Influence of Spin Coating Parameters on Gas Transport Properties of Thin-Film Composite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane Fabrication
2.3. Gas Permeance Measurement
2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Elemental Spectroscopy (EDX)
3. Results
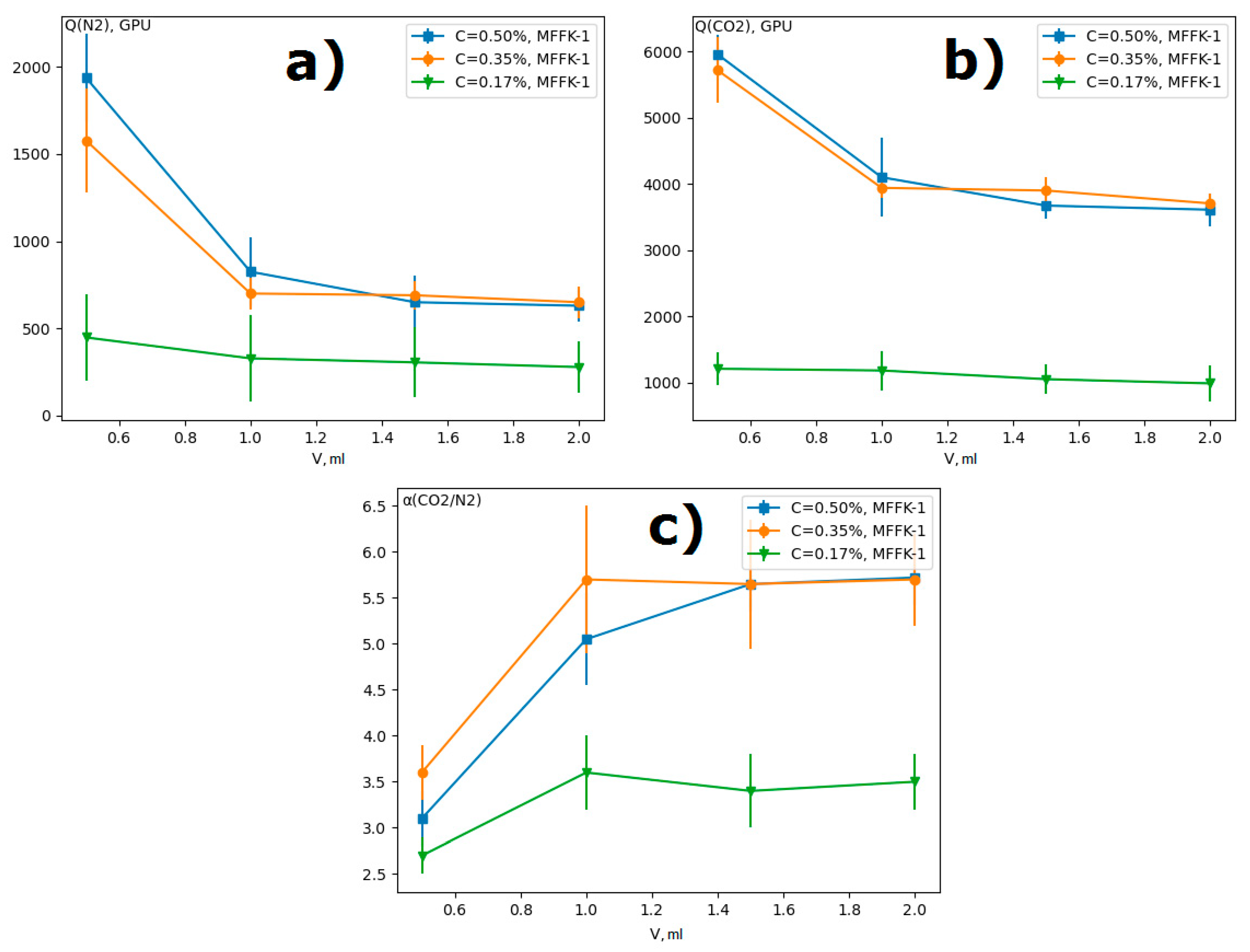
3.1. Volume of the Film-Forming Solution
3.2. Concentration of the Film-Forming Solution
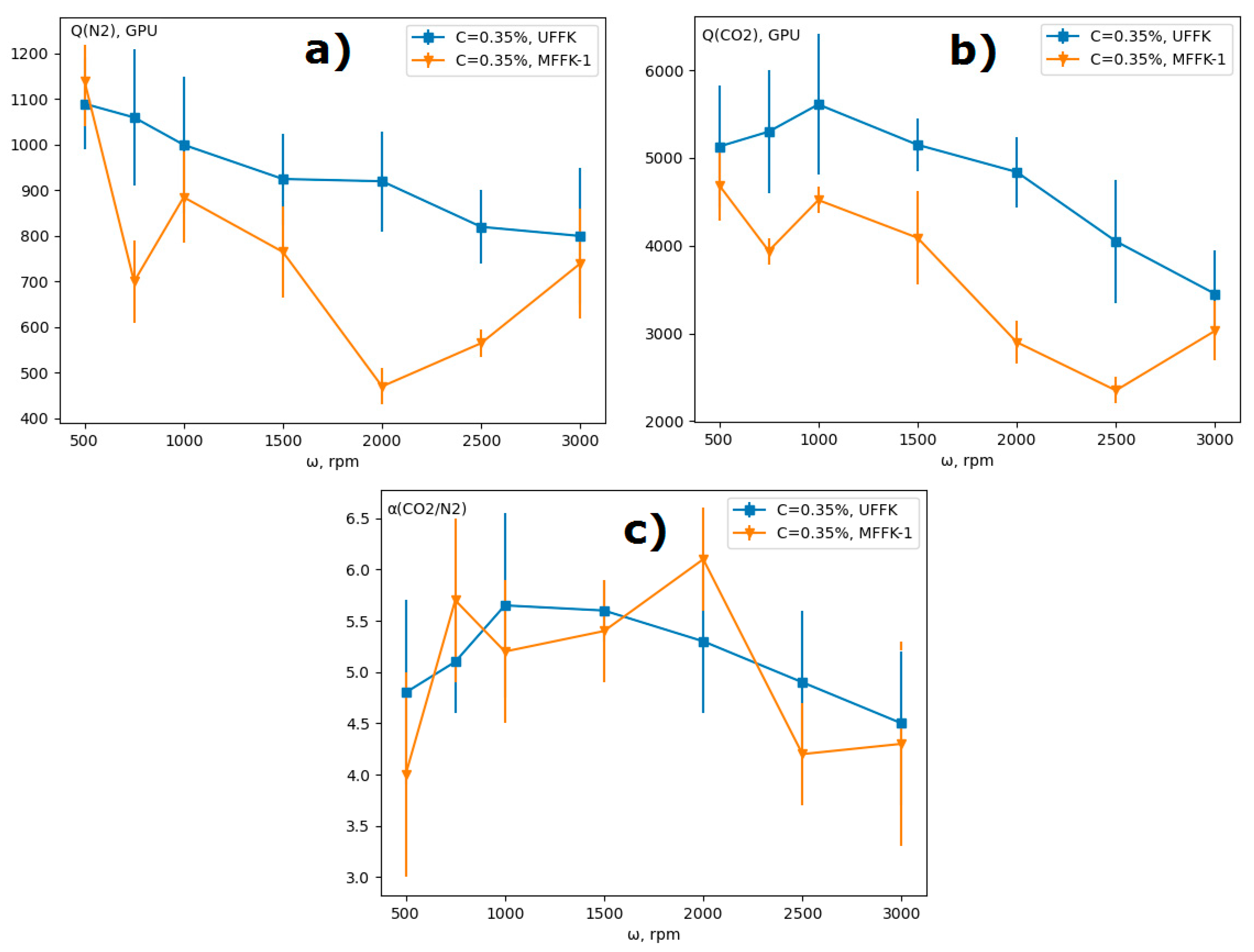
3.3. Rotation Speed
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, J.; Peinemann, K.-V. Multilayer composite membranes for gas separation based on crosslinked PTMSP gutter layer and partially crosslinked Matrimid® 5218 selective layer. J. Membr. Sci. 2009, 340, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Borisov, I.; Bakhtin, D.; Luque-Alled, J.M.; Rybakova, A.; Makarova, V.; Foster, A.B.; Harrison, W.J.; Volkov, V.; Polevaya, V.; Gorgojo, P.; et al. Synergistic enhancement of gas selectivity in thin film composite membranes of PIM-1. J. Mater. Chem. A 2019, 7, 6417–6430. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Pan, Y.; Peinemann, K.-V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Membr. Sci. 2013, 425, 235–242. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Membr. Sci. 2016, 524, 266–279. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, K.H.; Lee, J.H.; Kim, T.W.; Chung, C.W.; Cho, Y.H.; Park, H.B. Ultrathin gutter layer for high-performance thin-film composite membranes for CO2 separation. J. Membr. Sci. 2018, 566, 336–345. [Google Scholar] [CrossRef]
- Cook, M.; Gaffney, P.R.; Peeva, L.G.; Livingston, A.G. Roll-to-roll dip coating of three different PIMs for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 558, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Grushevenko, E.; Borisov, I.; Knyazeva, A.; Volkov, V. Polyalkylmethylsiloxanes composite membranes for hydrocarbon/methane separation: Eight component mixed-gas permeation properties. Sep. Purif. Technol. 2020, 241, 116696. [Google Scholar] [CrossRef]
- Wenten, I.; Khoiruddin, K.; Wardani, A.; Aryanti, P.; Astuti, D.; Komaladewi, A. Preparation of antifouling polypropylene/ZnO composite hollow fiber membrane by dip-coating method for peat water treatment. J. Water Process. Eng. 2020, 34, 101158. [Google Scholar] [CrossRef]
- Malakhov, A.; Bazhenov, S.; Vasilevsky, V.; Borisov, I.; Ovcharova, A.; Bildyukevich, A.; Volkov, V.; Giorno, L. Thin-film composite hollow fiber membranes for ethylene/ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2019, 219, 64–73. [Google Scholar] [CrossRef]
- Hamm, J.B.; Ambrosi, A.; Pollo, L.D.; Marcilio, N.R.; Tessaro, I.C. Thin polymer layer-covered porous alumina tubular membranes prepared via a dip-coating/phase-inversion process. Mater. Chem. Phys. 2021, 265, 124511. [Google Scholar] [CrossRef]
- Lu, M.; Hu, M.Z. Novel porous ceramic tube-supported polymer layer membranes for acetic acid/water separation by pervaporation dewatering. Sep. Purif. Technol. 2019, 236, 116312. [Google Scholar] [CrossRef]
- Bakhtin, D.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of thin-film composite membranes based on PTMSP loaded with porous aromatic frameworks. J. Membr. Sci. 2020, 10, 419. [Google Scholar] [CrossRef]
- He, G.; Huang, X.; Xu, R.; Zhu, B. An improved resistance model for gas permeation in composite membranes. J. Membr. Sci. 1996, 118, 1–7. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, W.; Kattula, M.; Ponnuru, K.; Furlani, E.P.; Lin, H. Effect of porous supports on the permeance of thin film composite membranes: Part I. Track-etched polycarbonate supports. J. Membr. Sci. 2016, 514, 684–695. [Google Scholar] [CrossRef]
- Ugrozov, V.V.; Bakhtin, D.S.; Balynin, A.V.; Polevaya, V.G.; Volkov, A.V. Effect of Support on Gas Transport Properties of PTMSP/UFFK and PTMSP/MFFK-1 Composite Membranes. Membr. Membr. Technol. 2019, 1, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Afshoun, H.R.; Chenar, M.P.; Ismail, A.F.; Matsuura, T. Effect of support layer on gas permeation properties of composite polymeric membranes. Korean J. Chem. Eng. 2017, 34, 3178–3184. [Google Scholar] [CrossRef]
- Le Roux, J.; Paul, D. Preparation of composite membranes by a spin coating process. J. Membr. Sci. 1992, 74, 233–252. [Google Scholar] [CrossRef]
- Burmann, P.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014, 107, 66–75. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J. Membr. Sci. 2018, 572, 38–60. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Olivieri, L.; Meneguzzo, S.; Ligi, S.; Saccani, A.; Giorgini, L.; Orsini, A.; Pettinau, A.; De Angelis, M.G. Reducing ageing of thin PTMSP films by incorporating graphene and graphene oxide: Effect of thickness, gas type and temperature. J. Membr. Sci. 2018, 555, 258–267. [Google Scholar] [CrossRef]
- Shu, L.; Xie, L.-H.; Meng, Y.; Liu, T.; Zhao, C.; Li, J.-R. A thin and high loading two-dimensional MOF nanosheet based mixed-matrix membrane for high permeance nanofiltration. J. Membr. Sci. 2020, 603, 118049. [Google Scholar] [CrossRef]
- Liu, G.; Cheng, L.; Chen, G.; Liang, F.; Liu, G.; Jin, W. Pebax-Based Membrane Filled with Two-Dimensional Mxene Nanosheets for Efficient CO2 Capture. Chem.–Asian J. 2019, 15, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Bakhtin, D.S.; Polevaya, V.G.; Balynin, A.V.; Maksimov, A.L.; Volkov, A.V. Friedel-Crafts Synthesis of New Porous Aromatic Frameworks for Stabilizing Gas Transport Properties of Highly Permeable Glassy Polymers. Russ. J. Appl. Chem. 2019, 92, 199–207. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Fan, J.; Chen, H.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
- Volkov, A.; Bakhtin, D.; Kulikov, L.; Terenina, M.; Golubev, G.; Bondarenko, G.; Legkov, S.; Shandryuk, G.; Volkov, V.; Khotimskiy, V.; et al. Stabilization of gas transport properties of PTMSP with porous aromatic framework: Effect of annealing. J. Membr. Sci. 2016, 517, 80–90. [Google Scholar] [CrossRef]
- Wei, W.; Xia, S.; Liu, G.; Gu, X.; Jin, W.; Xu, N. Interfacial adhesion between polymer separation layer and ceramic support for composite membrane. AIChE J. 2009, 56, 1584–1592. [Google Scholar] [CrossRef]
- Naik, P.V.; Bernstein, R.; Vankelecom, I.F.J. Influence of support layer and PDMS coating conditions on composite membrane performance for ethanol/water separation by pervaporation. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a Viscous Liquid on a Rotating Disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Lee, U.G.; Kim, W.-B.; Han, D.H.; Chung, H.S. A Modified Equation for Thickness of the Film Fabricated by Spin Coating. Symmetry 2019, 11, 1183. [Google Scholar] [CrossRef] [Green Version]
- Mellbring, O.; Øiseth, S.K.; Krozer, A.; Lausmaa, J.; Hjertberg, T. Spin Coating and Characterization of Thin High-Density Polyethylene Films. Macromolecules 2001, 34, 7496–7503. [Google Scholar] [CrossRef]
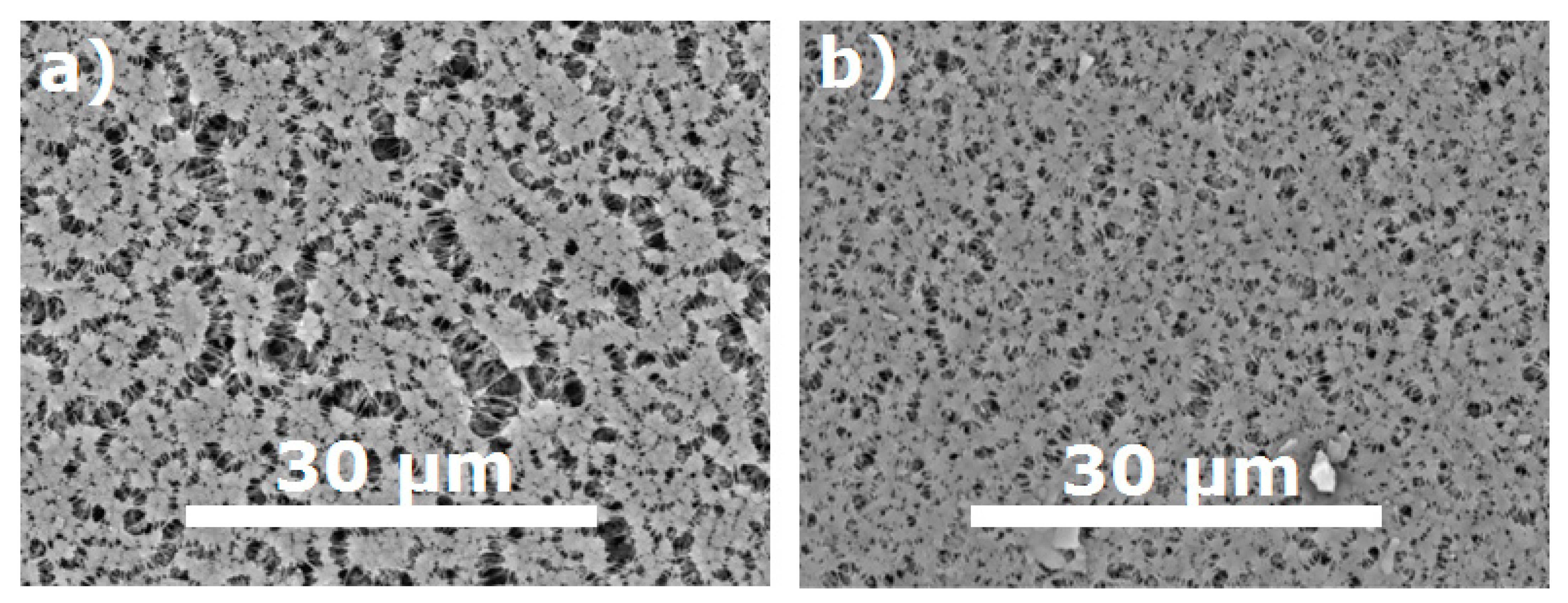
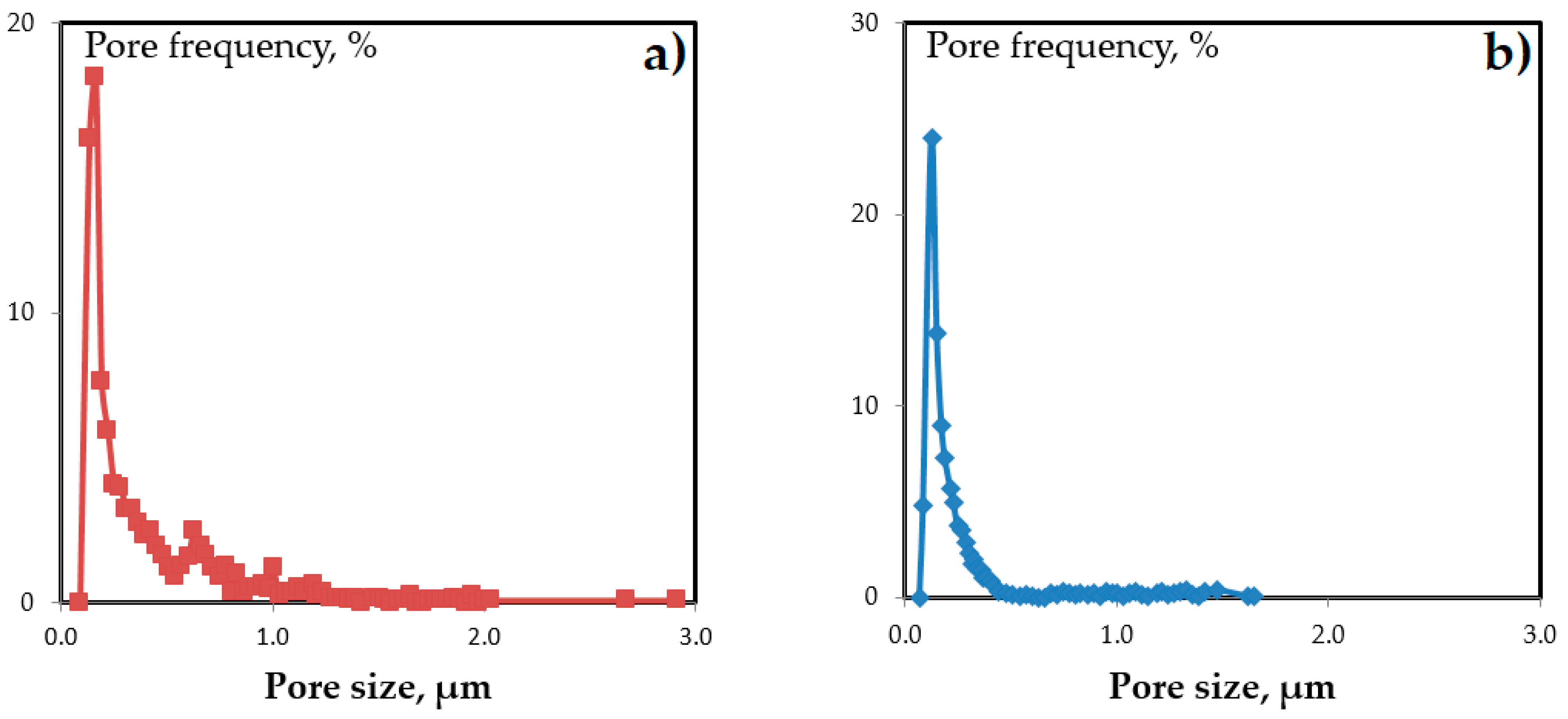
| Sample | Average Pore Size, nm | Modal Pore Size, nm | Surface Porosity, % |
|---|---|---|---|
| MFFK-1 | 290 | 160 | 66 |
| UFFK | 190 | 130 | 35 |
| PTMSP Concentration, wt % | Dynamic Viscosity, cP |
|---|---|
| 0.17 | 11 ± 3 |
| 0.25 | 34 ± 3 |
| 0.35 | 80 ± 3 |
| 0.50 | 300 ± 25 |
| Concentration, wt % | Rotation Speed, rpm | N2 Permeance, GPU | CO2 Permeance, GPU | Ideal Selectivity CO2/N2 |
|---|---|---|---|---|
| 0.17 | 500 | 220 ± 20 | 970 ± 100 | 4.4 ± 1.0 |
| 1000 | 130 ± 20 | 520 ± 100 | 4.0 ± 0.5 | |
| 3000 | 230 ± 30 | 870 ± 100 | 3.8 ± 0.6 | |
| 0.25 | 500 | 290 ± 50 | 1560 ± 150 | 5.4 ± 0.9 |
| 1000 | 125 ± 20 | 575 ± 150 | 4.0 ± 0.5 | |
| 3000 | 250 ± 50 | 1020 ± 200 | 4.1 ± 0.5 | |
| 0.35 | 500 | 1140 ± 100 | 4690 ± 400 | 4.1 ± 1.0 |
| 1000 | 875 ± 100 | 4520 ± 100 | 5.2 ± 0.7 | |
| 3000 | 740 ± 120 | 3130 ± 340 | 4.2 ± 1.0 | |
| 0.50 | 500 | 910 ± 150 | 5040 ± 500 | 5.5 ± 0.5 |
| 1000 | 890 ± 150 | 4550 ± 200 | 5.2 ± 1.0 | |
| 3000 | 970 ± 100 | 4400 ± 150 | 4.5 ± 0.5 |
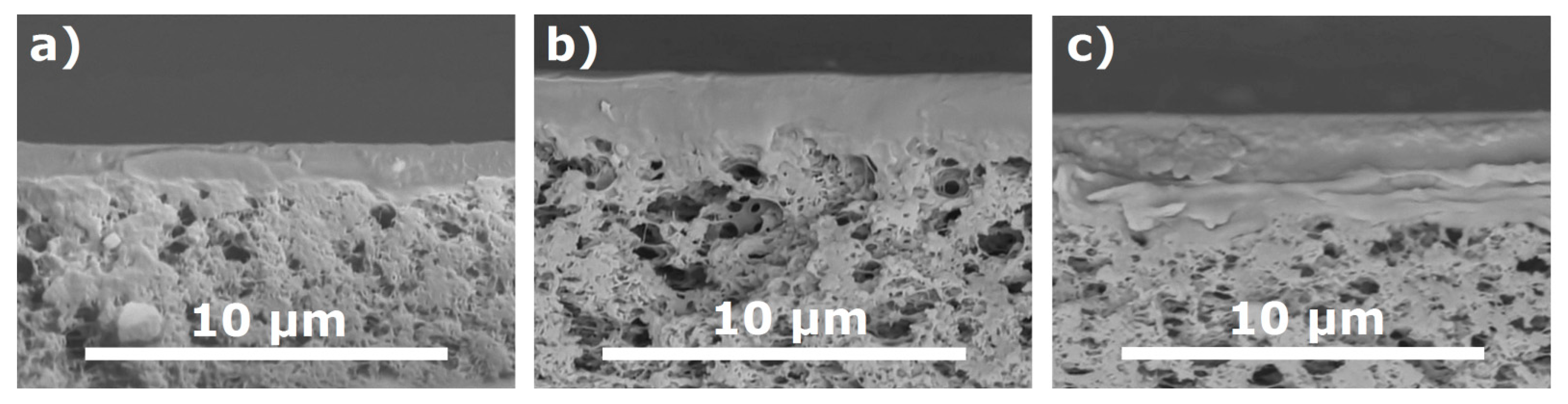
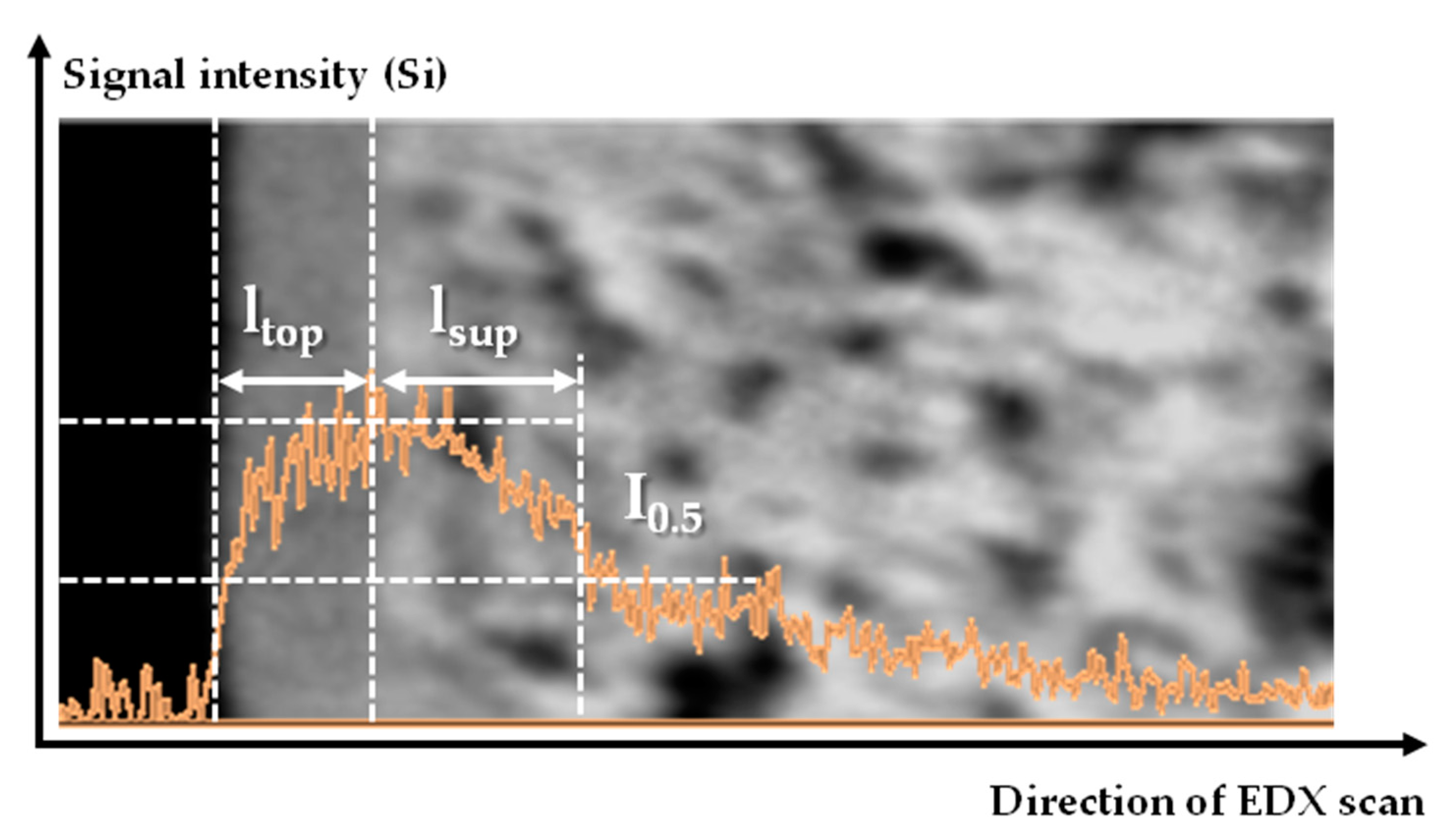
| Rotation Speed, rpm | ltop, µm | |
|---|---|---|
| PTMSP/MFFK-1 | PTMSP/MFFK | |
| 500 | 3.0 ± 0.5 | 1.7 ± 0.2 |
| 750 | 2.5 ± 0.2 | 1.6 ± 0.2 |
| 1000 | 2.2 ± 0.2 | 1.3 ± 0.2 |
| 1500 | 2.1 ± 0.8 | 1.3 ± 0.3 |
| 2000 | 1.6 ± 0.3 | 1.3 ± 0.1 |
| 2500 | 1.3 ± 0.4 | 1.2 ± 0.2 |
| 3000 | 1.0 ± 0.2 | 1.1 ± 0.2 |
| Rotation Speed, rpm | lsup, µm | |
|---|---|---|
| PTMSP/MFFK-1 | PTMSP/UFFK | |
| 500 | 1.6 | 0.9 |
| 1000 | 1.4 | 1.6 |
| 3000 | 1.9 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, S.; Balynin, A.; Bakhtin, D.; Borisov, I. Influence of Spin Coating Parameters on Gas Transport Properties of Thin-Film Composite Membranes. Materials 2021, 14, 5093. https://doi.org/10.3390/ma14175093
Sokolov S, Balynin A, Bakhtin D, Borisov I. Influence of Spin Coating Parameters on Gas Transport Properties of Thin-Film Composite Membranes. Materials. 2021; 14(17):5093. https://doi.org/10.3390/ma14175093
Chicago/Turabian StyleSokolov, Stepan, Alexey Balynin, Danila Bakhtin, and Ilya Borisov. 2021. "Influence of Spin Coating Parameters on Gas Transport Properties of Thin-Film Composite Membranes" Materials 14, no. 17: 5093. https://doi.org/10.3390/ma14175093






