-heterostructure electrodes for high-performance pseudocapacitor. Appl. Phys. Lett. 2014, 104, 133904. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO cauliflowers for supercapacitor application: Novel potentiodynamic deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Long, J.W.; Sassin, M.B.; Fischer, A.E.; Rolison, D.R.; Mansour, A.N.; Johnson, V.S.; Stallworth, P.E.; Greenbaum, S.G. Multifunctional MnO2 carbon nanoarchitectures exhibit battery and capacitor characteristics in alkaline electrolytes. J. Phys. Chem. C 2009, 113, 17595–17598. [Google Scholar] [CrossRef]
- Justin, P.; Meher, S.K.; Rao, G.R. Tuning of capacitance behavior of NiO using anionic, cationic, and nonionic surfactants by hydrothermal synthesis. J. Phys. Chem. 2010, 114, 5203–5210. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for high-performance supercapacitor applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Qu, Q.; Shi, Y.; Li, L.; Guo, W.; Wu, Y.; Zhang, H.; Guan, S.; Holze, R. V2O5 0.6 H2O nanoribbons as cathode material for asymmetric supercapacitor in K2SO4 solution. Electrochem. Commun. 2009, 11, 1325–1328. [Google Scholar] [CrossRef]
- Zou, G.F.; Li, H.; Zhang, D.W.; Xiong, K.; Dong, C.; Qian, Y.T. Well-aligned arrays of CuO nanoplatelets. J. Phys. Chem. B 2006, 110, 1632–1637. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Qiao, Y.Q.; Wang, X.L.; Zhong, J.; Zhang, D.; Gu, C.D. Electrochemical impedance analysis of a hierarchical CuO electrode composed of self-assembled nanoplates. J. Phys. Chem. C 2011, 115, 2505–2513. [Google Scholar] [CrossRef]
- Ke, F.S.; Huang, L.; Wei, G.-Z.; Xue, L.J.; Li, J.T.; Zhang, B.; Chen, S.R.; Fan, X.Y.; Sun, S.G. One-step fabrication of CuO nanoribbons array electrode and its excellent lithium storage performance. Electrochim. Acta 2009, 54, 5825–5829. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Zhang, L.; Zhou, Y.; Wang, X.L.; Shi, S.J. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–1319. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Kharistal, D.J.; Zhao, W.; Lalia, B.S.; Hng, H.H.; Yan, Q. High-power and high-energy-density flexible pseudocapacitor electrodes made from porous CuO nanobelts and single-walled carbon nanotubes. ACS Nano 2011, 5, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, J.; Chen, S.; Gao, Y.; Cao, D. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.S. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Jain, R.; Mishra, S. Electrical and electrochemical properties of graphene modulated through surface functionalization. RSC Adv. 2016, 6, 27404–27415. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Cho, M.H. Simple route for gram synthesis of less defective few layered graphene and its electrochemical performance. RSC Adv. 2015, 5, 44920–44927. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G. Flexible graphene devices related to energy conversion and storage. Energy Environ. Sci. 2015, 8, 790–823. [Google Scholar] [CrossRef]
- Yang, J.; Gunasekaran, S. Electrochemically reduced graphene oxide sheets for use in high performance supercapacitors. Carbon 2013, 51, 36–44. [Google Scholar] [CrossRef]
- Manga, K.K.; Wang, S.; Jaiswal, M.; Bao, Q.; Loh, K.P. High-gain graphene-titanium oxide photoconductor made from inkjet printable ionic solution. Adv. Mater. 2010, 22, 5265–5270. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Qu, M.; Warner, J.H. Superparamagnetic Fe3O4 nanocrystals@graphene composites for energy storage devices. J. Mater. Chem. 2011, 21, 5069–5075. [Google Scholar] [CrossRef]
- Kang, J.; Hirata, A.; Kang, L.; Zhang, X.; Hou, Y.; Chen, L.; Li, C.; Fujita, T.; Akagi, K.; Chen, M. Enhanced supercapacitor performance of MnO2 by atomic doping. Angew. Chem. Int. Ed. 2013, 125, 1708–1711. [Google Scholar] [CrossRef]
- Lu, X.; Zhai, T.; Zhang, X.; Shen, Y.; Yuan, L.; Hu, B.; Gong, L.; Chen, J.; Gao, Y.; Zhou, J.; et al. WO3−x@Au@MnO2 core-shell nanowires on carbon fabric for high-performance flexible supercapacitors. Adv. Mater. 2012, 24, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.Y.; Cho, M.H. Facile electrochemical assisted synthesis of ZnO/graphene nanosheets with enhanced photocatalytic activity. RSC Adv. 2015, 5, 97788–97797. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Rafique, S.; Jafer, R.; Mohamad, S.; Ramesh, K.; Ramesh, S. High performance supercapattery incorporating ternary nanocomposite of multiwalled carbon nanotubes decorated with Co3O4 nanograins and silver nanoparticles as electrode material. Electrochim. Acta 2018, 278, 72–82. [Google Scholar] [CrossRef]
- Ansari, M.O.; Oves, M.; Salah, N.; Asad, M.; Kumar, R.; Hasan, P.M.; Alshahrie, A.; Darwesh, R. DC electrical conductivity retention and antibacterial aspects of microwave-assisted ultrathin CuO@ polyaniline composite. Chem. Pap. 2020, 74, 3887–3898. [Google Scholar] [CrossRef]
- Wang, Z.; Pischedda, V.; Saxena, S.K.; Lazor, P. X-ray diffraction and Raman spectroscopic study of nanocrystalline CuO under pressure. Solid State Commun. 2002, 121, 275–279. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venugopalan, V.P.; Arunya, D.D.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm activity of nano sized CuO. In Proceedings of the International Conference on Nanoscience, Engineering and Technology IEEE (ICONSET 2011), Chennai, India, 28–30 November 2011; pp. 580–583. [Google Scholar]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Meti, S.; Rahman, M.R.; Ahmad, M.I.; Bhat, K.U. Chemical free synthesis of graphene oxide in the preparation of reduced graphene oxide-zinc oxide nanocomposite with improved photocatalytic properties. Appl. Surf. Sci. 2018, 451, 67–75. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.M.; Park, H.K.; Jung, I.S.; Jin, M.H.; Jeong, H.K.; Kim, J.M.; Choi, J.Y.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Qian, Z.; Cheng, Y.; Zhou, X.; Wu, J.; Xu, G. Fabrication of graphene oxide/Ag hybrids and their surface-enhanced Raman scattering characteristics. J. Colloid Interface Sci. 2013, 397, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.C.; Chen, D.H. Microwave-assisted green synthesis of Ag/reduced graphene oxide nanocomposite as a surface-enhanced Raman scattering substrate with high uniformity. Nanoscale Res. Lett. 2014, 913, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.N.; Dai, S.G.; Liu, G.L.; Xi, Y.; Hu, C.G.; Wang, X. CuO Nanoflowers growing on Carbon Fiber Fabric for Flexible High-Performance Supercapacitors. Electrochim. Acta 2016, 203, 1–8. [Google Scholar] [CrossRef]
- Duraisamy, N.; Numan, A.; Ramesh, K.; Choi, K.H.; Ramesh, S. Investigation on structural and electrochemical properties of binder free nanostructured nickel oxide thin film. Mater. Lett. 2015, 161, 694–697. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.L.; Shu, C.Y.; Guo, Y.G.; Wang, C.R. Synthesis of CuO/graphene nanocomposite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar] [CrossRef]
- Sridevi, A.; Balraj, B.; Senthilkumar, N.; Venkatesan, G.P. Synthesis of rGO/CuO/Ag Ternary Nanocomposites Via Hydrothermal Approach for Opto-electronics and Supercapacitor Applications. J. Supercond. Novel Magn. 2020, 33, 3501–3510. [Google Scholar] [CrossRef]
- Basu, P.; Mahesh, R.; Harish, S.; Joseph, S.; Sagayaraj, P. One-pot hydrothermal preparation of Cu2O-CuO/rGO nanocomposites with enhanced electrochemical performance for supercapacitor applications. Appl. Surf. Sci. 2018, 449, 474–484. [Google Scholar]
- Gholivand, M.B.; Heydari, H.; Abdolmaleki, A.; Hosseini, H. Nanostructured CuO/PANI composite as supercapacitor electrode material. Mater. Sci. Semicond. Process. 2015, 30, 157–161. [Google Scholar] [CrossRef]
- Kuang, M.; Li, T.T.; Chen, H.; Zhang, S.M.; Zhang, L.L.; Zhang, Y.X. Hierarchical Cu2O/CuO/Co3O4 core-shell nanowires: Synthesis and electrochemical properties. Nanotechnology 2015, 26, 304002. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Li, Y.; Ye, K.; Cheng, K.; Cao, D.; Pan, Y.; Kong, S.; Zhang, X.; Wang, G. Anchoring CuO nanoparticles on nitrogen-doped reduced graphene oxide nanosheets as electrode material for supercapacitors. J. Electroanal. Chem. 2014, 727, 154–162. [Google Scholar] [CrossRef]



| Element | Weight% | Atomic% |
|---|---|---|
| C K | 4.15 | 12.64 |
| O K | 20.82 | 47.63 |
| Cu L | 60.32 | 34.74 |
| Ag L | 14.71 | 4.99 |
| Electrode Material | Electrolyte | Specific Capacitance (Csp) Fg−1 | Current Density, Ag−1 | No. of Cycles | Retention % | Ref. |
|---|---|---|---|---|---|---|
| CuO NRr | 1M Na2 SO4 | 206.6 | 1 | 1000 | 88 | [48] |
| CuO Cauliflower | 1M Na2 SO4 | 179 | 2 mAcm−2 | 2000 | 81 | [10] |
| CuO/PANI | 1M Na2 SO4 | 185 | 5 mVs−1 | 2000 | 72 | [49] |
| rGO/Cu2O | 1M KOH | 98 | 1 | 1000 | 50 | [50] |
| GO/CuO | 1M Na2 SO4 | 245 | 0.1 | 1000 | 79 | [51] |
| CuO/N-rGO | 6M KOH | 340 | 0.5 | 500 | 80 | [52] |
| Ag-rGO@CuO | 2M KOH | 612.5 | 0.5 | 3000 | 92 | Present case |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications. Materials 2021, 14, 5032. https://doi.org/10.3390/ma14175032
Ansari AR, Ansari SA, Parveen N, Ansari MO, Osman Z. Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications. Materials. 2021; 14(17):5032. https://doi.org/10.3390/ma14175032
Chicago/Turabian StyleAnsari, Akhalakur Rahman, Sajid Ali Ansari, Nazish Parveen, Mohammad Omaish Ansari, and Zurina Osman. 2021. "Silver Nanoparticles Embedded on Reduced Graphene Oxide@Copper Oxide Nanocomposite for High Performance Supercapacitor Applications" Materials 14, no. 17: 5032. https://doi.org/10.3390/ma14175032






