Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration
Abstract
1. Introduction
2. Physicochemical Properties and Bioapplications of CNMs
3. Biocompatibility of CNMs
| Classification of CNM | Conjugation/Combination/Modification Material | Physicochemical Advances | Osteogenic/Antimicrobial Activities | Biological Evaluation (Species) | Reference |
|---|---|---|---|---|---|
| Graphene | Zinc oxide nanocomposite coating on the acrylic tooth | - | Antimicrobial and nontoxicity on human cell | In vitro (S. mutans, HEK-293 cell) | [32] |
| G nanoplatelet coating | - | Antimicrobial effect | In vitro (S. aureus) | [77] | |
| G-doped PMMA | - | Increased bone formation indexes (NBF, BMI, LBD, BIC, BAIT, and BAOT) | In vivo (rabbit) | [78] | |
| Composite with Y-Zr ceramics | Increased density, Vickers hardness, bending strength, fracture toughness, and wettability | - | - | [79] | |
| Graphene oxide | GO/3Y–ZrO2 composite | Reduced friction coefficient, wear rate, surface roughness. Increased wetting property. | Increased cell adhesion, proliferation, and ALP activity. | In vitro (MC3T3-E1 cell) | [80] |
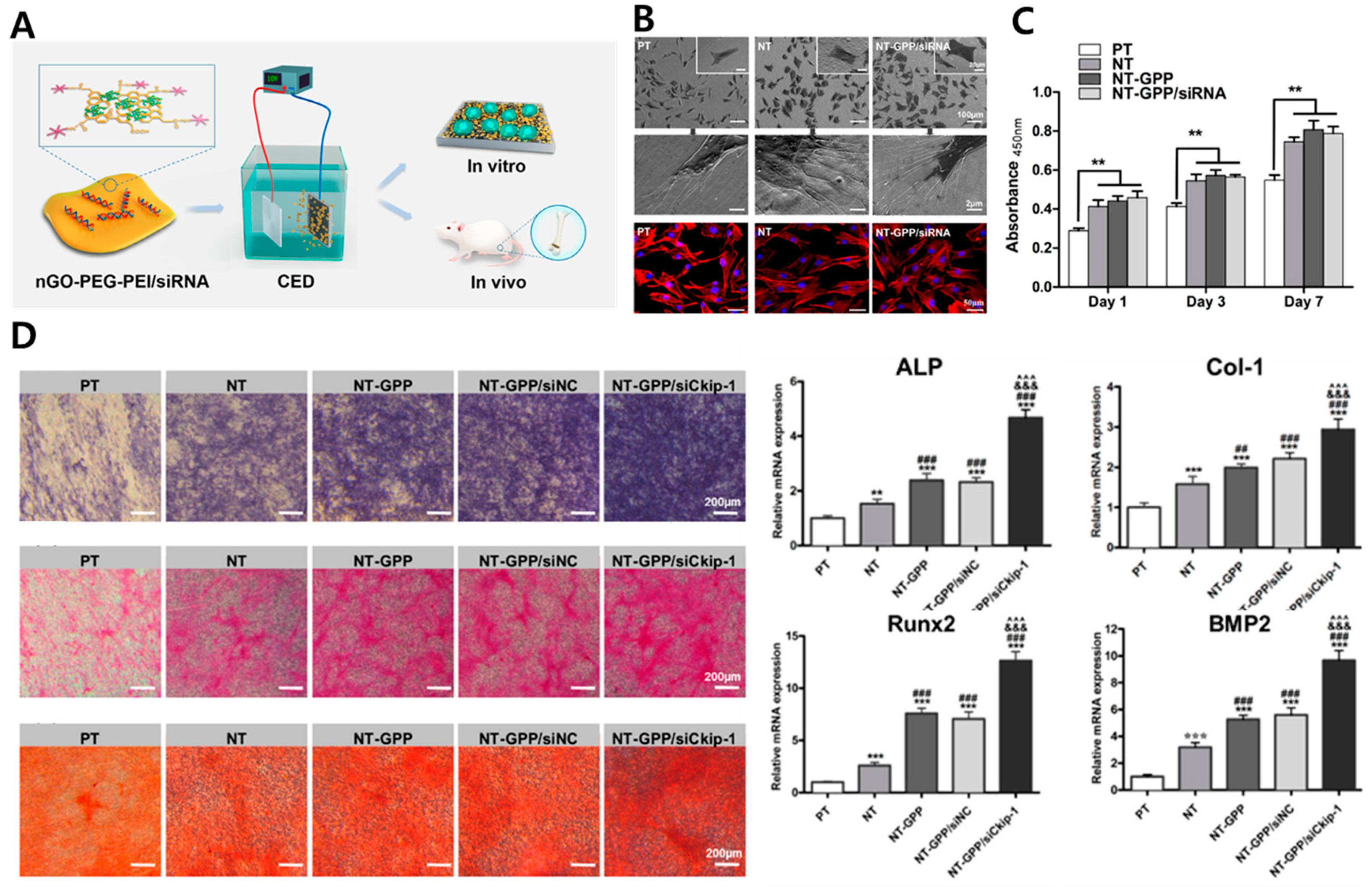
| NT/GO-PEG-PEI/siRNA | - | Enhanced cell adhesion, proliferation, uptake/knockdown efficiency, osteogenic gene expression, ALP activity, collagen secretion, ECM mineralization, and in vivo osseointegration | In vitro (MC3T3-E1 cell) and in vivo (mouse) | [81] | |
| MH-loaded GO film on Ti | - | Prevention and therapeutic effect on peri-implantitis | In vivo (Beagle dog) | [82] | |
| Nano GO-coated Ti/SLA surface | Rough and irregular surface, wettability, protein adsorption | Enhanced cell proliferation, cell area, focal adhesion formation, mineralization, and osteogenic gene expression via the FAK/MAPK signaling pathway | In vitro (rBMSC) and in vivo (SD rat) | [83] | |
| MMP-2/SP-loaded GO/Ti | Enhanced roughness and wettability | MMP-2/SP delivery facilitated new bone formation | In vivo (mouse) | [84] | |
| GO/PEEK | Surface roughness and wettability | Antibacterial ability, enhanced cell viability, proliferation, ALP activity, mineralization nodule formation, osteogenic gene expression | In vitro (MG-63 cell, E. coli and S. aureus) | [85] | |
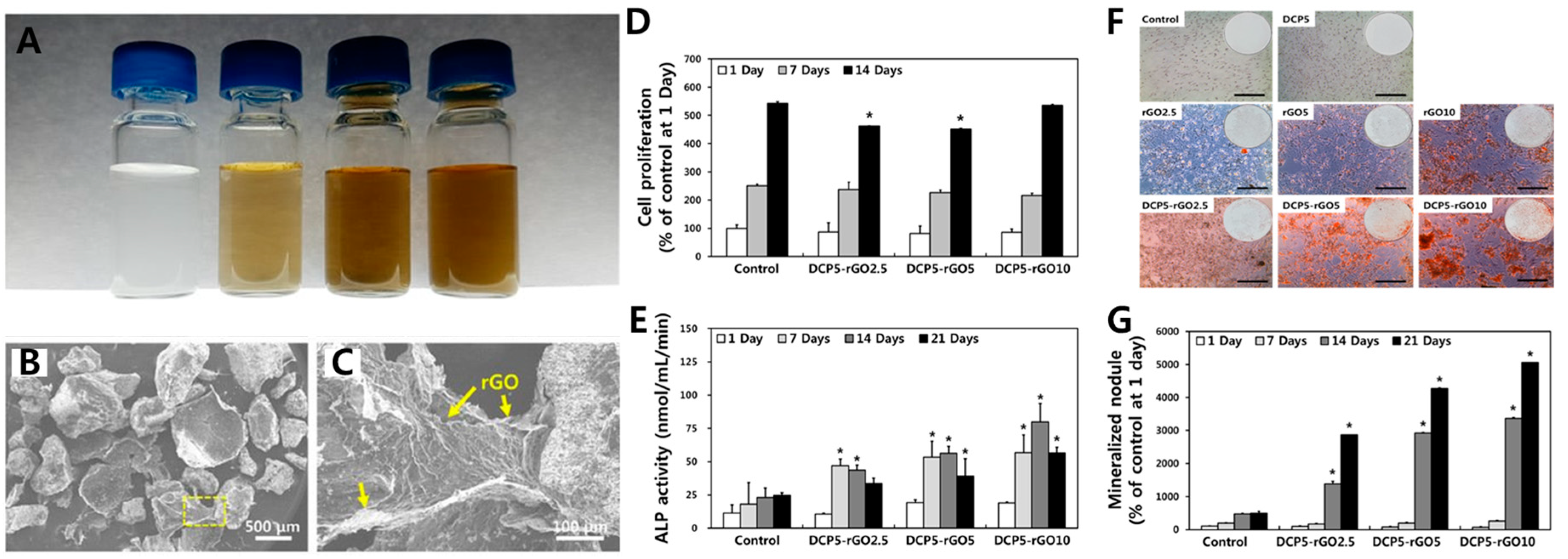
| Reduced graphene oxide | DCP-rGO composites | Controllable hybridization ratio | Cell proliferation, ALP activity, and mineralization | In vitro (MC3T3-E1 cell) | [86] |
| Dex/GO-Ti and Dex/rGO-Ti | Dex-loading capacity | Cell proliferation, osteogenic gene expression, and mineralization | In vitro (rBMSC) | [87] | |
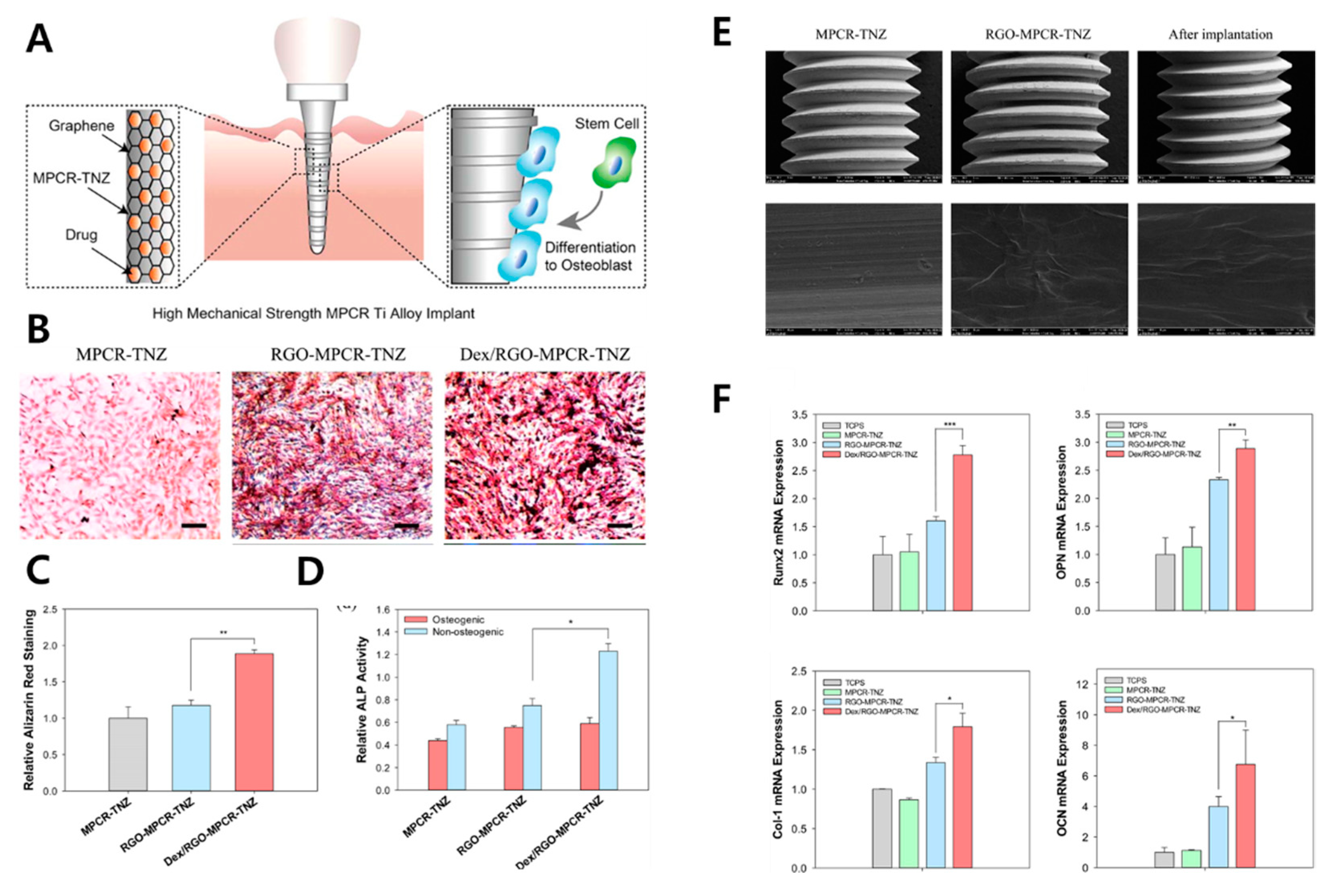
| Dex/rGO-coated Ti13Nb13Zr | Enhanced wettability and fatigue property | Enhanced cell viability, mineralization, and osteogenic gene upregulation | In vitro (MC3T3-E1 cell) | [88] | |
| rGO/FHAp composites | Enhanced mechanical strength (GPa, MPa), ion dissolution time | Enhanced cell proliferation, ALP activity, and anti-adhesion/proliferation on bacteria | In vitro (MC3T3-E1 cell and S. mutans) | [89] | |
| rGO-coated Ti6Al4V alloy | - | Enhanced cell viability, adhesion, proliferation, mineralization nodule formation, ALP activity, and osteogenic gene expression | In vitro (MC3T3-E1 cell) | [90] | |
| Carbon nanodot | Nitrogen-doped CND/HA composite | Enhanced cell proliferation, ALP activity, mineralization nodule formation, and osteogenic gene expression. Bone regeneration in zebrafish jawbone model | In vitro (MC3T3-E1 cell) and in vivo (zebreafish) | [91] | |
| CND/chitosan/HAp composite | Photothermal effect | Cell adhesion and osteogenesis, no lobulated neutrophils, osteocyte proliferation, tumor cell killing effects, and antibacterial effects | In vitro (rat BMSC, S. aureus and E. coli) and in vivo (mouse) | [92] | |
| Carbon nanotube | MWCNT-reinforced HAp coated Ti6Al4V implant | Cost-effective and rapid coating via electrophoresis. No microcracking, increased bond strength, and peeling resistance. | [93] | ||
| MWCNT-reinforced HAp/316L SS implant | High corrosion protection and corrosion current density | Antibacterial effects and nanoflake morphology for enhancing bioactive potential | In vitro (B. subtilis, S. aureus, S. flexneri and E. coli) | [94] | |
| Cu-HAp/MWCNT composite coating on 316L SS implant | High corrosion resistance | Antibacterial effect, maintained cell viability, hemolytic activity | In vitro (human osteoblast, human RBC, B. subtilis, E. coli, S. aureus, and S.mutans) | [95] | |
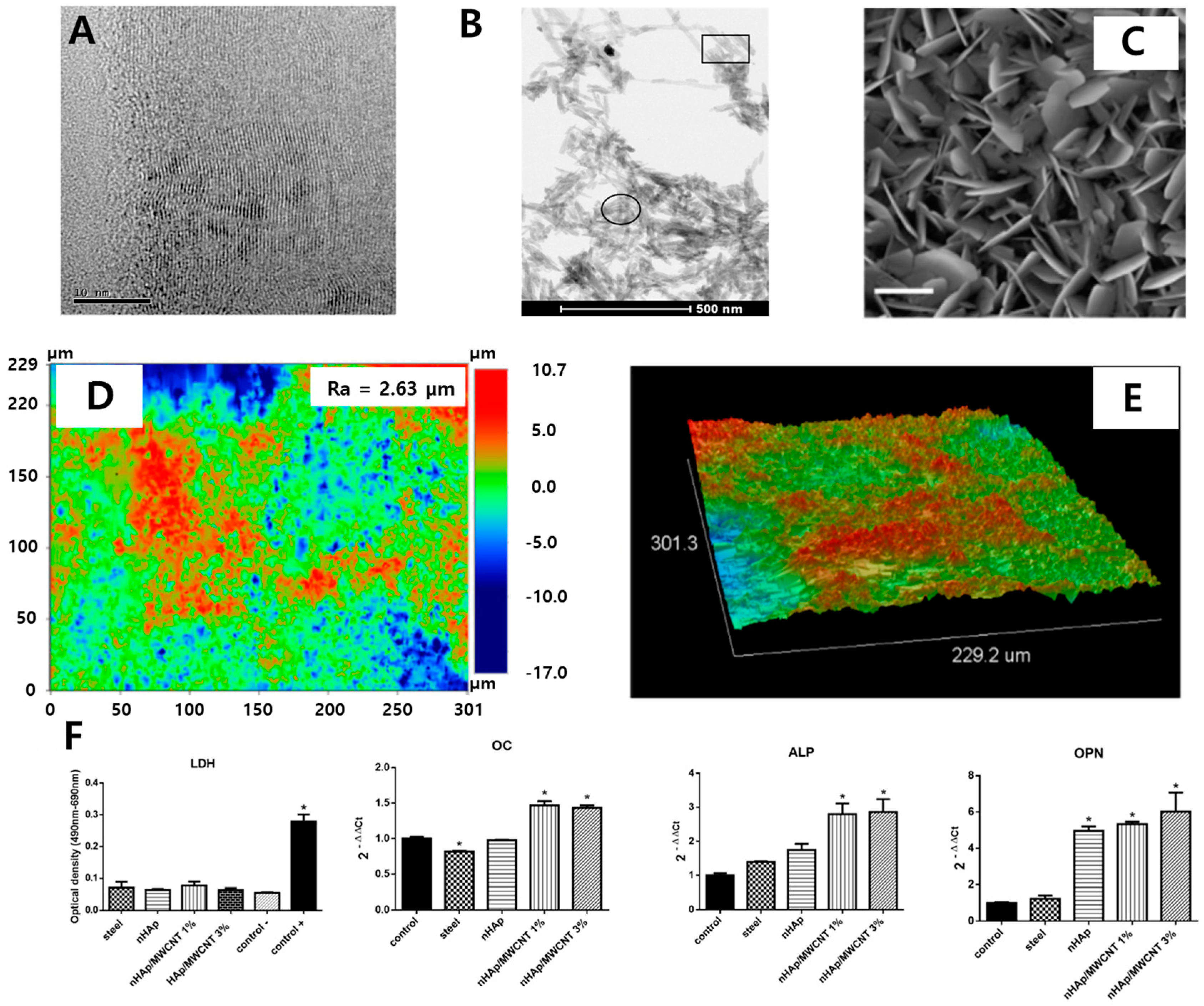
| Nano HAp/MWCNT coated stainless steel | Increased surface roughness | No damage on the cellular membrane and enhanced expression of osteogenic markers. | In vitro (MG-63 cell) | [96] | |
| Nanodiamond | ND/amorphous carbon composite | - | Enhanced fibronectin expression, attachment, proliferation, differentiation, calcium deposition, and ALP activity. | In vitro (EPC) | [97] |
| Icariin-functionalized ND composite | - | Icariin delivery, enhanced cell viability, particle uptake, ALP activity, calcium deposition, and osteogenic marker upregulation. | In vitro (MC3T3-E1 cell) | [98] | |
| Mg-nanodiamond composite | pH buffering, corrosion resistance, chemical passivation | Moderate cell viability | In vitro (L-929 cell) | [99] |
4. CNMs for Dental Application
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adya, N.; Alam, M.; Ravindranath, T.; Mubeen, A.; Saluja, B. Corrosion in titanium dental implants: Literature review. J. Indian Prosthodont. Soc. 2005, 5, 126–131. [Google Scholar] [CrossRef]
- Teigen, K.; Jokstad, A. Dental implant suprastructures using cobalt–chromium alloy compared with gold alloy framework veneered with ceramic or acrylic resin: A retrospective cohort study up to 18 years. Clin. Oral Implant. Res. 2012, 23, 853–860. [Google Scholar] [CrossRef]
- Karamian, E.; Motamedi, M.R.K.; Khandan, A.; Soltani, P.; Maghsoudi, S. An in vitro evaluation of novel NHA/zircon plasma coating on 316L stainless steel dental implant. Prog. Nat. Sci. 2014, 24, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Cheng, Y.; Zheng, Y.; Ruan, L. In vitro and in vivo studies on Ti-based bulk metallic glass as potential dental implant material. Mater. Sci. Eng. C 2013, 33, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.C.L.G.; Campos, M.I.G.; Line, S.R.P. Early dental implant failure: A review of the literature. Braz. J. Oral Sci. 2002, 1, 103–111. [Google Scholar]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Tillander, J.; Hagberg, K.; Hagberg, L.; Brånemark, R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin. Orthop. Relat. Res. 2010, 468, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2020, 122, 26–49. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014–2004041. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Kang, M.S.; Lee, J.H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Nanocomposites for enhanced osseointegration of dental and orthopedic implants revisited: Surface functionalization by carbon nanomaterial coatings. J. Compos. Sci. 2021, 5, 23. [Google Scholar] [CrossRef]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349–e12357. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1144–1150. [Google Scholar]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene family nanomaterials: Properties and potential applications in dentistry. Int. J. Biomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef]
- Portelli, M.; Gatto, E.; Matarese, G.; Militi, A.; Catalfamo, L.; Gherlone, E.; Lucchese, A. Unilateral condylar hyperplasia: Diagnosis, clinical aspects and operative treatment. Eur. J. Paediatr. Dent. 2015, 16, 100–103. [Google Scholar]
- Park, J.-W.; Hanawa, T.; Chung, J.-H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019, 14, 5697–5711. [Google Scholar] [CrossRef]
- Rosa, A.; Kato, R.; Castro Raucci, L.; Teixeira, L.; de Oliveira, F.; Bellesini, L.; de Oliveira, P.; Hassan, M.; Beloti, M. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J. Cell. Biochem. 2014, 115, 540–548. [Google Scholar] [CrossRef]
- Kim, E.J.; Boehm, C.A.; Mata, A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. Post microtextures accelerate cell proliferation and osteogenesis. Acta Biomater. 2010, 6, 160–169. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Shah, J.; Misra, R. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Kim, H.; Namgung, R.; Singha, K.; Oh, I.-K.; Kim, W.J. Graphene oxide–polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug. Chem. 2011, 22, 2558–2567. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Lin, Y. Graphene and graphene-like 2D materials for optical biosensing and bioimaging: A review. 2D Mater. 2015, 2, 032004–032013. [Google Scholar] [CrossRef]
- Zang, Z.; Zeng, X.; Wang, M.; Hu, W.; Liu, C.; Tang, X. Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sensor. Actuators B Chem. 2017, 252, 1179–1186. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, J.H.; Song, S.-J.; Shin, D.-M.; Jang, J.-H.; Hyon, S.-H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Graphene oxide-functionalized nanofibre composite matrices to enhance differentiation of hippocampal neuronal cells. Mater. Adv. 2020, 1, 3496–3506. [Google Scholar] [CrossRef]
- Shin, Y.C.; Song, S.-J.; Lee, J.H.; Park, R.; Kang, M.S.; Lee, Y.B.; Hong, S.W.; Han, D.-W. Different alignment between skeletal and smooth muscle cells on reduced graphene oxide-patterned arrays. Sci. Adv. Mater. 2020, 12, 474–480. [Google Scholar] [CrossRef]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 1–9. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Meena, R.; Singh, B.R.; Khan, A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 2014, 30, 1281–1294. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Zanden, C.; Liu, J. The promising application of graphene oxide as coating materials in orthopedic implants: Preparation, characterization and cell behavior. Biomed. Mater. 2015, 10, 015019–015028. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Lee, Y.-C.; Huang, K.-Y.; Huang, S.; Shrestha, S.; Conibeer, G.; Tang, J. Efficient electron transfer in carbon nanodot–graphene oxide nanocomposites. J. Mater. Chem. C 2014, 2, 2894–2901. [Google Scholar] [CrossRef]
- Chavez, J. Carbon Nanodot Cellular Uptake and Modulation of Tumor Necrosis Factor-Alpha-Induced Endothelial Dysfunction. Ph.D. Thesis, The University of North Carolina at Greensboro, Greensboro, NC, USA, 2018. [Google Scholar]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent advances in bioapplications of C-dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Carbon dots: Preparation, properties, and application. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Inamuddin, Asiri, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 651–676. [Google Scholar]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.H.; Kostarelos, K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.-S.; Park, J.-C.; Hong, S.W.; Han, D.-W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Kim, B.; Park, J.-C.; Han, D.-W. Synergistic effects of reduced graphene oxide and hydroxyapatite on osteogenic differentiation of MC3T3-E1 preosteoblasts. Carbon 2015, 95, 1051–1060. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Raphey, V.; Henna, T.; Nivitha, K.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.C.P.; Fearon, P.K.; Hsu, W.K.; Billingham, N.C.; Kroto, H.W.; Walton, D.R.M. Carbon nanotubes as polymer antioxidants. J. Mater. Chem. 2003, 13, 491–495. [Google Scholar] [CrossRef]
- Savage, T.; Bhattacharya, S.; Sadanadan, B.; Gaillard, J.; Tritt, T.; Sun, Y.; Wu, Y.; Nayak, S.; Car, R.; Marzari, N.; et al. Photoinduced oxidation of carbon nanotubes. J. Phys. Condens. Matter 2003, 15, 5915–5922. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Torres, V.M.; Srdjenovic, B. Biomedical application of fullerenes. In Handbook on Fullerene: Synthesis, Properties and Applications; Verner, R.F., Benvegnu, C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 199–239. [Google Scholar]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the surfaces of nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond. Nanotechnology 2017, 28, 252001–252028. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res. A 2014, 102, 3282–3290. [Google Scholar] [CrossRef]
- Lobo, A.O.; Antunes, E.; Machado, A.; Pacheco-Soares, C.; Trava-Airoldi, V.; Corat, E. Cell viability and adhesion on as grown multi-wall carbon nanotube films. Mater. Sci. Eng. C 2008, 28, 264–269. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Baik, K.Y.; Park, S.Y.; Heo, K.; Lee, K.B.; Hong, S. Carbon nanotube monolayer cues for osteogenesis of mesenchymal stem cells. Small 2011, 7, 741–745. [Google Scholar] [CrossRef]
- Rajesh, R.; Ravichandran, Y.D. Development of new graphene oxide incorporated tricomponent scaffolds with polysaccharides and hydroxyapatite and study of their osteoconductivity on MG-63 cell line for bone tissue engineering. RSC Adv. 2015, 5, 41135–41143. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.; Apicella, A.; Petrescu, F.I. Nano-diamond hybrid materials for structural biomedical application. Am. J. Biochem. Biotechnol. 2016, 13, 34–41. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Yan, J.; Zhang, Q.; Dang, B.; Wang, Z.; Yao, Y.; Lin, K.; Guo, Z.; Bi, L.; et al. Evaluation of the osteogenesis and osseointegration of titanium alloys coated with graphene: An in vivo study. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, C.; Li, Y.; Li, Y.; Chen, G.; He, Y.; Yi, C.; Wang, C.; Yu, D. Dose-dependent cytotoxicity induced by pristine graphene oxide nanosheets for potential bone tissue regeneration. J. Biomed. Mater. Res. A 2020, 108, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Yu, S.-J.; Kang, M.-W.; Chang, H.-C.; Chen, K.-M.; Yu, Y.-C. Bright fluorescent nanodiamonds: No photobleaching and low cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605. [Google Scholar] [CrossRef]
- Paget, V.; Sergent, J.; Grall, R.; Altmeyer-Morel, S.; Girard, H.; Petit, T.; Gesset, C.; Mermoux, M.; Bergonzo, P.; Arnault, J.-C.; et al. Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 2014, 8, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Duch, M.C.; Budinger, G.S.; Liang, Y.T.; Soberanes, S.; Urich, D.; Chiarella, S.E.; Campochiaro, L.A.; Gonzalez, A.; Chandel, N.S.; Hersam, M.C.; et al. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011, 11, 5201–5207. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Ioannides, N.; Trzebicka, B.; Hampel, S.; Rümmeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 1–26. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Møller, P.; Clausen, P.A.; Saber, A.T.; Micheletti, C.; Jensen, K.A.; Wallin, H.; Vogel, U. Biodistribution of carbon nanotubes in animal models. Basic Clin. Pharmacol. Toxicol. 2017, 121, 30–43. [Google Scholar] [CrossRef]
- Elgrabli, D.; Floriani, M.; Abella-Gallart, S.; Meunier, L.; Gamez, C.; Delalain, P.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008, 5, 1–13. [Google Scholar] [CrossRef]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial activity against staphylococcus aureus of titanium surfaces coated with graphene nanoplatelets to prevent peri-implant diseases. an in-vitro pilot study. Int. J. Environ. Res. Public Health 2020, 17, 1568. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Orsini, T.; Di Carlo, F.; Valbonetti, L.; Lorusso, F. Graphene-doped poly (methyl-methacrylate) (PMMA) implants: A micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2021, 22, 1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Z.; Zhao, L.; Liu, W.; Si, P.; Lan, J. Synthesis and characterization of multilayer graphene oxide on yttria-zirconia ceramics for dental implant. J. Mater. Res. 2020, 35, 2466–2477. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Song, W.; Wu, K.; Zhang, Y.; Zhao, Y. Dual-functionalized graphene oxide based siRNA delivery system for implant surface biomodification with enhanced osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 34722–34735. [Google Scholar] [CrossRef]
- Qian, W.; Qiu, J.; Liu, X. Minocycline hydrochloride-loaded graphene oxide films on implant abutments for peri-implantitis treatment in beagle dogs. J. Periodontol. 2020, 91, 792–799. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 signaling pathways in mediating the enhanced osteogenesis induced by nano-graphene oxide modification on titanium implant surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.-S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116. [Google Scholar]
- Ouyang, L.; Deng, Y.; Yang, L.; Shi, X.; Dong, T.; Tai, Y.; Yang, W.; Chen, Z.G. Graphene-oxide-decorated microporous polyetheretherketone with superior antibacterial capability and in vitro osteogenesis for orthopedic implant. Macromol. Biosci. 2018, 18, 1800036. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Shin, Y.C.; Song, S.J.; Cha, J.M.; Hong, S.W.; Lim, Y.-J.; Jeong, S.J.; Han, D.-W.; Kim, B. Dicalcium phosphate coated with graphene synergistically increases osteogenic differentiation in vitro. Coatings 2018, 8, 13. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface modification of multipass caliber-rolled Ti alloy with dexamethasone-loaded graphene for dental applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloy. Compd. 2016, 688, 657–667. [Google Scholar] [CrossRef]
- Li, X.; Lin, K.; Wang, Z. Enhanced growth and osteogenic differentiation of MC3T3-E1 cells on Ti6Al4V alloys modified with reduced graphene oxide. RSC Adv. 2017, 7, 14430–14437. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Kumar, V.B.; Gigi, D.; Gedanken, A.; Karasik, D. Accelerated bone regeneration by nitrogen-doped carbon dots functionalized with hydroxyapatite nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19373–19385. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Li, M.; Lin, Z.; Wang, L.; Zhang, Y.; Yin, Q.; Xia, H.; Han, G. Zero-dimensional carbon dots enhance bone regeneration, osteosarcoma ablation, and clinical bacterial eradication. Bioconjug. Chem. 2018, 29, 2982–2993. [Google Scholar] [CrossRef]
- Kaya, C.; Singh, I.; Boccaccini, A.R. Multi-walled carbon nanotube-reinforced hydroxyapatite layers on Ti6Al4V medical implants by electrophoretic deposition (EPD). Adv. Eng. Mater. 2008, 10, 131–138. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K. Novel synthesis of bioactive hydroxyapatite/f-multiwalled carbon nanotube composite coating on 316L SS implant for substantial corrosion resistance and antibacterial activity. J. Alloy. Compd. 2019, 777, 1340–1346. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K.; Ganeshkumar, A.; Rajaram, R. Tailoring Cu substituted hydroxyapatite/functionalized multiwalled carbon nanotube composite coating on 316L SS implant for enhanced corrosion resistance, antibacterial and bioactive properties. Int. J. Pharm. 2020, 590, 119946–119957. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.M.; Ribeiro, M.J.G.; Ricci, R.; Marques, M.A.; Lobo, A.O.; Marciano, F.R. In vitro osteogenesis stimulation via nano-hydroxyapatite/carbon nanotube thin films on biomedical stainless steel. Materials 2018, 11, 1555. [Google Scholar] [CrossRef]
- Ivanova, L.; Popov, C.; Kolev, I.; Shivachev, B.; Karadjov, J.; Tarassov, M.; Kulisch, W.; Reithmaier, J.; Apostolova, M. Nanocrystalline diamond containing hydrogels and coatings for acceleration of osteogenesis. Diam. Relat. Mater. 2011, 20, 165–169. [Google Scholar] [CrossRef]
- Choi, S.; Noh, S.H.; Lim, C.O.; Kim, H.-J.; Jo, H.-S.; Min, J.S.; Park, K.; Kim, S.E. Icariin-functionalized nanodiamonds to enhance osteogenic capacity in vitro. Nanomaterials 2020, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Anasori, B.; Dennison, C.R.; Wang, K.; Kumbur, E.C.; Strich, R.; Zhou, J.G. Fabrication, biodegradation behavior and cytotoxicity of Mg-nanodiamond composites for implant application. J. Mater. Sci. Mater. Med. 2015, 26, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e5. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Koegel, S.; Waltimo, T.; Zimmermann, A.; Walker, C.; Hauser-Gerspach, I.; Jung, C. Antimicrobial efficacy of copper-doped titanium surfaces for dental implants. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Gosau, M.; Haupt, M.; Thude, S.; Strowitzki, M.; Schminke, B.; Buergers, R. Antimicrobial effect and biocompatibility of novel metallic nanocrystalline implant coatings. J. Biomed. Mater. Res. B 2016, 104, 1571–1579. [Google Scholar] [CrossRef]
- Campos, K.d.P.L.; Viana, G.M.; Cabral, L.M.; Portela, M.B.; Junior, R.H.; Cavalcante, L.M.; Lourenço, E.J.V.; de Moraes Telles, D. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: Cytotoxicity, antimicrobial, physical, and mechanical properties. Dent. Mater. 2020, 36, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Annibali, S.; Ripari, M.; La Monaca, G.; Tonoli, F.; Cristalli, M.P. Local accidents in dental implant surgery: Prevention and treatment. Int. J. Periodontics Restor. Dent. 2009, 29–36. [Google Scholar]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; De Leon, A.C.C.; Rodrigues, D.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Kang, M.S.; Kang, J.I.; Le Thi, P.; Park, K.M.; Hong, S.W.; Choi, Y.S.; Han, D.-W.; Park, K.D. Three-dimensional printable gelatin hydrogels incorporating graphene oxide to enable spontaneous myogenic differentiation. ACS Macro Lett. 2021, 10, 426–432. [Google Scholar] [CrossRef]
- Park, K.O.; Lee, J.H.; Park, J.H.; Shin, Y.C.; Huh, J.B.; Bae, J.-H.; Kang, S.H.; Hong, S.W.; Kim, B.; Yang, D.J.; et al. Graphene oxide-coated guided bone regeneration membranes with enhanced osteogenesis: Spectroscopic analysis and animal study. Appl. Spectrosc. Rev. 2016, 51, 540–551. [Google Scholar] [CrossRef]
- Daniyal, M.; Liu, B.; Wang, W. Comprehensive review on graphene oxide for use in drug delivery system. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Sun, S.; Guo, H.; Zhang, J.; Yu, B.; Sun, K.; Jin, Q. Adenovirus-mediated expression of bone morphogenetic protein-2 activates titanium particle-induced osteoclastogenesis and this effect occurs in spite of the suppression of TNF-α expression by siRNA. Int. J. Mol. Med. 2013, 32, 403–409. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Tran, K.K.; Shen, H.; Grainger, D.W. Selective local delivery of RANK siRNA to bone phagocytes using bone augmentation biomaterials. Biomaterials 2012, 33, 8540–8547. [Google Scholar] [CrossRef]
- Bland, P.S.; Goodson, J.M.; Gunsolley, J.C.; Grossi, S.G.; Otomo-Corgel, J.; Doherty, F.; Comiskey, J.L. Association of antimicrobial and clinical efficacy: Periodontitis therapy with minocycline microspheres. J. Int. Acad. Periodontol. 2010, 12, 11–19. [Google Scholar]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.; Zajonkovsky, I.; Valles, J.M.; Baschini, M.T. Antimicrobial properties of tetracycline and minocycline-montmorillonites. Appl. Clay Sci. 2010, 49, 194–199. [Google Scholar] [CrossRef]
- Hylden, J.L.; Wilcox, G.L. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981, 217, 212–215. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Maturavongsadit, P.; Chen, X.; Sitasuwan, P.; Lu, L.; Su, J.; Wang, Q. Nanotopographical cues mediate osteogenesis of stem cells on virus substrates through BMP-2 intermediate. Nano Lett. 2019, 19, 8372–8380. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhou, Y.; Wang, J.; Liu, C.; Xiao, Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis. Tissue Eng. Part A 2018, 24, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chang, H.; Chen, S.; Lai, C.; Khademhosseini, A.; Wu, H. Regulating cellular behavior on few-layer reduced graphene oxide films with well-controlled reduction states. Adv. Funct. Mater. 2012, 22, 751–759. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, I.; Miyaji, H.; Takita, H.; Nishida, E.; Tsuji, M.; Fugetsu, B.; Sun, L.; Inoue, K.; Ibara, A.; Akasaka, T.; et al. Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int. J. Nanomed. 2014, 9, 3363–3373. [Google Scholar]
- Shao, D.; Lu, M.; Xu, D.; Zheng, X.; Pan, Y.; Song, Y.; Xu, J.; Li, M.; Zhang, M.; Li, J.; et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2017, 5, 1820–1827. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Eklund, P. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Treacy, M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Papo, M.J.; Catledge, S.A.; Vohra, Y.K.; Machado, C. Mechanical wear behavior of nanocrystalline and multilayer diamond coatings on temporomandibular joint implants. J. Mater. Sci. Mater. Med. 2004, 15, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, M.; Bacakova, L.; Vacik, J.; Fendrych, F.; Vorlicek, V.; Kratochvilova, I.; Lisa, V.; Van Hove, E.; Mer, C.; Bergonzo, P.; et al. Improved adhesion, growth and maturation of human bone-derived cells on nanocrystalline diamond films. Phys. Status Solidi A 2008, 205, 2146–2153. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Jang, H.J.; Lee, S.H.; Lee, J.E.; Jo, H.J.; Jeong, S.J.; Kim, B.; Han, D.-W. Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration. Materials 2021, 14, 5104. https://doi.org/10.3390/ma14175104
Kang MS, Jang HJ, Lee SH, Lee JE, Jo HJ, Jeong SJ, Kim B, Han D-W. Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration. Materials. 2021; 14(17):5104. https://doi.org/10.3390/ma14175104
Chicago/Turabian StyleKang, Moon Sung, Hee Jeong Jang, Seok Hyun Lee, Ji Eun Lee, Hyo Jung Jo, Seung Jo Jeong, Bongju Kim, and Dong-Wook Han. 2021. "Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration" Materials 14, no. 17: 5104. https://doi.org/10.3390/ma14175104
APA StyleKang, M. S., Jang, H. J., Lee, S. H., Lee, J. E., Jo, H. J., Jeong, S. J., Kim, B., & Han, D.-W. (2021). Potential of Carbon-Based Nanocomposites for Dental Tissue Engineering and Regeneration. Materials, 14(17), 5104. https://doi.org/10.3390/ma14175104








