Influence of Solution Treatment Time on Precipitation Behavior and Mechanical Properties of Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
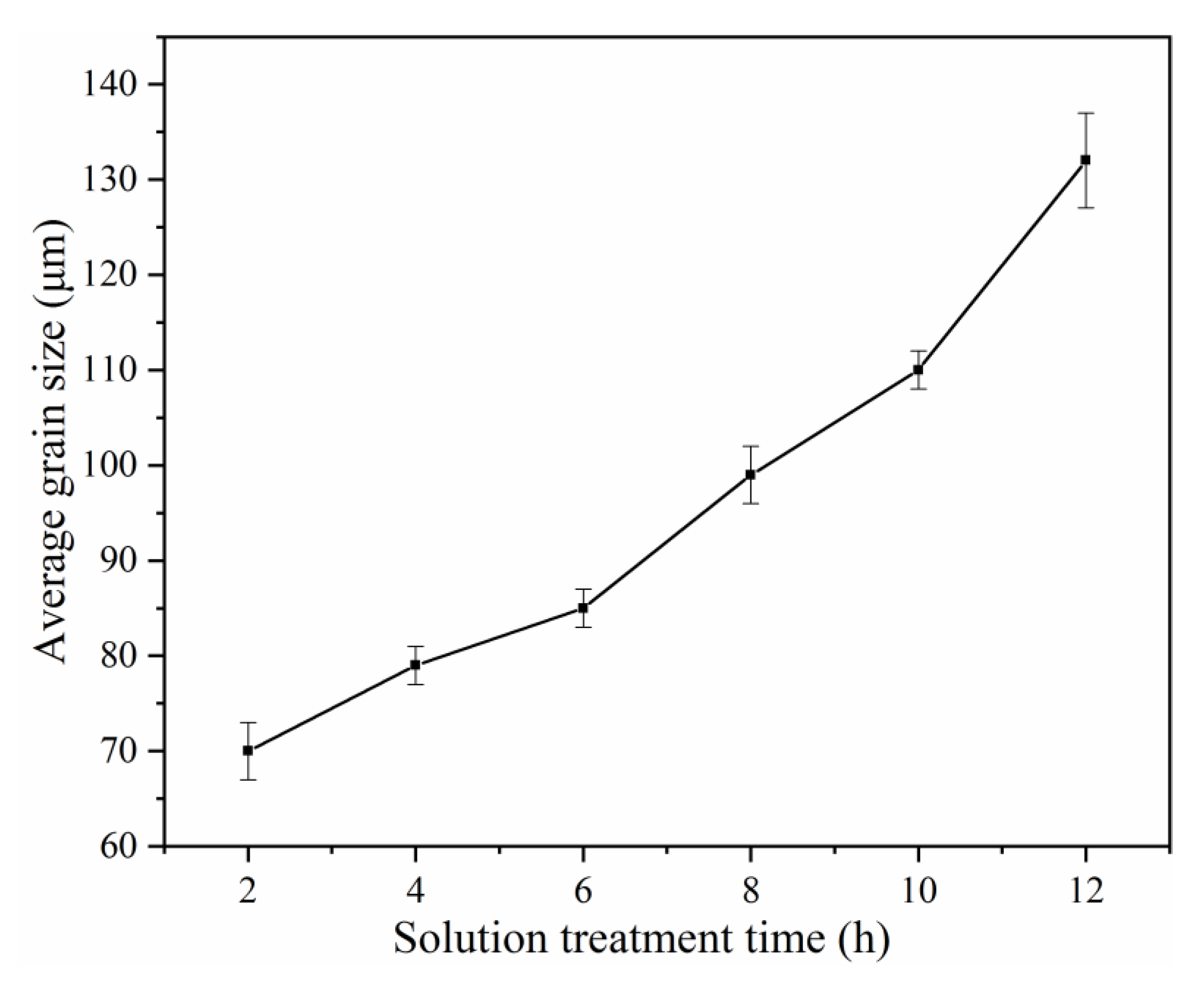
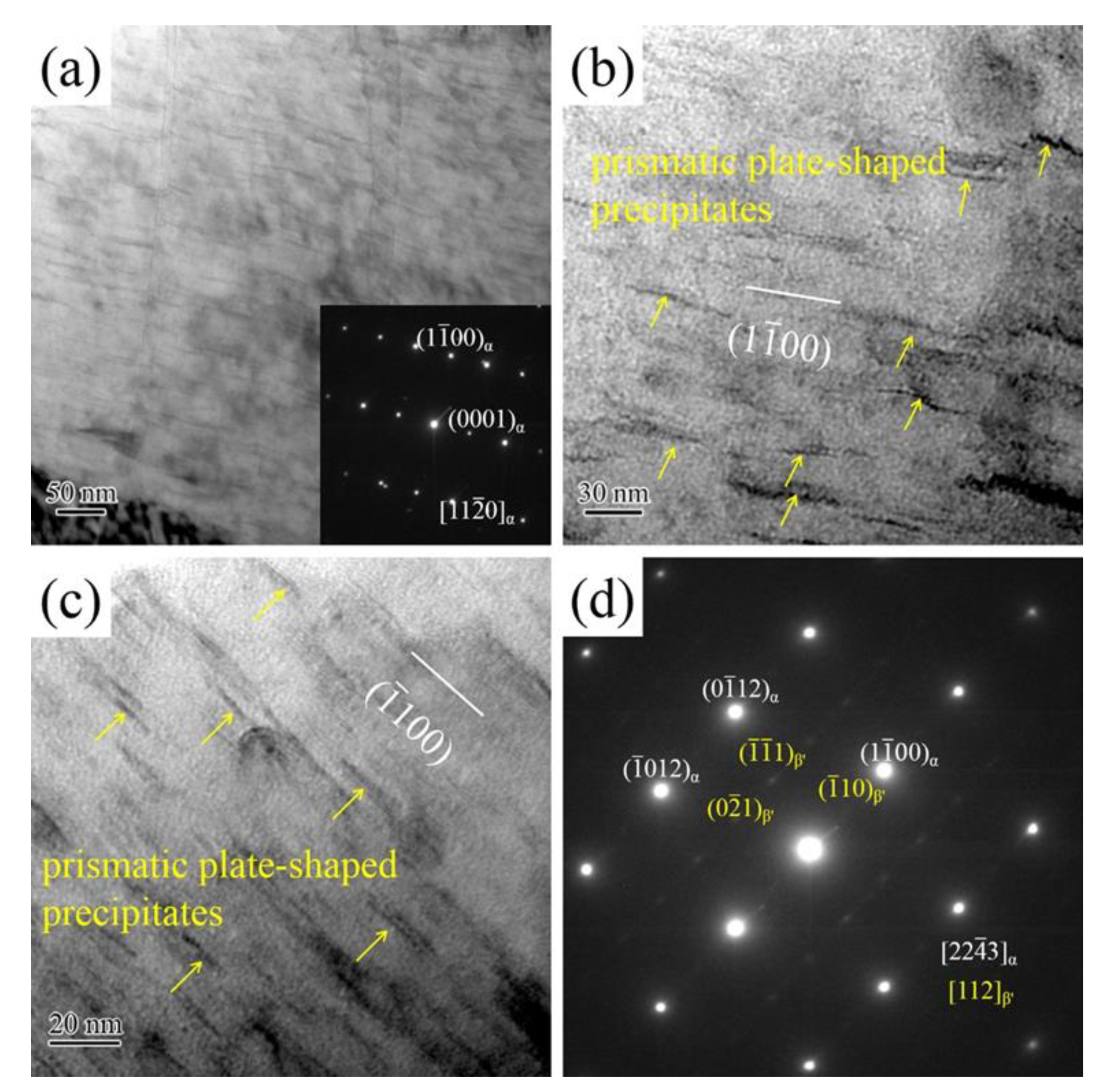
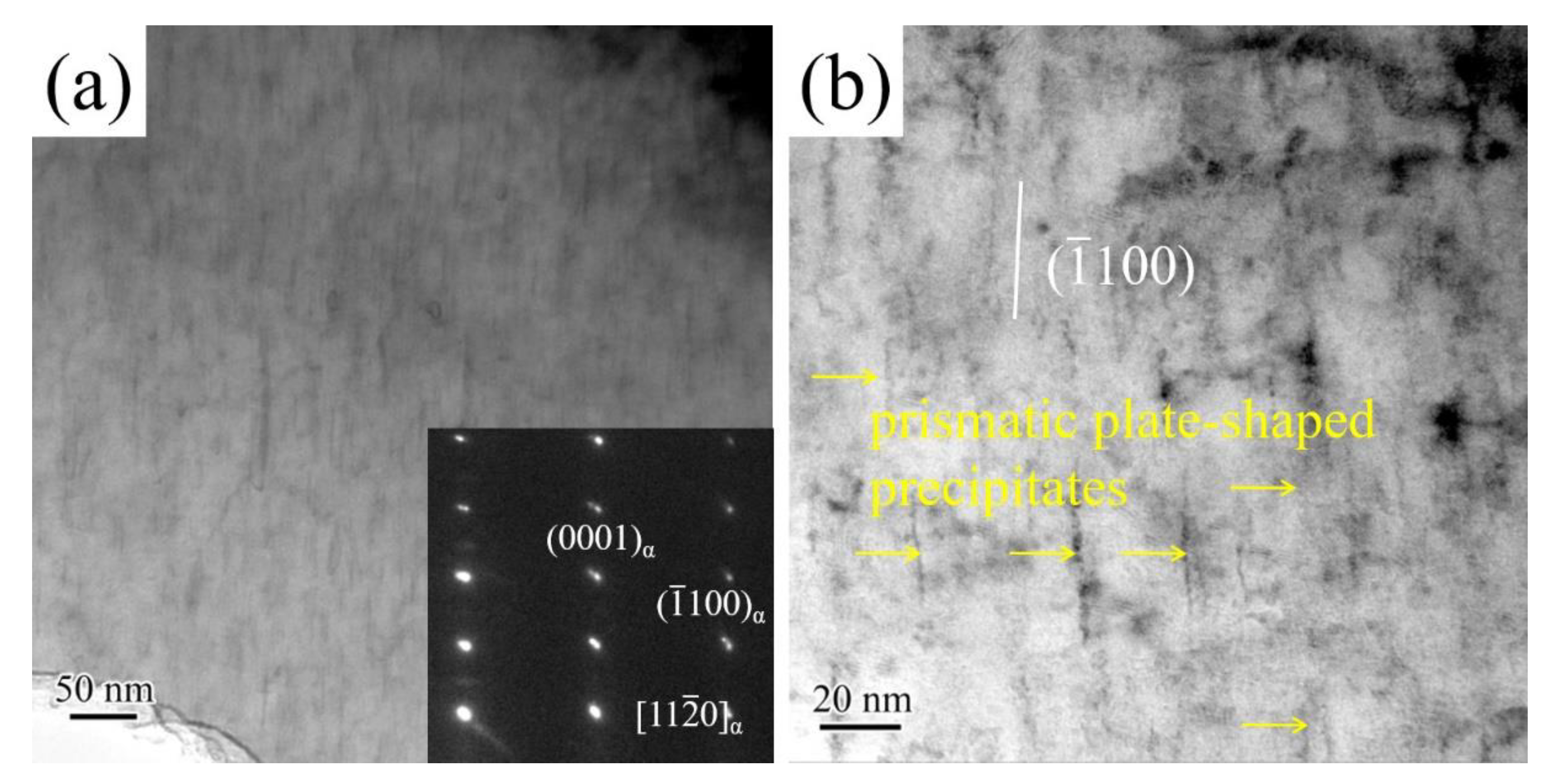
3.1. Microstructure
3.2. Mechanical Properties
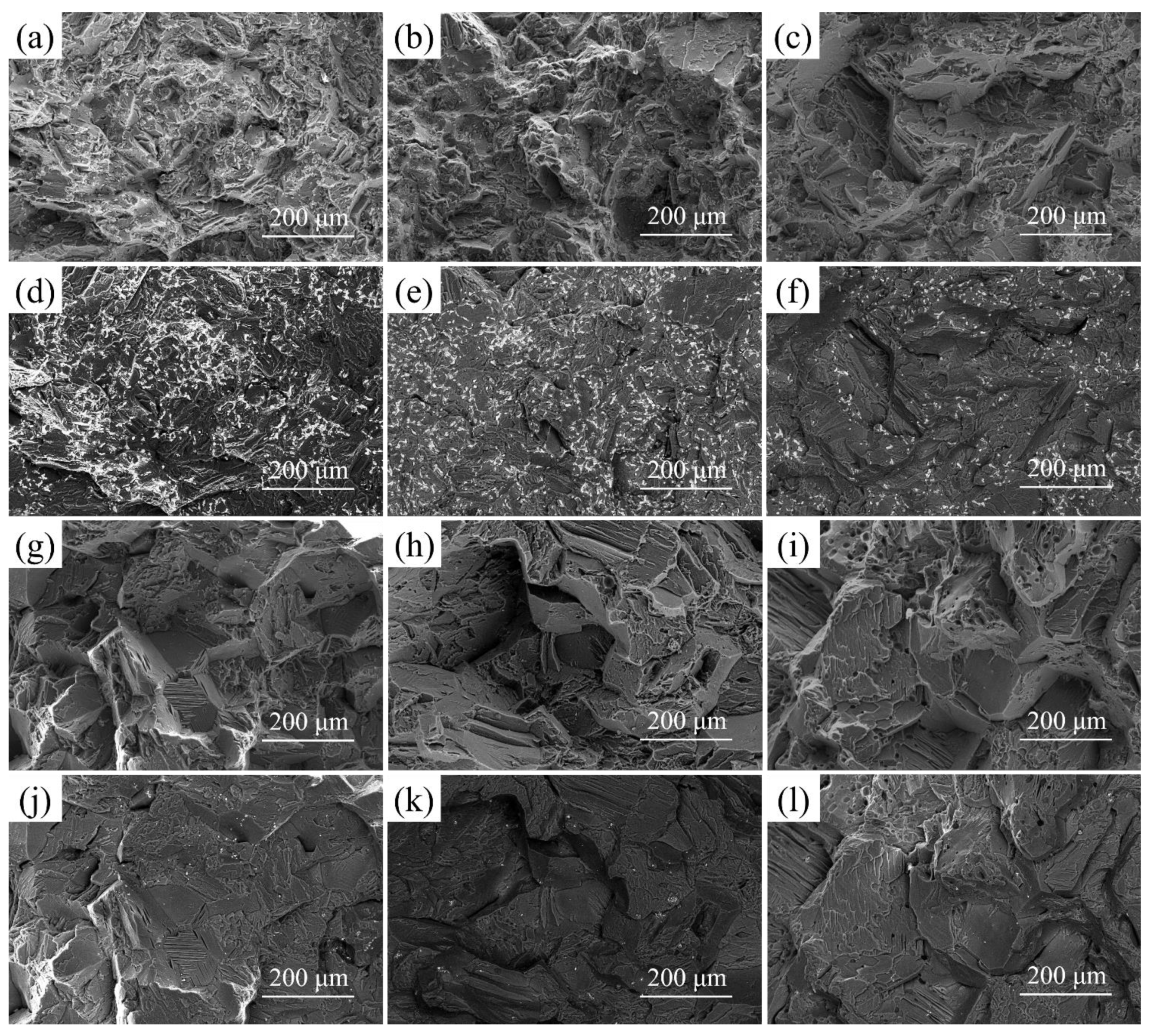
3.3. Fractography
4. Conclusions
- The as-cast alloy mainly consists of α-Mg matrix and network-like β-Mg12(Nd, Sm, Zn) phase. With the increase of solution treatment time from 2 h to 12 h at 788 K, the grain size increases gradually, and the amount of β phase decrease gradually.
- Precipitates morphologies in aged alloys include spherical-shaped precipitates, basal plate-shaped precipitates, and prismatic plate-shaped precipitates. The solution treatment time is carried out for 2 h, the α-Mg matrix mainly contains basal plate-shaped precipitates and spherical-shaped precipitates. As solution treatment time exceeds 8 h, a large number of prismatic plate-shaped β′ precipitates exist in the α-Mg matrix.
- Upon solution treatment time increase from 2 h to 8 h, the improvement of mechanical properties is related to the key strengthening phase transforming from basal plate-shaped precipitates and spherical-shaped precipitates to prismatic plate-shaped β′ precipitates. Further increasing of solution treatment time to 12 h, the decrease of mechanical properties of aged alloys is attributed to grain coarsening.
- The optimal solution treatment time for Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr alloy is 8 h, the YS and UTS of the alloy are 154 ± 1.5 MPa, and 261 ± 4.1 MPa, respectively, and the elongation rate is 5.8 ± 0.1%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naghdi, F.; Mahmudi, G. Effect of solution treatment on the microstructural evolution and mechanical properties of an aged Mg-4Zn-0.3Ca alloy. Mater. Sci. Eng. A 2015, 631, 144–152. [Google Scholar] [CrossRef]
- Gui, Z.Z.; Kang, Z.X.; Li, Y.Y. Evolution of the microstructure and fracture characteristics of a Mg-Nd-Zn-Zr-Mn alloy through heat treatment and extrusion. J. Alloys Comp. 2018, 765, 470–479. [Google Scholar] [CrossRef]
- Xiao, H.C.; Yang, Z.J.; Li, J.; Wang, Y.C. Micro-Shear Bands and Their Enhancement on High Temperature Strength of Mg-Gd-Y-Zr Alloy. Materials 2021, 14, 3262. [Google Scholar] [CrossRef]
- Zhao, S.C.; Guo, E.J.; Cao, G.J.; Wang, L.P.; Lun, Y.C.; Feng, Y.C. Microstructure and mechanical properties of Mg-Nd-Zn-Zr alloy processed by integrated extrusion and equal channel angular pressing. J. Alloys Comp. 2017, 705, 118–125. [Google Scholar] [CrossRef]
- Nodooshan, H.R.J.; Wu, G.H.; Liu, W.C.; Wei, G.L.; Li, Y.L.; Zhang, S. Effect of Gd content on high temperature mechanical properties of Mg-Gd-Y-Zr alloy. Mater. Sci. Eng. A 2016, 651, 840–847. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Microstructure and strengthening mechanism of high strength Mg-10Gd-2Y-0.5Zr alloy. J. Alloys Comp. 2007, 427, 316–323. [Google Scholar] [CrossRef]
- Xiao, H.C.; Yang, Z.J.; Li, J.; Wang, Y.C. Deformation Mechanism, Microstructure, and Mechanical Properties Evolution of Mg-Gd-Y-Zr Alloy during Cold Torsion. Materials 2021, 14, 2067. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Z.; Xu, W.C.; Yang, Z.Z.; Yuan, C.; Shan, D.B.; Teng, D.B.; Jin, B.C. Analysis of abnormal texture formation and strengthening mechanism in an extruded Mg-Gd-Y-Zn-Zr alloy. J. Mater. Sci. Technol. 2020, 45, 133–145. [Google Scholar] [CrossRef]
- Li, B.S.; Meng, F.Z.; Lv, S.H.; Guan, K.; Yang, Q.; Qin, P.F.; Qiu, X.; Tian, Z.; Zhang, D.D.; Meng, J. Influence of various Yb additions on microstructures of a casting Mg-8Gd-1.2Zn-0.5Zr alloy. J. Alloys Comp. 2019, 789, 720–729. [Google Scholar] [CrossRef]
- Tang, C.P.; Wu, K.; Liu, W.H.; Feng, D.; Wang, X.Z.; Miao, G.D.; Yang, M.M.; Liu, X.; Li, Q. Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy. Metal 2018, 8, 790. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, G.; Liu, W.; Liang, Z.; Zhang, H.; Cui, W. Effects of minor Y addition on microstructure and mechanical properties of Mg-Nd-Zn-Zr alloy. J. Mater. Res. 2017, 32, 3712–3722. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Peng, L.M.; Zeng, X.Q. Characterization of phases in a Mg-6Gd-4Sm-0.4Zr (wt.%) alloy during solution treatment. Mater. Charact. 2009, 60, 555–559. [Google Scholar] [CrossRef]
- Zhang, D.P.; Yang, Q.; Zhang, D.D.; Guan, K.; Bu, F.Q.; Zhou, H.; Meng, J. Effects of substitution of Nd in a sand-cast Mg-2.5Nd-0.6Zn-0.5Zr alloy with x wt.% Sm (x = 2.5, 4, and 6). J. Rare Earths 2017, 35, 1261–1267. [Google Scholar] [CrossRef]
- Chang, J.W.; Fu, P.H.; Guo, X.W.; Peng, L.M.; Ding, W.J. The effects of heat treatment and zirconium on the corrosion behaviour of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy. Corros. Sci. 2007, 49, 2612–2617. [Google Scholar] [CrossRef]
- Yuan, M.; Zheng, Z.Q. Effects of heat treatment on microstructure and mechanical properties of Mg-2.6Sm- 1.3Gd-0.6Zn-0.5Zr alloy. Mater. Sci. Technol. 2014, 30, 261–267. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, Y.C.; Wang, J.; Zhang, J. Microstructure Evolution of Mg 4.3Zn 0.7Y 0.6Zr Alloy during Solution Heat Treatment. Mater. Trans. 2014, 55, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.D.; Hu, S.S.; Li, S.; Chai, D.L.; Zhu, X.R. Influence of Heat Treatment on the Microstructure and Property of Mg-Nd-Gd-Zn-Zr Alloy. Rare Metal Mater. Eng. 2011, 40, 1133–1137. [Google Scholar]
- Liu, X.Y.; Pan, Q.L.; Lu, Z.L.; Cao, S.F.; He, Y.B.; Li, W.B. Effects of solution treatment on the microstructure and mechanical properties of Al-Cu-Mg-Ag alloy. Mater. Des. 2010, 31, 4392–4397. [Google Scholar] [CrossRef]
- Cao, G.J.; Zhu, N.; Zhao, S.C.; Feng, Y.C.; Guo, E.J.; Wang, L.P. Evolution of the hardening precipitates with an enclosed structure in pre-deformed Mg-Sm-Nd-Zn-Zr alloy. Mater. Lett. 2019, 246, 117–120. [Google Scholar] [CrossRef]
- Gao, X.; Nie, J.F. Enhanced precipitation-hardening in Mg-Gd alloys containing Ag and Zn. Scr. Mater. 2008, 58, 619–622. [Google Scholar] [CrossRef]
- Ning, H.Y.; Yu, Y.D.; Gao, B.; Xiao, L.R.; Wen, L.H.; Yan, Z.H.; Li, L.; Chen, X.F. Grain Refinement and Aging Hardening of the Mg-10Gd-3Y-2Ag-0.4Zr Alloy Produced by a Two-Step Forming Process. Materials 2018, 11, 757. [Google Scholar] [CrossRef] [Green Version]
- Honma, T.; Ohkubo, T.; Kamado, S.; Hono, K. Effect of Zn additions on the age-hardening of Mg-2.0Gd-1.2Y-0.2Zr alloys. Acta Mater. 2007, 55, 4137–4150. [Google Scholar] [CrossRef]
- Xia, X.; Sun, W.; Luo, A.A.; Stone, D.S. Precipitation evolution and hardening in Mg-Sm-Zn-Zr alloys. Acta Mater. 2016, 111, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.X.; Zhu, Y.Y.; Zeng, X.Q.; Chen, B. Segregation of solute atoms in Mg-Ce binary alloy: Atomic-scale novel structures observed by HAADF-STEM. Philos. Mag. 2017, 97, 1498–1508. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and Hardening in Magnesium Alloys. Metall. Mater. Trans. A 2012, 43, 3891–3939. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Rong, W.; Wu, Y.J.; Peng, L.M. Achieving ultra-high strength in Mg-Gd-Ag-Zr wrought alloy via bimodal-grained structure and enhanced precipitation. J. Mater. Sci. Technol. 2020, 54, 160–170. [Google Scholar] [CrossRef]
- Kim, J.K.; Sandlöbes, S.; Raabe, D. On the room temperature deformation mechanisms of a Mg-Y-Zn alloy with long-period-stacking-ordered structures. Acta Mater. 2015, 82, 414–423. [Google Scholar] [CrossRef]
- Nie, J.F. Effects of precipitate shape and orientation on dispersion strengthening in magnesium alloys. Scr. Mater. 2003, 48, 1009–1015. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Zhao, S.; Wang, L.; Wang, Z.; Guo, E.; Feng, Y.; Li, J. Influence of Solution Treatment Time on Precipitation Behavior and Mechanical Properties of Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr Alloy. Materials 2021, 14, 5037. https://doi.org/10.3390/ma14175037
Ma T, Zhao S, Wang L, Wang Z, Guo E, Feng Y, Li J. Influence of Solution Treatment Time on Precipitation Behavior and Mechanical Properties of Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr Alloy. Materials. 2021; 14(17):5037. https://doi.org/10.3390/ma14175037
Chicago/Turabian StyleMa, Tao, Sicong Zhao, Liping Wang, Zhiwei Wang, Erjun Guo, Yicheng Feng, and Jingfang Li. 2021. "Influence of Solution Treatment Time on Precipitation Behavior and Mechanical Properties of Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr Alloy" Materials 14, no. 17: 5037. https://doi.org/10.3390/ma14175037
APA StyleMa, T., Zhao, S., Wang, L., Wang, Z., Guo, E., Feng, Y., & Li, J. (2021). Influence of Solution Treatment Time on Precipitation Behavior and Mechanical Properties of Mg-2.0Nd-2.0Sm-0.4Zn-0.4Zr Alloy. Materials, 14(17), 5037. https://doi.org/10.3390/ma14175037





