Influence of Carbon on the Microstructure Evolution and Hardness of Fe–13Cr–xC (x = 0–0.7 wt.%) Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Solidification Behavior
3.2. Dissolution of Carbides
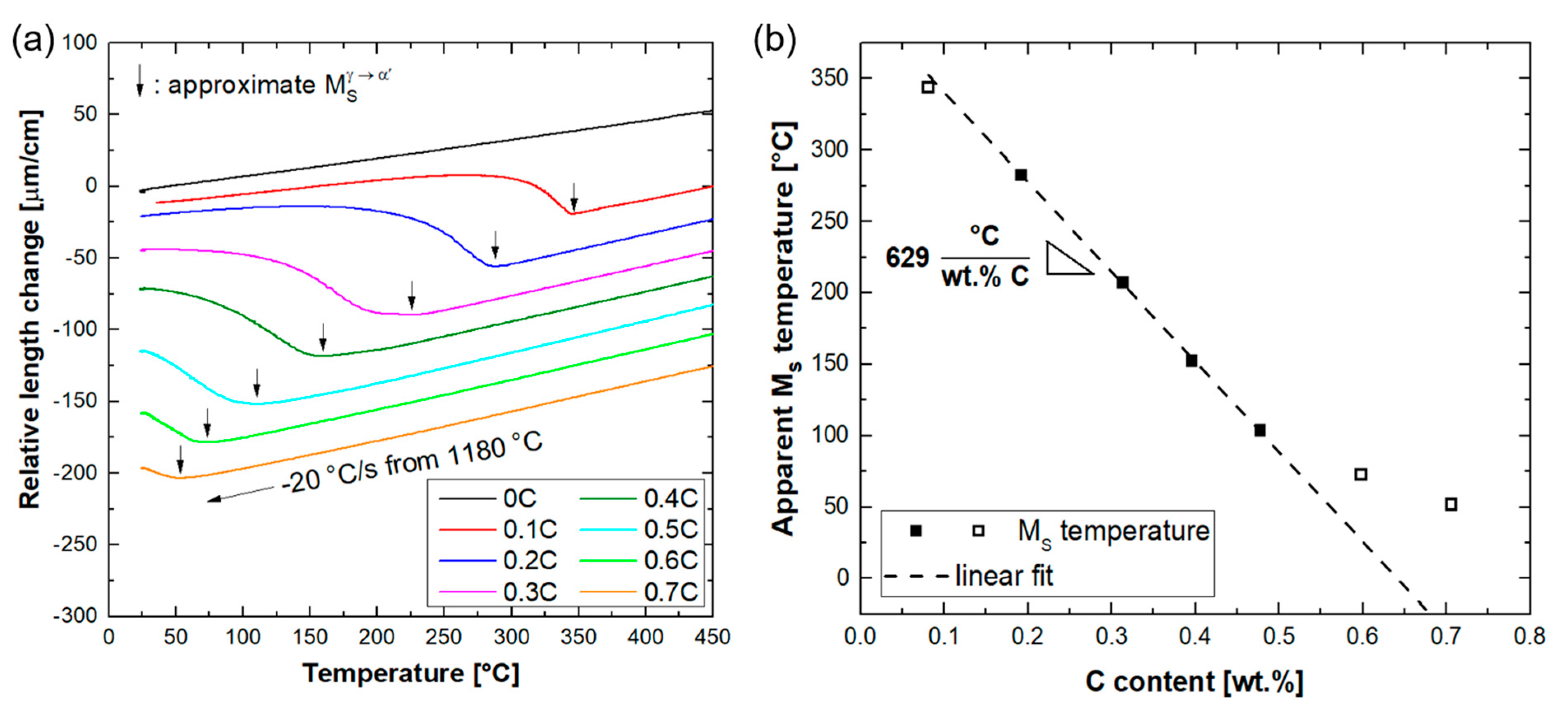
3.3. Martensitic Transformation
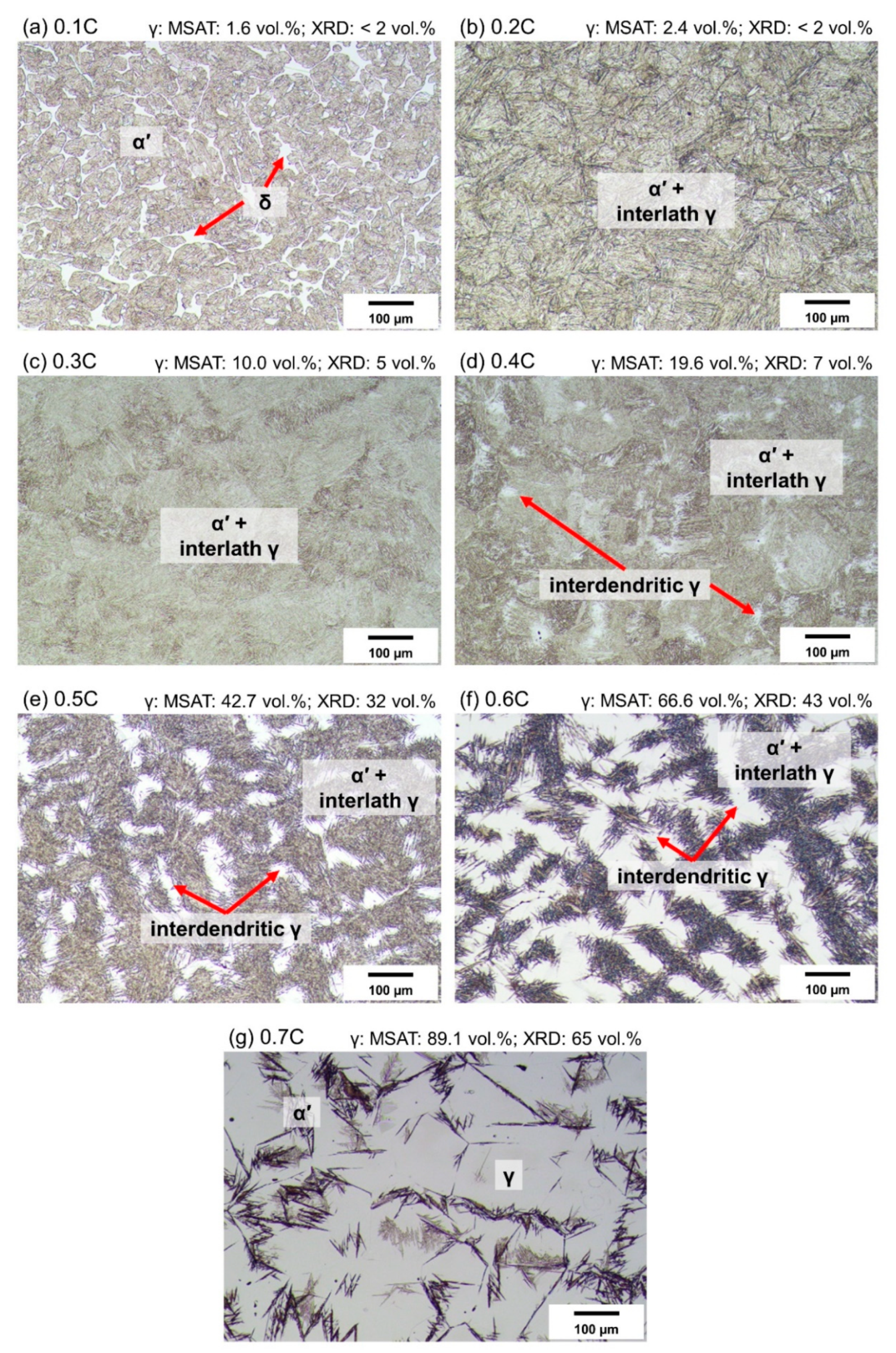
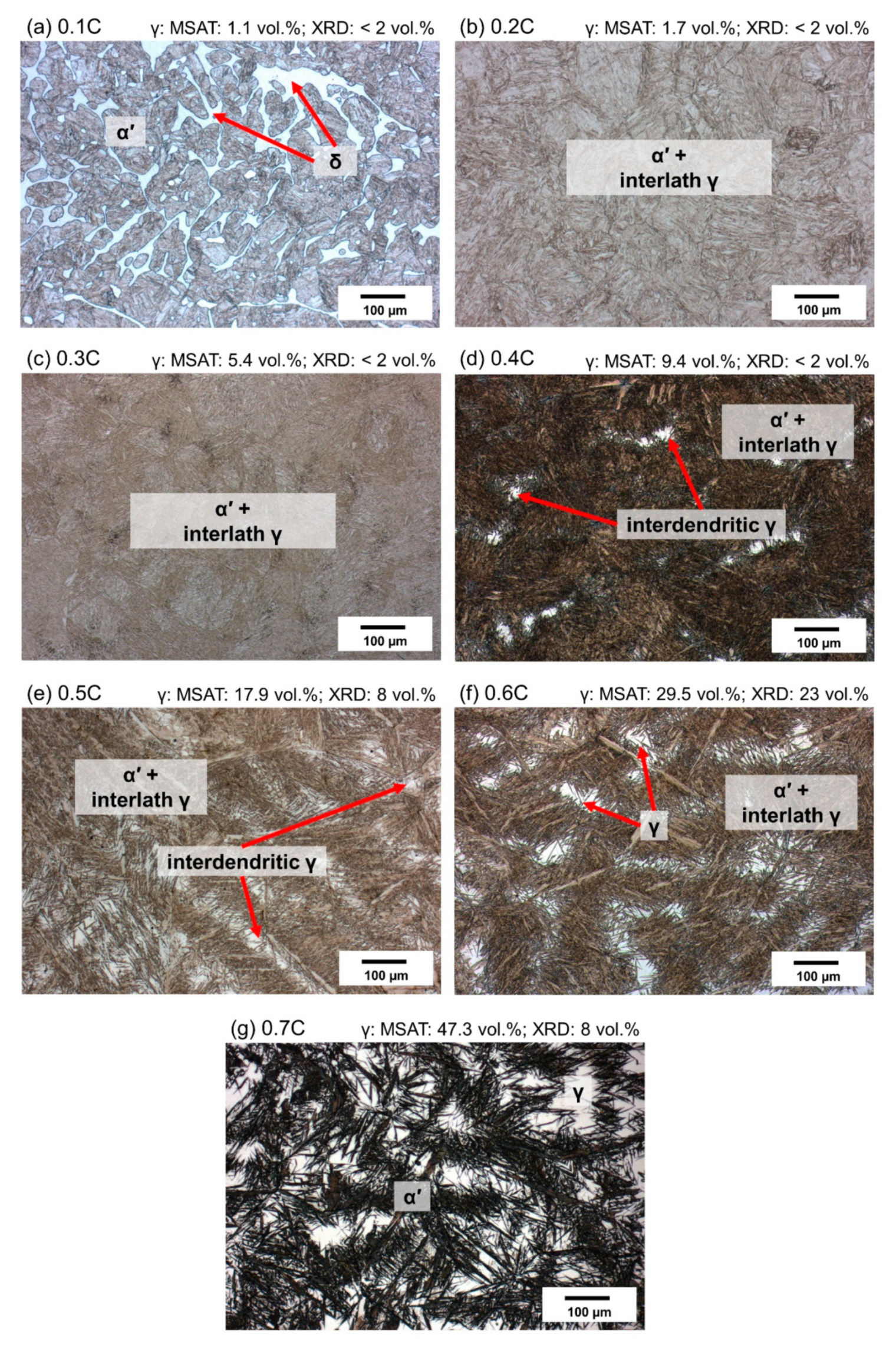
3.4. As-Quenched Condition
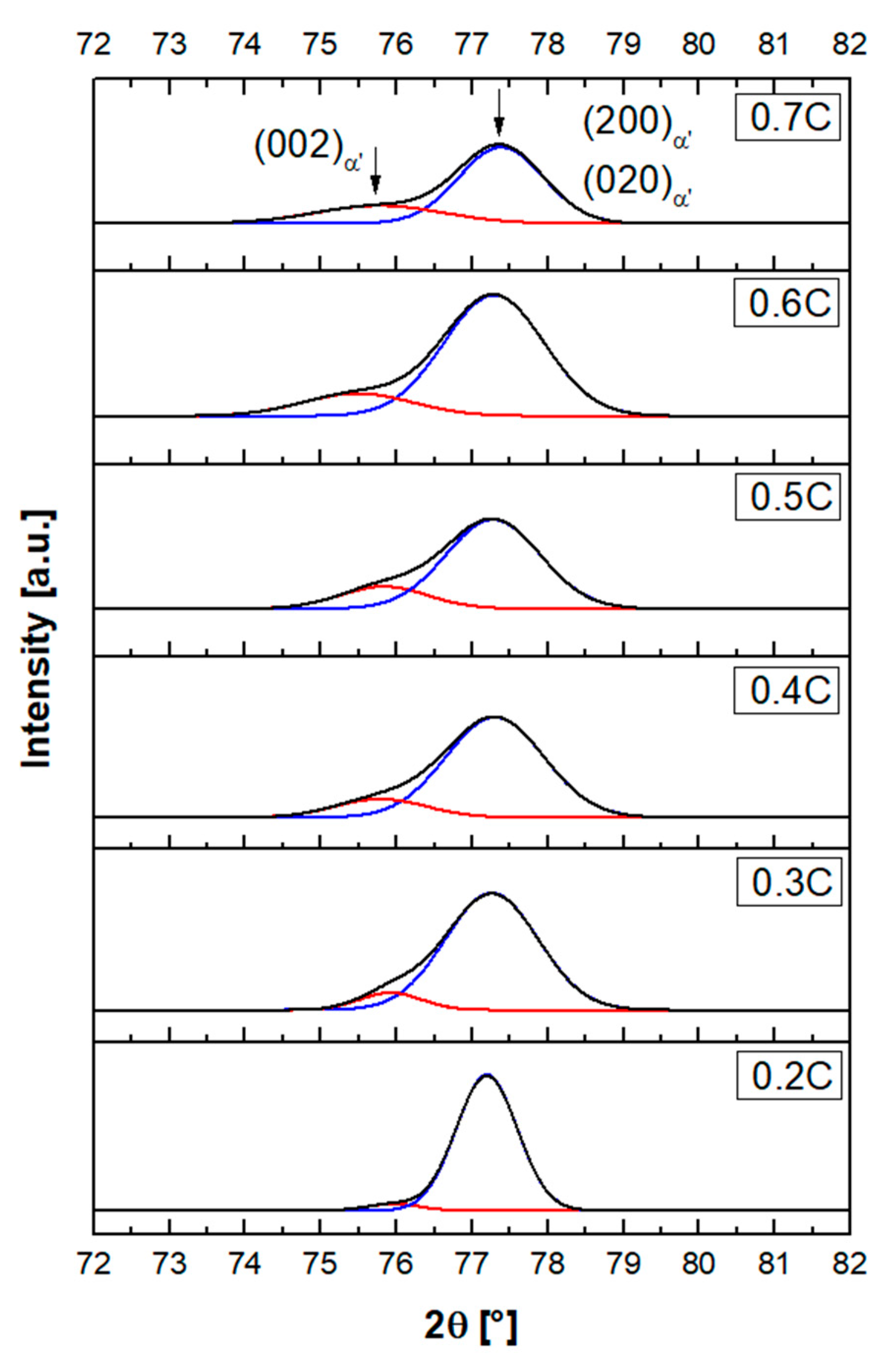
3.5. Lattice Parameters for Martensite and Austenite
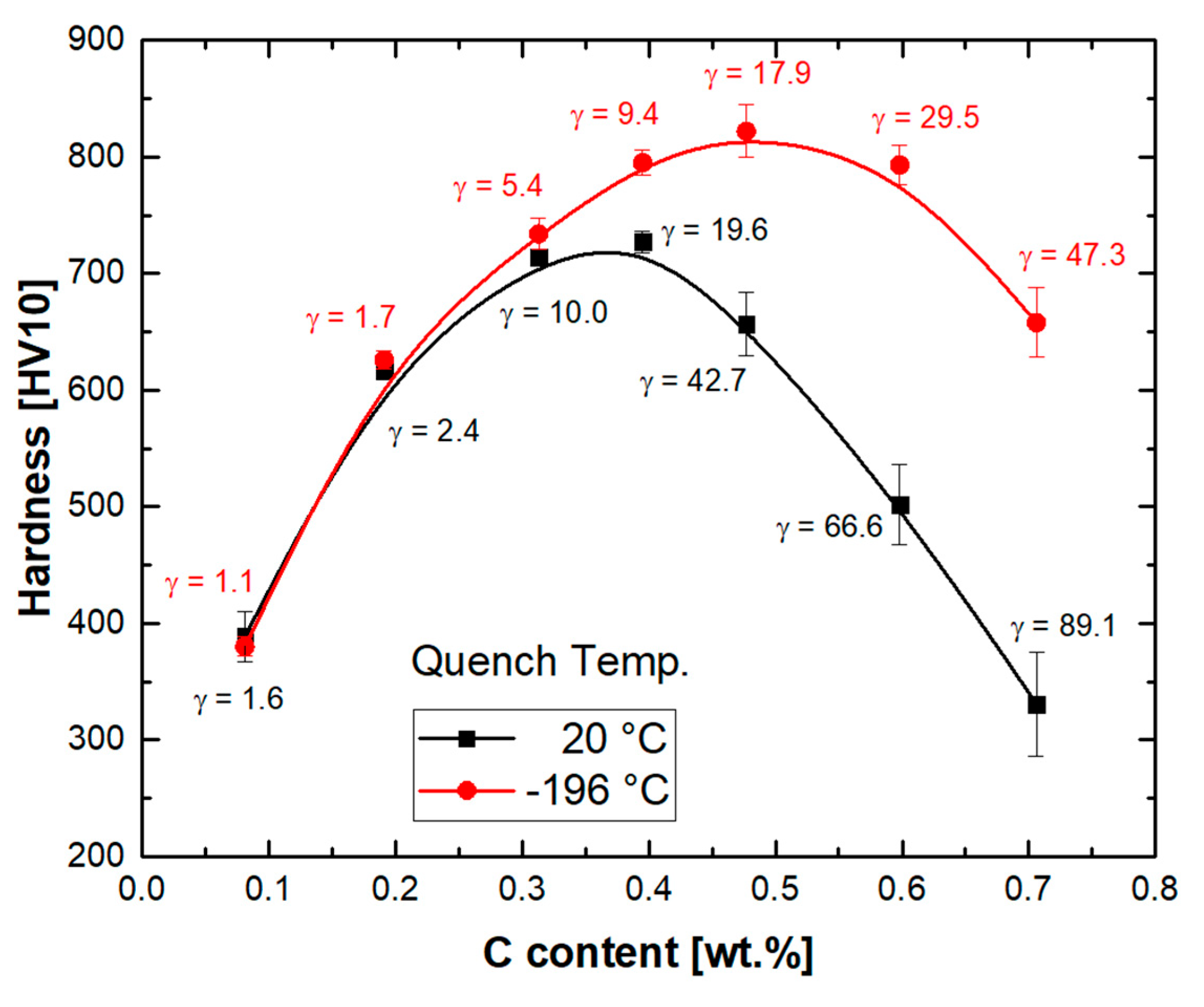
3.6. Hardness of Martensite and Austenite Mixtures
4. Conclusions
- By DSC measurements, the solidification mode was found to change from ferritic to ferritic–austenitic as the carbon concentration increased. The experimental results were in reasonable agreement with the thermodynamic calculations. The solidus–liquidus interval was, however, displaced and broader in the DSC measurements.
- Based on the DSC results, aided by microstructural examinations in the as-quenched state, alloys containing nearly 0% C and 0.1%C exhibited fully ferritic and austenitic–ferritic high-temperature microstructures, respectively. Alloys containing 0.2–0.7%C, on the other hand, exhibited a fully austenitic phase field.
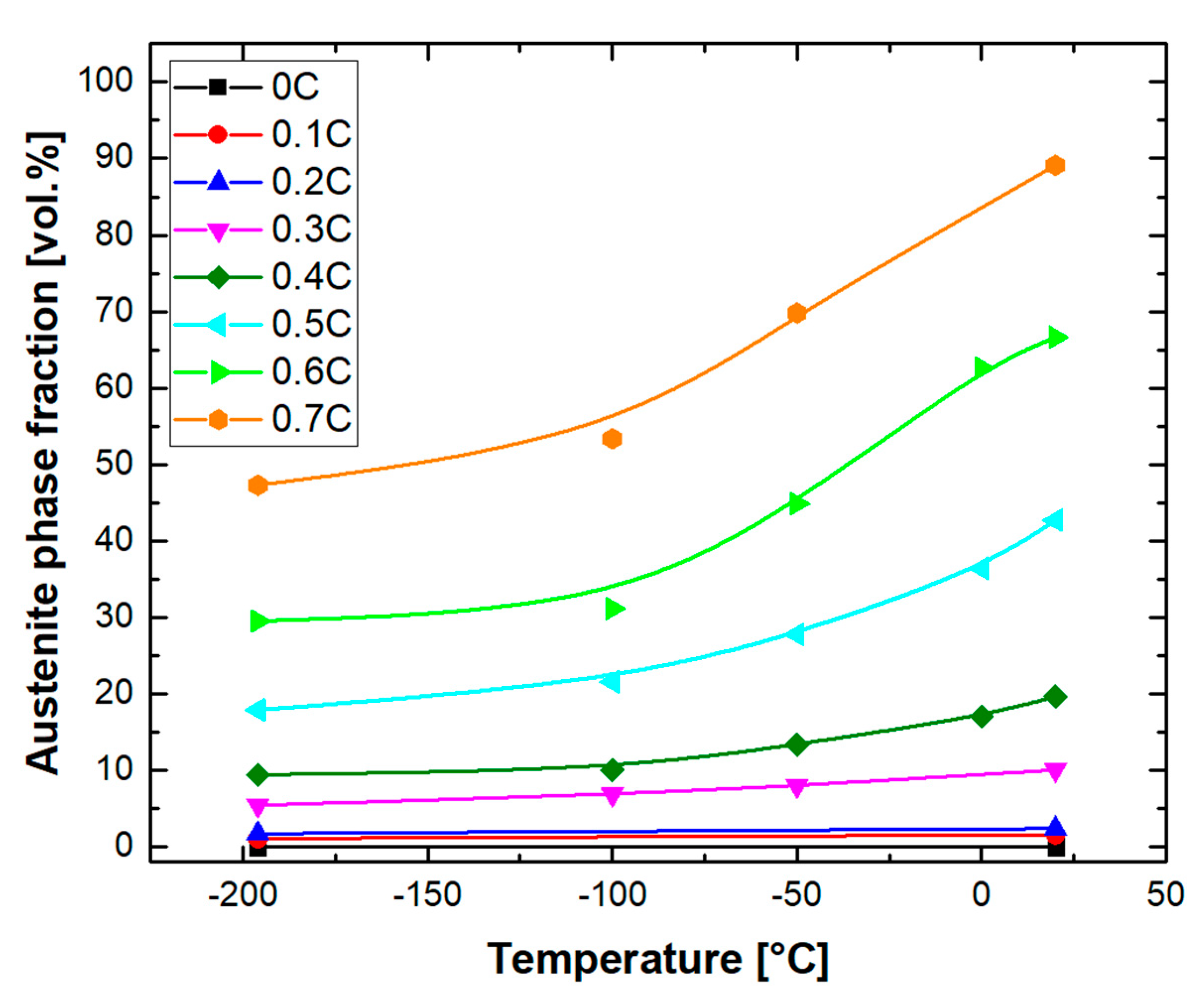
- Quenching to RT after heat treatment of alloys in the austenite range resulted in the partial transformation of austenite to athermal martensite. The fraction of retained austenite increased with the carbon concentration. This was due to the reduction in the martensite start temperature, by about 629 °C per wt.%C. The phase fraction of retained austenite was reduced by cooling to −196 °C.
- According to the XRD results, the tetragonality of the martensite unit cell becomes detectable at a carbon concentration of 0.2%, and continuously increases at higher carbon concentrations. The variations in lattice parameters with carbon concentration for martensite ( and ) and austenite () were expressed by linear equations in the following form: , and , with denoting carbon concentration in wt.% and lattice parameters expressed in angstrom. The equations are valid for 13%Cr steels with carbon contents between 0.313 and 0.706 wt.%.
- The variation in hardness with carbon concentration for steels with martensitic–austenitic microstructures indicated a maximum at intermediate carbon concentrations. Given the steady increase in the tetragonality of the martensitic constituent at higher carbon concentrations, as confirmed by the X-ray diffraction measurements, the observed variation in hardness with carbon concentration was justified by the amount and stability of the coexisting austenite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durand-Charre, M. Microstructure of Steels and Cast Irons; Springer: Berlin, Germany, 2004; ISBN 978-3-540-20963-8. [Google Scholar]
- Davis, J.R. Stainless Steels, 2nd ed.; ASM International: Materials Park, OH, USA, 1996; ISBN 978-0-87170-503-7. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2006; ISBN 978-0-7506-5924-6. [Google Scholar]
- Hougardy, H.P.; Yamazaki, K. An improved calculation of the transformation of steels. Steel Res. 1986, 57, 466–471. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Tobata, J.; Tao, T.; Nakada, N.; Takaki, S. Quenching and partitioning treatment of a low-carbon martensitic stainless steel. Mater. Sci. Eng. A 2012, 532, 585–592. [Google Scholar] [CrossRef]
- Harwarth, M.; Chen, G.; Rahimi, R.; Biermann, H.; Zargaran, A.; Duffy, M.; Zupan, M.; Mola, J. Aluminum-alloyed lightweight stainless steels strengthened by B2-(Ni,Fe)Al precipitates. Mater. Des. 2021, 206, 109813. [Google Scholar] [CrossRef]
- Akhiate, A.; Braud, E.; Thibault, D.; Brochu, M. Carbon content and heat treatment effects on microstructures and mechanical properties of 13%Cr-4%Ni martensitic stainless steel. In Proceedings of the COM 2014, 53rd Annual Conference of Metallurgists, Vancouver, BC, Canada, 28 September–1 October 2014; Davis, B., Ed.; Canadian Institute of Mining Metallurgy and Petroleum: Westmount, QC, Canada, 2014. ISBN 9781926872247. [Google Scholar]
- Shen, Y.F.; Dong, X.X.; Song, X.T.; Jia, N. Carbon content-tuned martensite transformation in low-alloy TRIP steels. Sci. Rep. 2019, 9, 7559. [Google Scholar] [CrossRef]
- Suutala, N. Effect of solidification conditions on the solidification mode in austenitic stainless steels. Metall. Trans. A 1983, 14, 191–197. [Google Scholar] [CrossRef]
- Akhavan Tabatabae, B.; Ashrafizadeh, F.; Hassanli, A.M. Influence of Retained Austenite on the Mechanical Properties of Low Carbon Martensitic Stainless Steel Castings. ISIJ Int. 2011, 51, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Amuda, M.; Akinlabi, E.T.; Mridha, S. Ferritic stainless steels: Metallurgy, application and weldability. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar]
- Liu, J.H.; Binot, N.; Delagnes, D.; Jahazi, M. Influence of the cooling rate below Ms on the martensitic transformation in a low alloy medium-carbon steel. J. Mater. Res. Technol. 2021, 12, 234–242. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; Estrin, Y.; De Cooman, B.C. Microstructure-mechanical properties relationships for quenching and partitioning (Q&P) processed steel. Acta Mater. 2016, 113, 124–139. [Google Scholar] [CrossRef]
- Pardal, J.M.; Tavares, S.S.M.; Tavares, M.T.; Garcia, P.S.P.; Velasco, J.A.C.; Abreu, H.F.G.; Pardal, J.P. Influence of carbon content on the martensitic transformation of titanium stabilized austenitic stainless steels. Int. J. Adv. Manuf. Technol. 2020, 108, 345–356. [Google Scholar] [CrossRef]
- Wendler, M.; Weiß, A.; Krüger, L.; Mola, J.; Franke, A.; Kovalev, A.; Wolf, S. Effect of Manganese on Microstructure and Mechanical Properties of Cast High Alloyed CrMnNi-N Steels. Adv. Eng. Mater. 2013, 15, 558–565. [Google Scholar] [CrossRef]
- Berns, H.; Gavriljuk, V.; Shanina, B. Intensive Interstitial Strengthening of Stainless Steels. Adv. Eng. Mater. 2008, 10, 1083–1093. [Google Scholar] [CrossRef]
- Berns, H.; Nabiran, N.; Mujica, L. High-Interstitial Stainless Austenitic Steel Castings. Steel Res. Int. 2013, 84, 119–128. [Google Scholar] [CrossRef]
- Lee, T.-H.; Ha, H.-Y.; Hwang, B.; Kim, S.-J.; Shin, E. Effect of Carbon Fraction on Stacking Fault Energy of Austenitic Stainless Steels. Metall. Mater. Trans. A 2012, 43, 4455–4459. [Google Scholar] [CrossRef]
- Brofman, P.J.; Ansell, G.S. On the Effect of Carbon on the Stacking Fault Energy of Austenitic Stainless Steels. Metall. Trans. A 1978, 9, 879–880. [Google Scholar] [CrossRef]
- Mola, J.; Luan, G.; Huang, Q.; Ullrich, C.; Volkova, O.; Estrin, Y. Dynamic strain aging mechanisms in a metastable austenitic stainless steel. Acta Mater. 2021, 212, 116888. [Google Scholar] [CrossRef]
- Chen, G.; Rahimi, R.; Xu, G.; Biermann, H.; Mola, J. Impact of Al addition on deformation behavior of Fe–Cr–Ni–Mn–C austenitic stainless steel. Mater. Sci. Eng. A 2020, 797, 140084. [Google Scholar] [CrossRef]
- Ogawa, T.; Koyama, M.; Tasan, C.C.; Tsuzaki, K.; Noguchi, H. Effects of martensitic transformability and dynamic strain age hardenability on plasticity in metastable austenitic steels containing carbon. J. Mater. Sci. 2017, 52, 7868–7882. [Google Scholar] [CrossRef]
- Talonen, J.; Nenonen, P.; Hänninen, H. Static strain ageing of cold-worked austenitic stainless steel. In Proceedings of the 7th International Conference on High Nitrogen Steels 2004, HNS 2004, Ostend, Belgium, 19–22 September 2004; Akdut, N., Cooman, B.C., de Foct, J., Eds.; GRIPS Media: Bad Harzburg, Germany, 2004. ISBN 3937057099. [Google Scholar]
- Khvan, A.V.; Hallstedt, B.; Broeckmann, C. A thermodynamic evaluation of the Fe–Cr–C system. Calphad 2014, 46, 24–33. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Liu, J.; Song, Y.; Li, S.; Zhang, R.; Li, T.; Liang, Y. The influence of chromium on the pearlite-austenite transformation kinetics of the Fe–Cr–C ternary steels. J. Alloy. Compd. 2015, 648, 494–499. [Google Scholar] [CrossRef]
- Wieczerzak, K.; Bala, P.; Dziurka, R.; Tokarski, T.; Cios, G.; Koziel, T.; Gondek, L. The effect of temperature on the evolution of eutectic carbides and M 7 C 3 → M 23 C 6 carbides reaction in the rapidly solidified Fe-Cr-C alloy. J. Alloy. Compd. 2017, 698, 673–684. [Google Scholar] [CrossRef]
- Liu, W.; Chen, C.; Tang, Y.; Long, Q.; Wei, S.; Zhang, G.; Mao, F.; Jiang, Q.; Zhang, T.; Liu, M. Thermodynamic evaluation and investigation of solidification microstructure in the Fe–Cr–Ni–C system. Calphad 2020, 69, 101763. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Fan, Y.; Zhang, T.; Dong, B.; Chen, L.; Wang, Y. Influence of microstructure on the corrosion behavior of super 13Cr martensitic stainless steel under heat treatment. Mater. Charact. 2021, 175, 111066. [Google Scholar] [CrossRef]
- Shahriari, A.; Ghaffari, M.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Amirkhiz, B.S.; Mohammadi, M. Corrosion resistance of 13wt.% Cr martensitic stainless steels: Additively manufactured CX versus wrought Ni-containing AISI 420. Corros. Sci. 2021, 184, 109362. [Google Scholar] [CrossRef]
- Schneider, R.; Perko, J.; Reithofer, G. Heat Treatment of Corrosion Resistant Tool Steels for Plastic Moulding. Mater. Manuf. Process. 2009, 24, 903–908. [Google Scholar] [CrossRef]
- Terry, J.C. Materials and design in Gillette razors. Mater. Des. 1991, 12, 277–281. [Google Scholar] [CrossRef]
- Li, J.; He, T.; Zhang, P.; Cheng, L.; Wang, L. Effect of large-size carbides on the anisotropy of mechanical properties in 11Cr-3Co-3W martensitic heat-resistant steel for turbine high temperature blades in ultra-supercritical power plants. Mater. Charact. 2020, 159, 110025. [Google Scholar] [CrossRef]
- Sá de Sousa, J.M.; Lobato, M.Q.; Garcia, D.N.; Machado, P.C. Abrasion resistance of Fe–Cr–C coating deposited by FCAW welding process. Wear 2021, 476, 203688. [Google Scholar] [CrossRef]
- Barlow, L.D.; Du Toit, M. Effect of Austenitizing Heat Treatment on the Microstructure and Hardness of Martensitic Stainless Steel AISI 420. J. Mater. Eng. Perform. 2012, 21, 1327–1336. [Google Scholar] [CrossRef]
- Lei, X.; Feng, Y.; Zhang, J.; Fu, A.; Yin, C.; Macdonald, D.D. Impact of Reversed Austenite on the Pitting Corrosion Behavior of Super 13Cr Martensitic Stainless Steel. Electrochim. Acta 2016, 191, 640–650. [Google Scholar] [CrossRef]
- Wendler, M.; Hauser, M.; Fabrichnaya, O.; Krüger, L.; Weiß, A.; Mola, J. Thermal and deformation-induced phase transformation behavior of Fe–15Cr–3Mn–3Ni–0.1N–(0.05–0.25)C austenitic and austenitic–martensitic cast stainless steels. Mater. Sci. Eng. A 2015, 645, 28–39. [Google Scholar] [CrossRef]
- Yang, H.-S.; Bhadeshia, H.K.D.H. Uncertainties in dilatometric determination of martensite start temperature. Mater. Sci. Technol. 2007, 23, 556–560. [Google Scholar] [CrossRef]
- García de Andrés, C. Application of dilatometric analysis to the study of solid–solid phase transformations in steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Bonyár, A.; Renkó, J.; Kovács, D.; Szabó, P.J. Understanding the mechanism of Beraha-I type color etching: Determination of the orientation dependent etch rate, layer refractive index and a method for quantifying the angle between surface normal and the 〈100〉, 〈111〉 directions for individual grains. Mater. Charact. 2019, 156, 109844. [Google Scholar] [CrossRef]
- Metallische Werkstoffe—Härteprüfung nach Vickers—Teil 1: Prüfverfahren; DIN EN ISO 6507-1:2018-07; Deutsche Fassung EN ISO 6507-1:2018; Beuth Verlag GmbH: Berlin, Germany, 2018.
- Huang, Q.; Yao, M.; Timokhina, I.; Schimpf, C.; Biermann, H.; Volkova, O.; de Cooman, B.C.; Mola, J. Tempering Reactions and Elemental Redistribution During Tempering of Martensitic Stainless Steels. Metall. Mater. Trans. A 2019, 50, 3663–3673. [Google Scholar] [CrossRef]
- Huang, Q.; Volkova, O.; Biermann, H.; Mola, J. Dilatometry Analysis of Dissolution of Cr-Rich Carbides in Martensitic Stainless Steels. Metall. Mater. Trans. A 2017, 48, 5771–5777. [Google Scholar] [CrossRef]
- Suutala, N.; Takalo, T.; Moisio, T. Single-phase ferritic solidification mode in austenitic-ferritic stainless steel welds. Metall. Trans. A 1979, 10, 1183–1190. [Google Scholar] [CrossRef]
- Suutala, N.; Takalo, T.; Moisio, T. Ferritic-austenitic solidification mode in austenitic stainless steel welds. Metall. Trans. A 1980, 11, 717–725. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Burlington, MA, USA, 2017; ISBN 9780081002704. [Google Scholar]
- Presoly, P.; Pierer, R.; Bernhard, C. Linking up of HT-LSCM and DSC measurements to characterize phase diagrams of steels. IOP Conf. Ser. Mater. Sci. Eng. 2012, 33, 12064. [Google Scholar] [CrossRef]
- Chapman, L.; Day, A.; Quested, P.N. Reference Materials for Calibration of Temperatures in DTA/DSC Instruments; NPL Report CMMT(A)157; National Physical Laboratory: London, UK, 1999. [Google Scholar]
- Gulliver, G. The quantitative effect of rapid cooling upon the constitution of binary alloy. J. Inst. Met. 1913, 9, 120–157. [Google Scholar]
- Scheil, E. Bemerkungen zur Schichtkristallbildung. Z. Metallkd. 1942, 34, 70–72. [Google Scholar]
- Kozeschnik, E. A scheil-gulliver model with back-diffusion applied to the microsegregation of chromium in Fe-Cr-C alloys. Metall. Mater. Trans. A 2000, 31, 1682–1684. [Google Scholar] [CrossRef]
- Kato, T.; Jones, H.; Kirkwood, D.H. Segregation and eutectic formation in solidification of Fe-1C-1 5-Cr steel. Mater. Sci. Technol. 2003, 19, 1070–1076. [Google Scholar] [CrossRef]
- Masoumi, F.; Jahazi, M.; Shahriari, D.; Cormier, J. Coarsening and dissolution of γ′ precipitates during solution treatment of AD730™ Ni-based superalloy: Mechanisms and kinetics models. J. Alloy. Compd. 2016, 658, 981–995. [Google Scholar] [CrossRef]
- Masoumi, F.; Jahazi, M.; Cormier, J.; Shahriari, D. Dissolution kinetics and morphological changes of γ’ in AD730 TM superalloy. MATEC Web Conf. 2014, 14, 13005. [Google Scholar] [CrossRef] [Green Version]
- Masoumi, F.; Shahriari, D.; Jahazi, M.; Cormier, J.; Devaux, A. Kinetics and Mechanisms of γ’ Reprecipitation in a Ni-based Superalloy. Sci. Rep. 2016, 6, 28650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Ponge, D.; Wittig, J.; Choi, P.; Jiménez, J.A.; Raabe, D. Nanoscale austenite reversion through partitioning, segregation and kinetic freezing: Example of a ductile 2GPa Fe–Cr–C steel. Acta Mater. 2012, 60, 2790–2804. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Volkova, O.; Biermann, H.; Mola, J. Thermal Analysis of the Formation and Dissolution of Cr-Rich Carbides in Al-Alloyed Stainless Steels. Adv. Eng. Mater. 2019, 21, 1800658. [Google Scholar] [CrossRef]
- Mola, J.; Luan, G.; Huang, Q.; Schimpf, C.; Rafaja, D. Cementite evolution in medium manganese twinning-induced plasticity steels. Materialia 2018, 2, 138–147. [Google Scholar] [CrossRef]
- García de Andrés, C.; Caruana, G.; Alvarez, L. Control of M23C6 carbides in 0.45C–13Cr martensitic stainless steel by means of three representative heat treatment parameters. Mater. Sci. Eng. A 1998, 241, 211–215. [Google Scholar] [CrossRef]
- Garcia, C.; Alvarez, L.F.; Carsi, M. Effects of heat-treatment parameters on non-equilibrium transformations and properties of X45Cr13 and X60Cr14MoV martensitic stainless steels. Weld. Int. 1992, 6, 612–621. [Google Scholar] [CrossRef]
- Alvarez, L.F.; Garcia, C. Etude des transformations au chauffage continu des aciers inoxydables martensitiques types Cr13 et CrMoV14. Rev. Met. Paris 1993, 90, 245–254. [Google Scholar] [CrossRef]
- Mola, J.; Ullrich, C.; Kuang, B.; Rahimi, R.; Huang, Q.; Rafaja, D.; Ritzenhoff, R. Austenitic Nickel- and Manganese-Free Fe-15Cr-1Mo-0.4N-0.3C Steel: Tensile Behavior and Deformation-Induced Processes between 298 K and 503 K (25 °C and 230 °C). Metall. Mater. Trans. A 2017, 48, 1033–1052. [Google Scholar] [CrossRef]
- Huang, Q.; Schröder, C.; Biermann, H.; Volkova, O.; Mola, J. Influence of Martensite Fraction on Tensile Properties of Quenched and Partitioned (Q&P) Martensitic Stainless Steels. Steel Res. Int. 2016, 87, 1082–1094. [Google Scholar] [CrossRef] [Green Version]
- Sourmail, T.; Garcia-Mateo, C. Critical assessment of models for predicting the Ms temperature of steels. Comput. Mater. Sci. 2005, 34, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 60, 721–727. [Google Scholar]
- Han, Y.; Li, C.; Ren, J.; Qiu, C.; Zhang, Y.; Wang, J. Dendrite Segregation Changes in High Temperature Homogenization Process of As-cast H13 Steel. ISIJ Int. 2019, 59, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Suutala, N. Effect of manganese and nitrogen on the solidification mode in austenitic stainless steel welds. METALL. TRANS. A 1982, 13, 2121–2130. [Google Scholar] [CrossRef]
- Hauser, M.; Wendler, M.; Ghosh Chowdhury, S.; Weiß, A.; Mola, J. Quantification of deformation induced α’-martensite in Fe–19Cr–3Mn–4Ni–0.15C–0.15N austenitic steel by in situ magnetic measurements. Mater. Sci. Technol. 2015, 31, 1473–1478. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction, 2nd ed.; Addison-Wesley: Reading, MA, USA, 1978; ISBN 0-201-01174-3. [Google Scholar]
- Dickson, M.J. The significance of texture parameters in phase analysis by X-ray diffraction. J. Appl. Crystallogr. 1969, 2, 176–180. [Google Scholar] [CrossRef]
- Villa, M.; Hansen, M.F.; Somers, M. Martensite formation in Fe-C alloys at cryogenic temperatures. Scr. Mater. 2017, 141, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Euser, V.K.; Williamson, D.L.; Clarke, A.J.; Speer, J.G. Limiting Retained Austenite Decomposition in Quenched and Tempered Steels: Influences of Rapid Tempering and Silicon. ISIJ Int. 2020, 60, 2990–3000. [Google Scholar] [CrossRef]
- Hauser, M.; Wendler, M.; Fabrichnaya, O.; Volkova, O.; Mola, J. Anomalous stabilization of austenitic stainless steels at cryogenic temperatures. Mater. Sci. Eng. A 2016, 675, 415–420. [Google Scholar] [CrossRef]
- Hauser, M.; Wendler, M.; Weiß, A.; Volkova, O.; Mola, J. On the Critical Driving Force for Deformation-Induced α′-Martensite Formation in Austenitic Cr–Mn–Ni Steels. Adv. Eng. Mater. 2019, 21, 1800676. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Huang, Q.; de Cooman, B.C.; Biermann, H.; Mola, J. Influence of Martensite Fraction on the Stabilization of Austenite in Austenitic–Martensitic Stainless Steels. Metall. Mater. Trans. A 2016, 47, 1947–1959. [Google Scholar] [CrossRef]
- Balbi, M.; Alvarez-Armas, I.; Armas, A. Effect of holding time at an intercritical temperature on the microstructure and tensile properties of a ferrite-martensite dual phase steel. Mater. Sci. Eng. A 2018, 733, 1–8. [Google Scholar] [CrossRef]
- Speich, G.R.; Leslie, W.C. Tempering of steel. Metall. Trans. A 1972, 3, 1043–1054. [Google Scholar] [CrossRef]
- Chen, P.C.; Winchell, P.G. Martensite lattice changes during tempering. Metall. Trans. A 1980, 11, 1333–1339. [Google Scholar] [CrossRef]
- Taylor, K.A.; Cohen, M. Aging of ferrous martensites. Prog. Mater. Sci. 1992, 36, 151–272. [Google Scholar] [CrossRef]
- Roberts, C.S. Effect of carbon on the volume fractions and lattice parameters of retained austenite and martensite. J. Met. 1953, 5, 203–204. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.; Sisson, R.D. The effect of carbon content on the c/a ratio of as-quenched martensite in Fe-C alloys. Mater. Sci. Eng. A 2017, 700, 592–597. [Google Scholar] [CrossRef]
- Paterson, M.S. X-Ray Diffraction by Face-Centered Cubic Crystals with Deformation Faults. J. Appl. Phys. 1952, 23, 805–811. [Google Scholar] [CrossRef]
- Lobodyuk, V.A.; Meshkov, Y.Y.; Pereloma, E.V. On Tetragonality of the Martensite Crystal Lattice in Steels. Metall. Mater. Trans. A 2019, 50, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Krauss, G. Martensite in steel: Strength and structure. Mater. Sci. Eng. A 1999, 273–275, 40–57. [Google Scholar] [CrossRef]
- Mola, J.; de Cooman, B.C. Quenching and Partitioning (Q&P) Processing of Martensitic Stainless Steels. Metall. Mater. Trans. A 2013, 44, 946–967. [Google Scholar] [CrossRef]
- Saeed-Akbari, A.; Mosecker, L.; Schwedt, A.; Bleck, W. Characterization and Prediction of Flow Behavior in High-Manganese Twinning Induced Plasticity Steels: Part I. Mechanism Maps and Work-Hardening Behavior. Metall. Mater. Trans. A 2012, 43, 1688–1704. [Google Scholar] [CrossRef]
- Jacques, P.J.; Delannay, F.; Ladrière, J. On the influence of interactions between phases on the mechanical stability of retained austenite in transformation-induced plasticity multiphase steels. Metall. Mater. Trans. A 2001, 32, 2759–2768. [Google Scholar] [CrossRef]
- Mola, J. Considerations in the design of formable austenitic stainless steels based on deformation-induced processes: 2. In Austenitic Stainless Steels; Borek, W., Tanski, T., Brytan, Z., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Seo, E.J.; Cho, L.; Kim, J.K.; Mola, J.; Zhao, L.; de Cooman, B.C. Constituent-specific properties in quenching and partitioning (Q&P) processed steel. Mater. Sci. Eng. A 2019, 740–741, 439–444. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, R.; Muránsky, O.; Beladi, H.; Kabra, S.; Schimpf, C.; Volkova, O.; Biermann, H.; Mola, J. Neutron diffraction analysis of stress and strain partitioning in a two-phase microstructure with parallel-aligned phases. Sci. Rep. 2020, 10, 13536. [Google Scholar] [CrossRef]
- Kim, H.S. On the rule of mixtures for the hardness of particle reinforced composites. Mater. Sci. Eng. A 2000, 289, 30–33. [Google Scholar] [CrossRef]

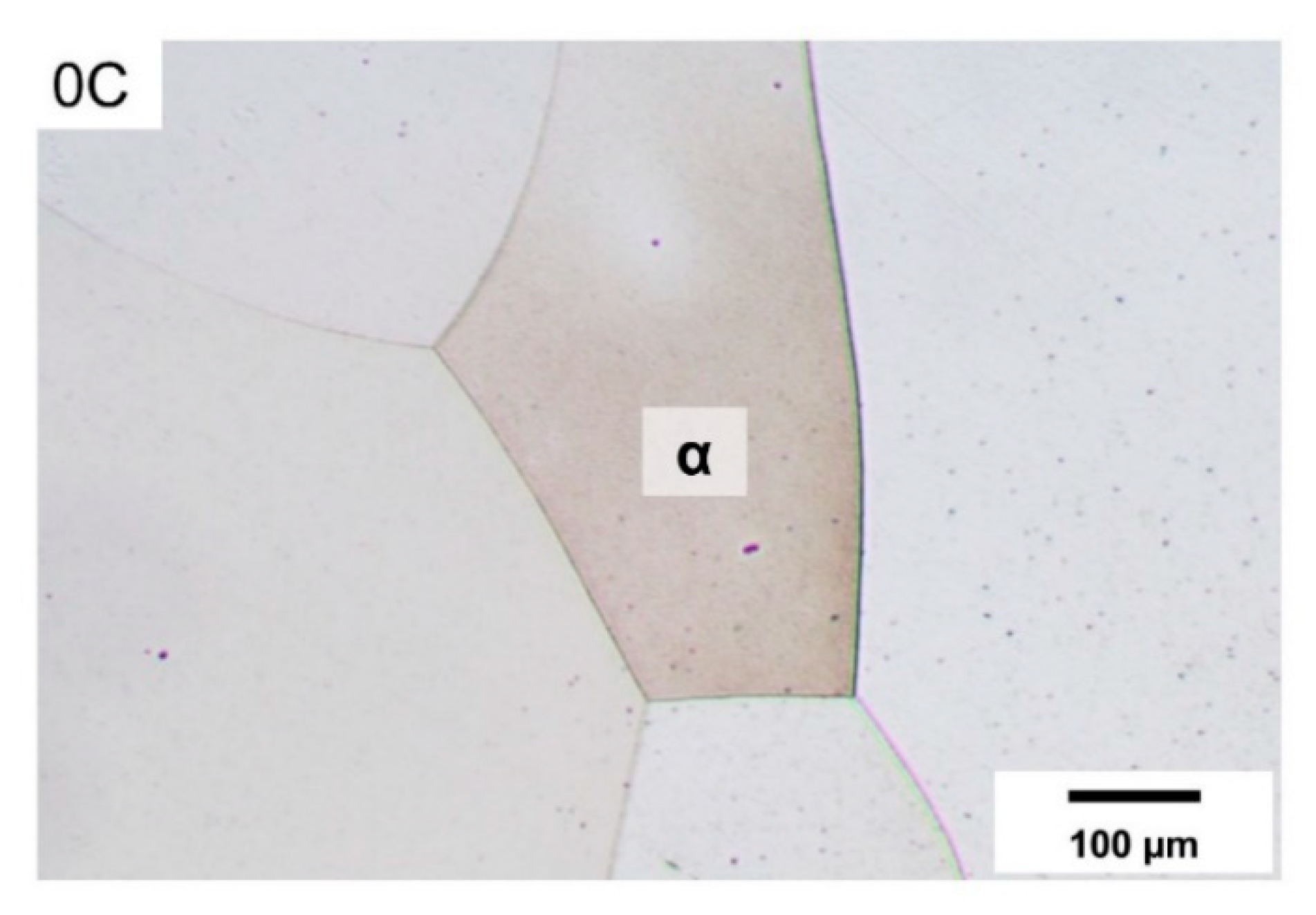
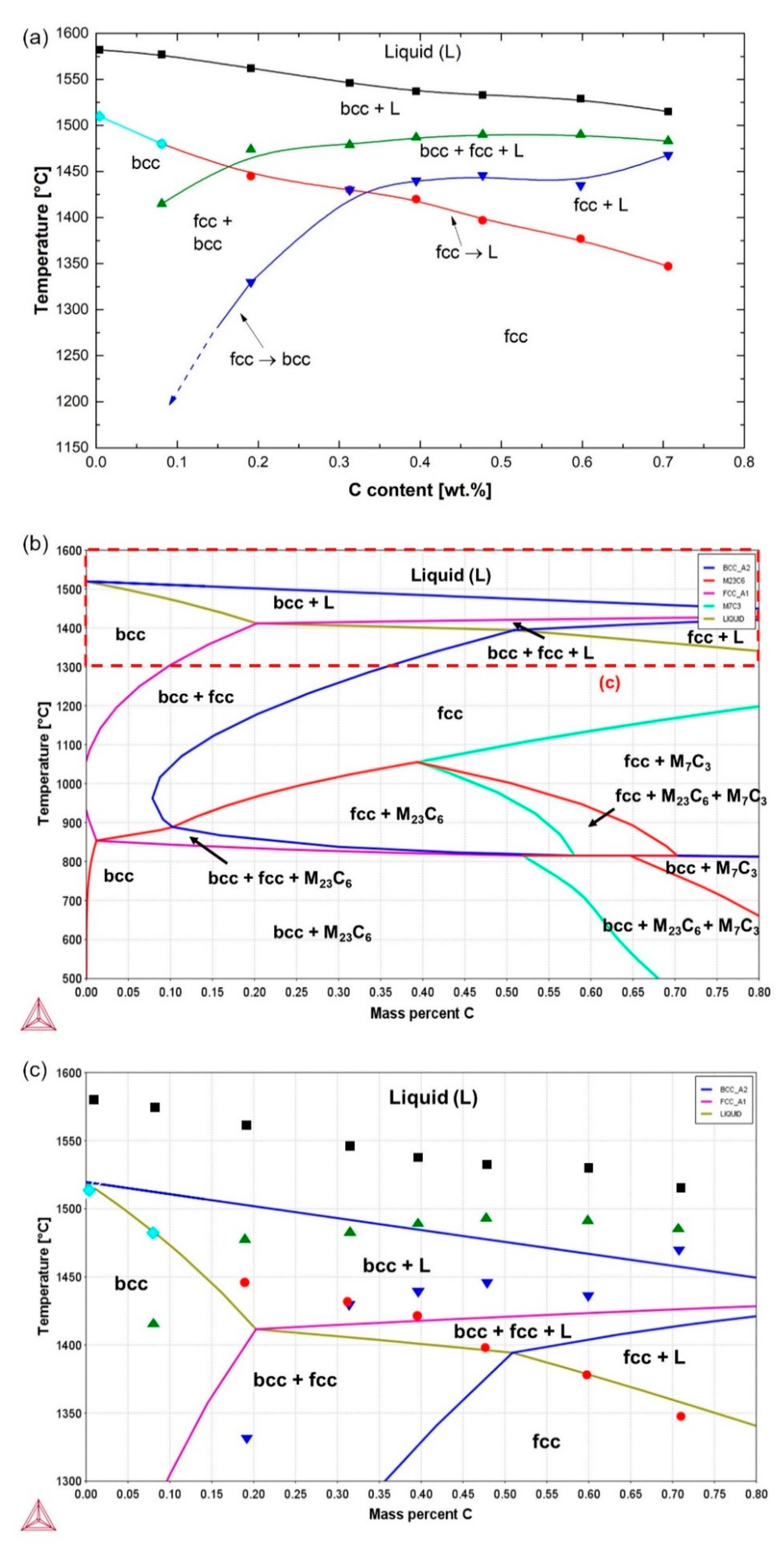
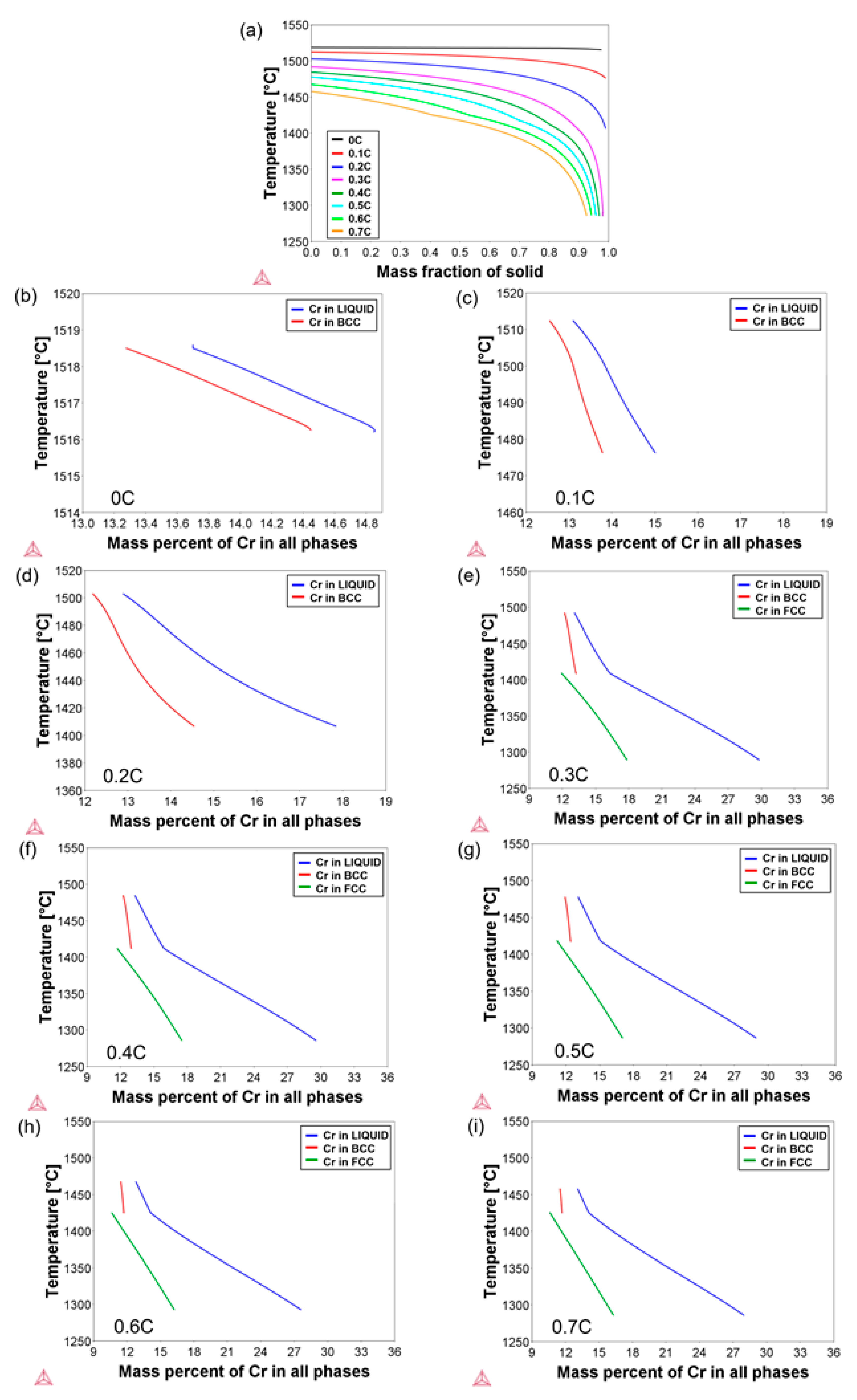
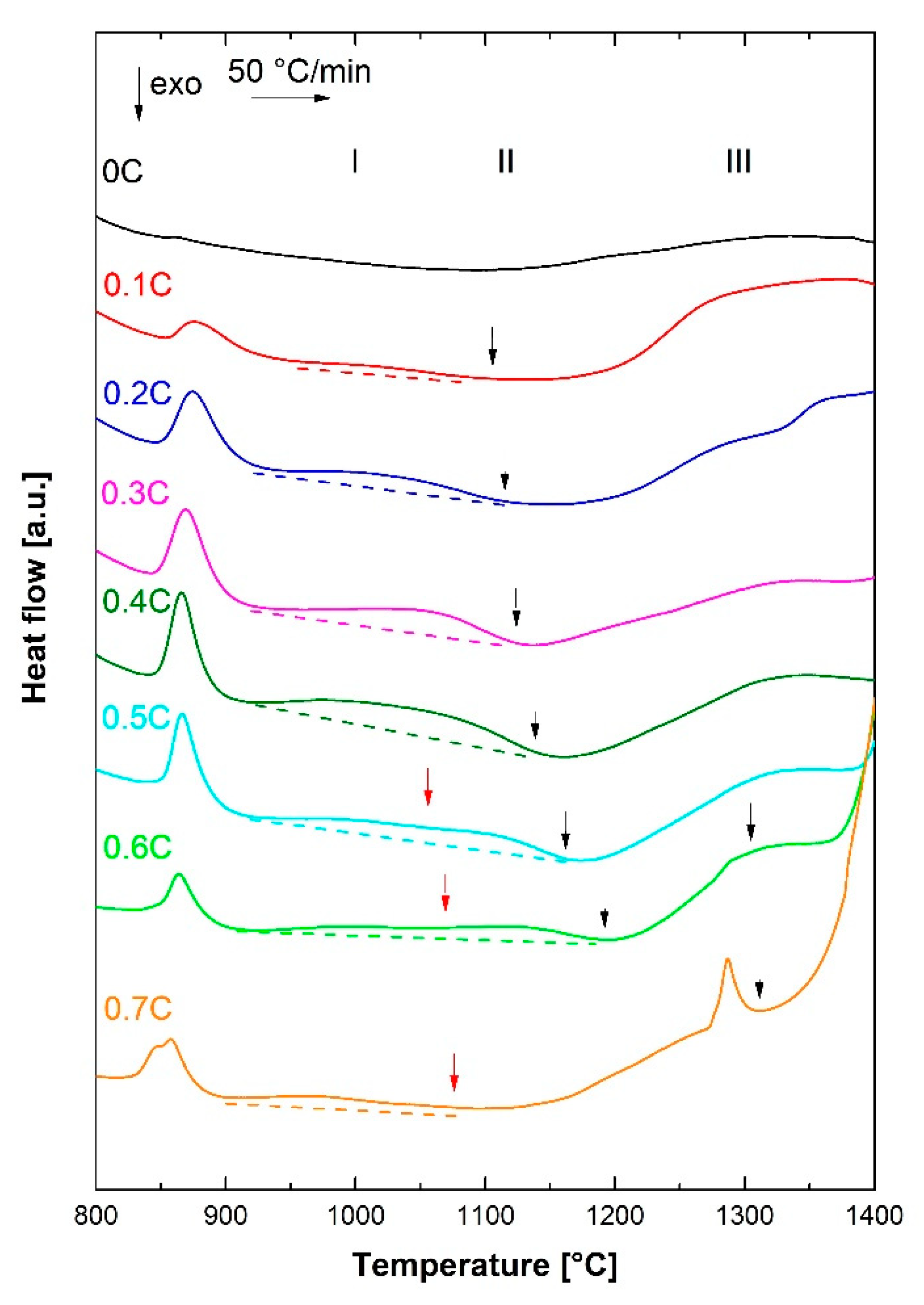

| Alloy ID | C | Cr | Fe + Trace Elements |
|---|---|---|---|
| 0C | 0.004 | 13.7 | balance |
| 0.1C | 0.081 | 13.1 | balance |
| 0.2C | 0.191 | 12.9 | balance |
| 0.3C | 0.313 | 13.1 | balance |
| 0.4C | 0.395 | 13.3 | balance |
| 0.5C | 0.477 | 13.1 | balance |
| 0.6C | 0.598 | 12.8 | balance |
| 0.7C | 0.706 | 13.1 | balance |
| Alloy ID | Austenitization Temperature (°C) | Holding Time (min) |
|---|---|---|
| 0.1C | 1100 | 10 |
| 0.2C | 1100 | 10 |
| 0.3C | 1150 | 10 |
| 0.4C | 1150 | 10 |
| 0.5C | 1200 | 10 |
| 0.6C | 1300 | 5 |
| 0.7C | 1300 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harwarth, M.; Brauer, A.; Huang, Q.; Pourabdoli, M.; Mola, J. Influence of Carbon on the Microstructure Evolution and Hardness of Fe–13Cr–xC (x = 0–0.7 wt.%) Stainless Steel. Materials 2021, 14, 5063. https://doi.org/10.3390/ma14175063
Harwarth M, Brauer A, Huang Q, Pourabdoli M, Mola J. Influence of Carbon on the Microstructure Evolution and Hardness of Fe–13Cr–xC (x = 0–0.7 wt.%) Stainless Steel. Materials. 2021; 14(17):5063. https://doi.org/10.3390/ma14175063
Chicago/Turabian StyleHarwarth, Michael, Adam Brauer, Qiuliang Huang, Mehdi Pourabdoli, and Javad Mola. 2021. "Influence of Carbon on the Microstructure Evolution and Hardness of Fe–13Cr–xC (x = 0–0.7 wt.%) Stainless Steel" Materials 14, no. 17: 5063. https://doi.org/10.3390/ma14175063
APA StyleHarwarth, M., Brauer, A., Huang, Q., Pourabdoli, M., & Mola, J. (2021). Influence of Carbon on the Microstructure Evolution and Hardness of Fe–13Cr–xC (x = 0–0.7 wt.%) Stainless Steel. Materials, 14(17), 5063. https://doi.org/10.3390/ma14175063








