Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers
Abstract
:1. Introduction
2. Methylcellulose and Cellulose Nanocrystals
2.1. Preparation and General Properties of MC Hydrogels
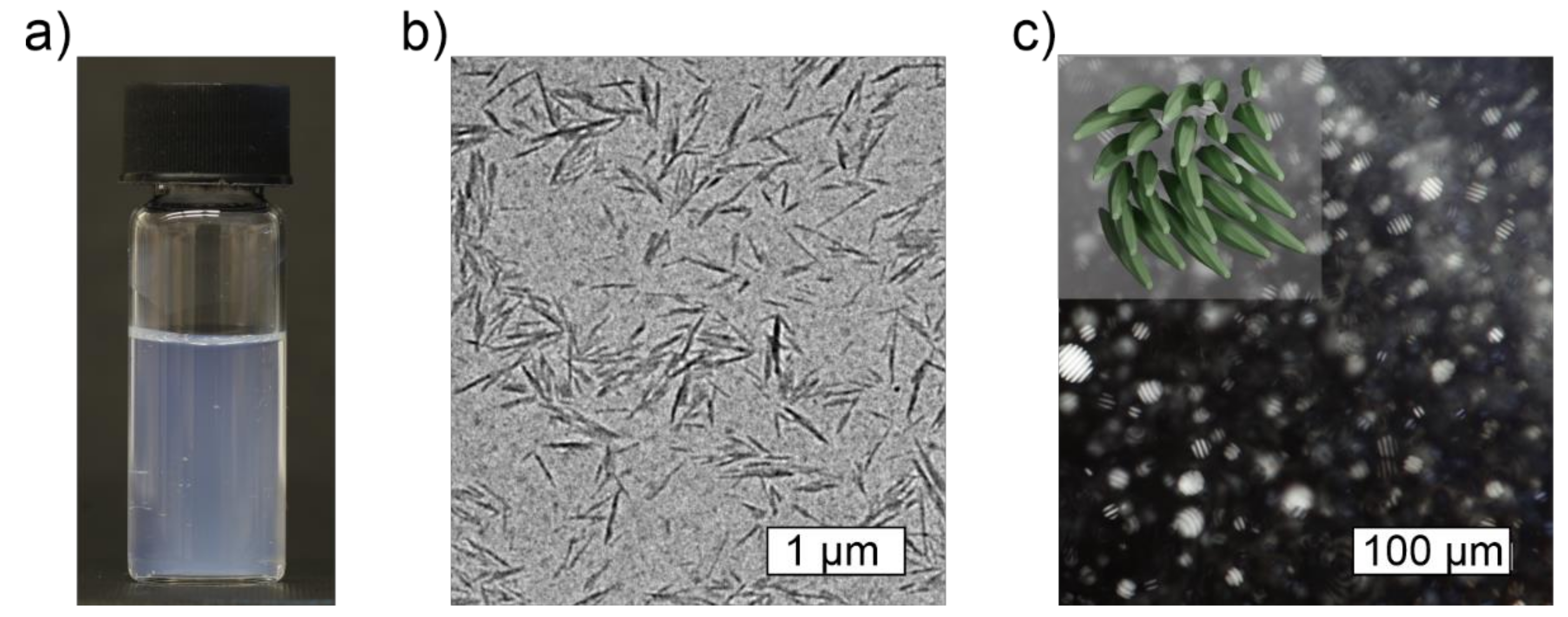
2.2. Characteristics of Cellulose Nanocrystals
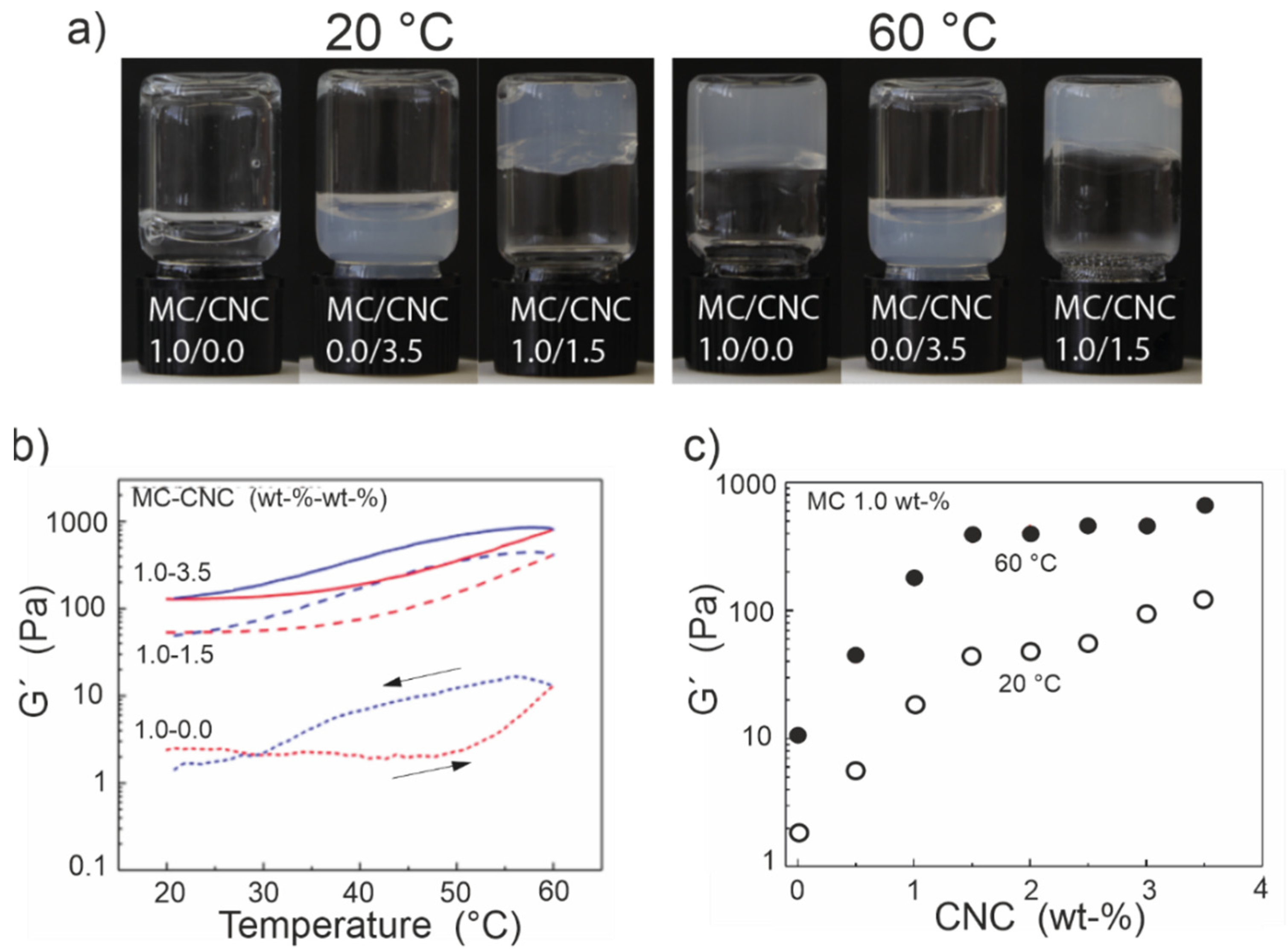
3. Mechanical Tunability of MC-CNC Composite Hydrogels
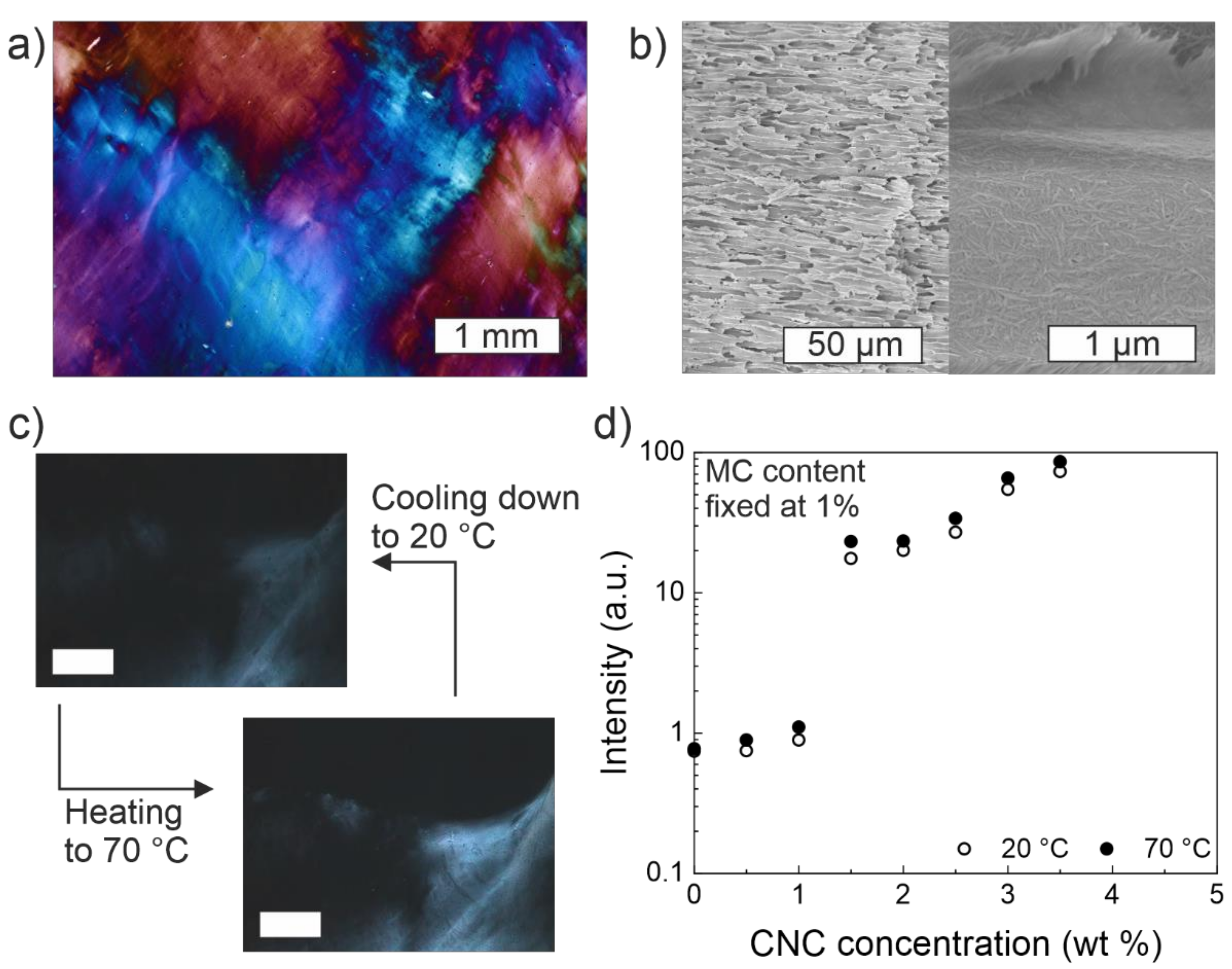
4. Optical Birefringence in MC-CNC Composite Hydrogels
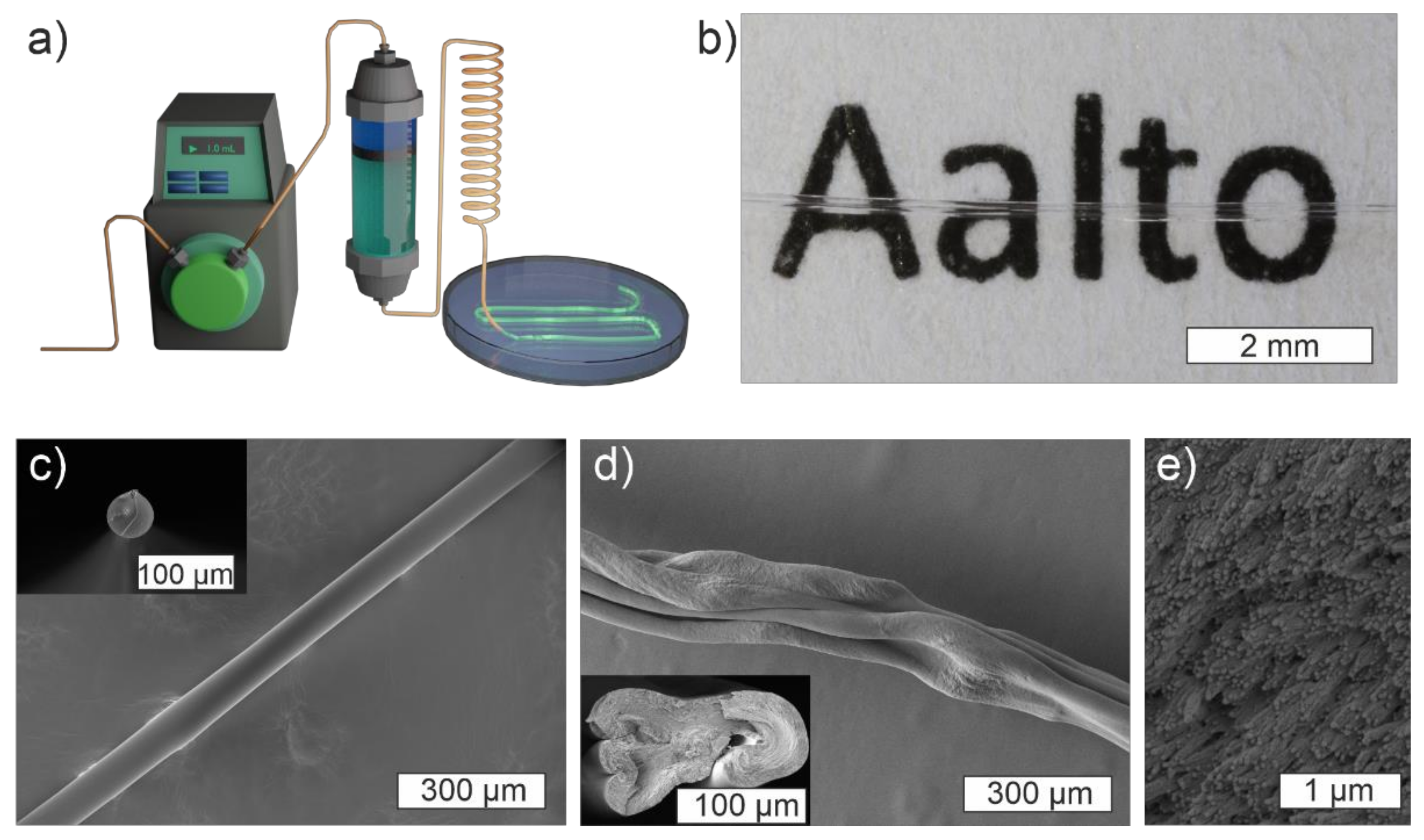
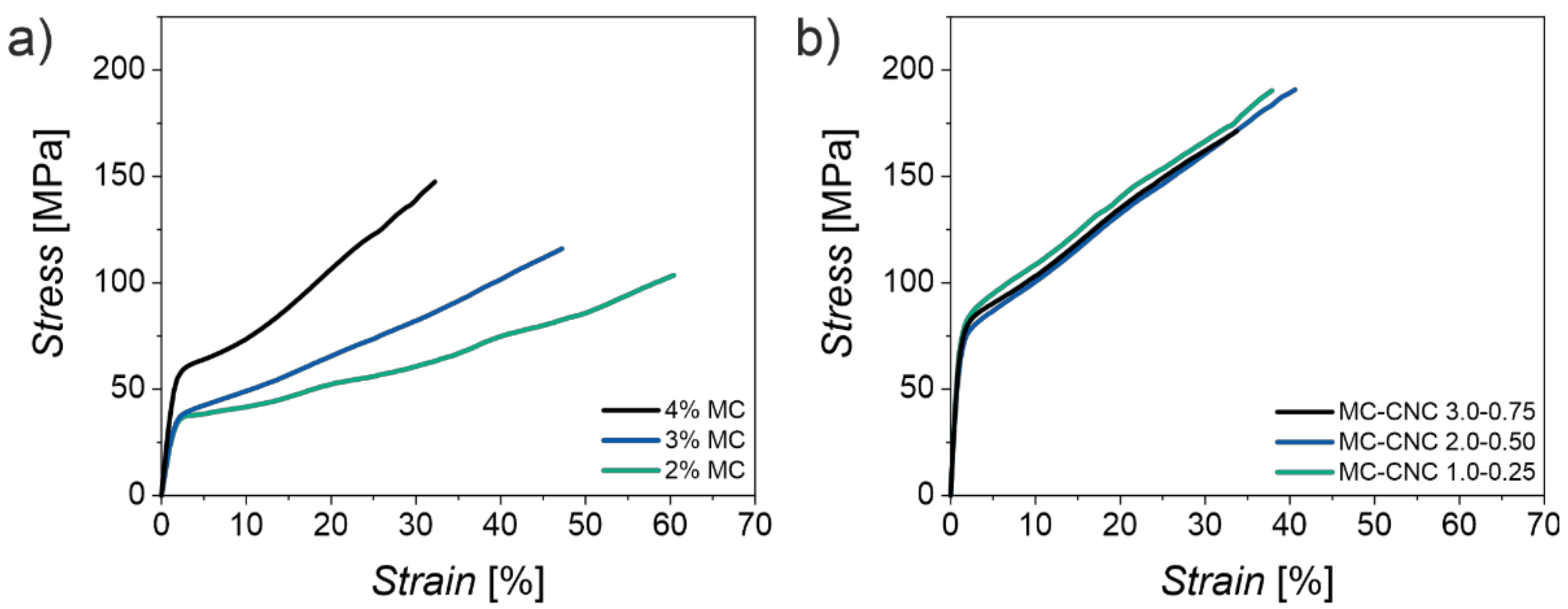
5. MC-CNC Composite Fibers
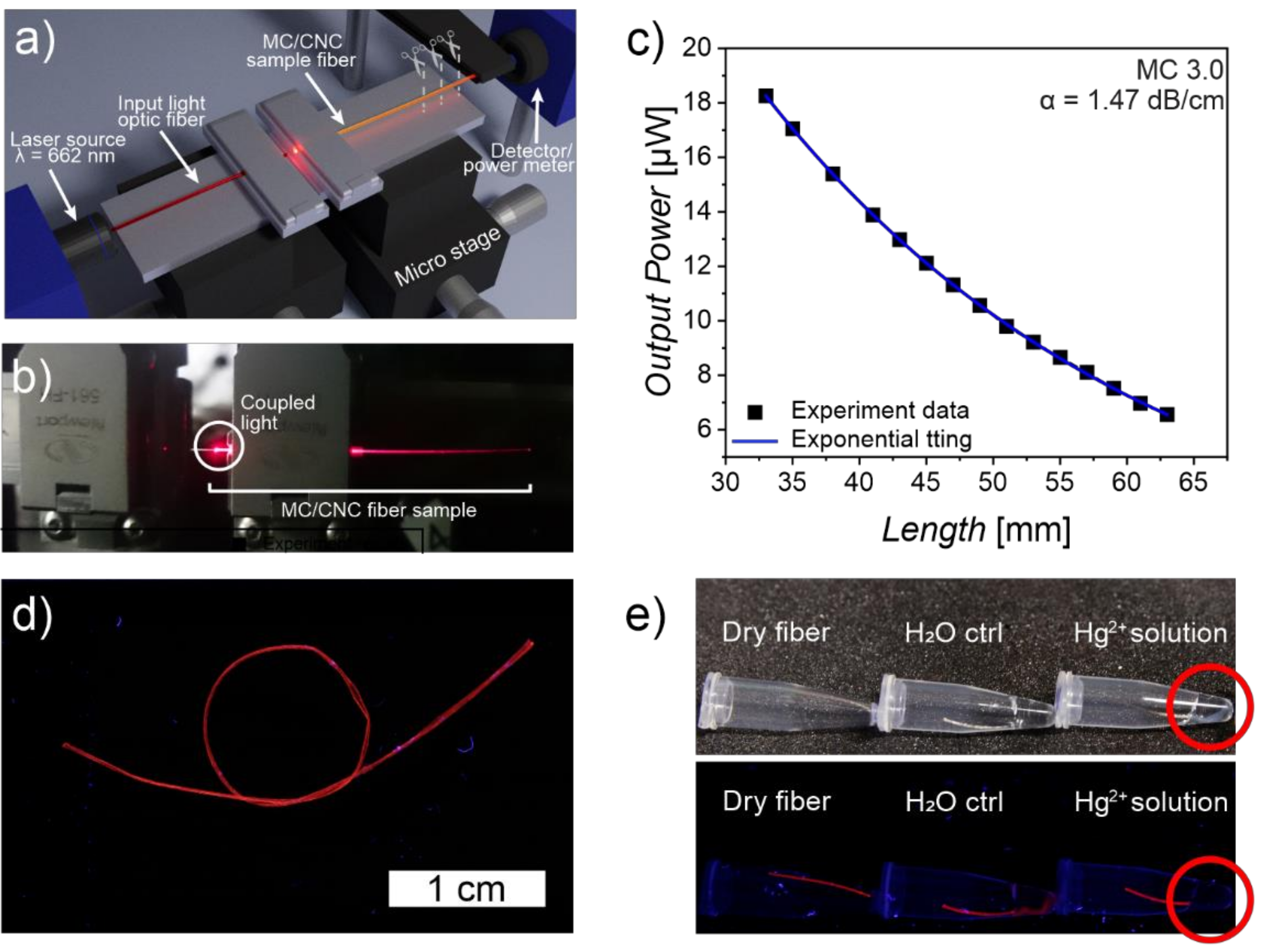
6. MC-Based Biopolymeric Optical Fibers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Kontturi, E.; Allahverdiyeva, Y.; Tammelin, T.; Linder, M.B.; Ikkala, O. Nanocellulose: Recent Fundamental Advances and Emerging Biological and Biomimicking Applications. Adv. Mater. 2021, 33, 2004349. [Google Scholar] [CrossRef]
- Myllymäki, T.T.T.; Guliyeva, A.; Korpi, A.; Kostiainen, M.A.; Hynninen, V.; Rannou, P.; Ikkala, O.; Halila, S. Lyotropic Liquid Crystals and Linear Supramolecular Polymers of End-Functionalized Oligosaccharides. Chem. Commun. 2019, 55, 11739–11742. [Google Scholar] [CrossRef]
- Majoinen, J.; Hassinen, J.; Haataja, J.S.; Rekola, H.T.; Kontturi, E.; Kostiainen, M.A.; Ras, R.H.A.; Törmä, P.; Ikkala, O. Chiral Plasmonics Using Twisting along Cellulose Nanocrystals as a Template for Gold Nanoparticles. Adv. Mater. 2016, 28, 5262–5267. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pötschke, P.; Pionteck, J.; Voit, B.; Qi, H. Smart Cellulose/Graphene Composites Fabricated by: In Situ Chemical Reduction of Graphene Oxide for Multiple Sensing Applications. J. Mater. Chem. A 2018, 6, 7777–7785. [Google Scholar] [CrossRef]
- Mohammadi, P.; Sesilja Aranko, A.; Landowski, C.P.; Ikkala, O.; Jaudzems, K.; Wagermaier, W.; Linder, M.B. Biomimetic Composites with Enhanced Toughening Using Silk-Inspired Triblock Proteins and Aligned Nanocellulose Reinforcements. Sci. Adv. 2019, 5, eaaw2541. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Liquid Crystalline Tactoidal Microphases in Ferrofluids: Spatial Positioning and Orientation by Magnetic Field Gradients. Chem 2019, 5, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Shariatnia, S.; Kumar, A.V.; Kaynan, O.; Asadi, A. Hybrid Cellulose Nanocrystal-Bonded Carbon Nanotubes/Carbon Fiber Polymer Composites for Structural Applications. ACS Appl. Nano Mater. 2020, 3, 5421–5436. [Google Scholar] [CrossRef]
- Chakraborty, A.; Mondal, B.; Chaudhari, K.; Rekola, H.; Hynninen, V.; Kostiainen, M.A.; Ras, R.H.A.; Pradeep, T. Near-Infrared Chiral Plasmonic Microwires through Precision Assembly of Gold Nanorods on Soft Biotemplates. J. Phys. Chem. C 2021, 125, 3267. [Google Scholar] [CrossRef]
- Zhao, T.H.; Parker, R.M.; Williams, C.A.; Lim, K.T.P.; Frka-Petesic, B.; Vignolini, S. Printing of Responsive Photonic Cellulose Nanocrystal Microfilm Arrays. Adv. Funct. Mater. 2019, 29, 1804531. [Google Scholar] [CrossRef] [Green Version]
- Kose, O.; Tran, A.; Lewis, L.; Hamad, W.Y.; MacLachlan, M.J. Unwinding a Spiral of Cellulose Nanocrystals for Stimuli-Responsive Stretchable Optics. Nat. Commun. 2019, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, R.; Cranston, E.D.; Pelton, R.H. Stable Aqueous Foams from Cellulose Nanocrystals and Methyl Cellulose. Biomacromolecules 2016, 17, 4095–4099. [Google Scholar] [CrossRef]
- Tardy, B.L.; Richardson, J.J.; Greca, L.G.; Guo, J.; Ejima, H.; Rojas, O.J. Exploiting Supramolecular Interactions from Polymeric Colloids for Strong Anisotropic Adhesion between Solid Surfaces. Adv. Mater. 2020, 32, 1906886. [Google Scholar] [CrossRef] [Green Version]
- Espinha, A.; Guidetti, G.; Serrano, M.C.; Frka-Petesic, B.; Dumanli, A.G.; Hamad, W.Y.; Blanco, Á.; López, C.; Vignolini, S. Shape Memory Cellulose-Based Photonic Reflectors. ACS Appl. Mater. Interfaces 2016, 8, 31935–31940. [Google Scholar] [CrossRef] [Green Version]
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Reimer, M.; Zollfrank, C. Cellulose for Light Manipulation: Methods, Applications, and Prospects. Adv. Energy Mater. 2021, 2003866. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Heise, K.; Delepierre, G.; King, A.W.T.; Kostiainen, M.A.; Zoppe, J.; Weder, C.; Kontturi, E. Chemical Modification of Reducing End-Groups in Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 66–87. [Google Scholar] [CrossRef]
- Mohammadi, P.; Gandier, J.-A.; Wagermaier, W.; Miserez, A.; Penttilä, M. Bioinspired Functionally Graded Composite Assembled Using Cellulose Nanocrystals and Genetically Engineered Proteins with Controlled Biomineralization. Adv. Mater. 2021. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.R.; Morris, G.A.; de la Torre, J.G.; Ortega, A.; Mischnick, P.; Harding, S.E. Molecular Flexibility of Methylcelluloses of Differing Degree of Substitution by Combined Sedimentation and Viscosity Analysis. Macromol. Biosci. 2008, 8, 1108–1115. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Hiroe, M.; Asai, I.; Takahashi, R.; Nishinari, K. Thermal Aggregation of Methylcellulose with Different Molecular Weights. Food Hydrocoll. 2007, 21, 46–58. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Lott, J.R.; McAllister, J.W.; Zhang, J.; Bates, F.S.; Lodge, T.P.; Sammler, R.L.; Li, Y.; Brackhagen, M. Interplay of Phase Separation and Thermoreversible Gelation in Aqueous Methylcellulose Solutions. Macromolecules 2013, 46, 300–309. [Google Scholar] [CrossRef]
- McAllister, J.W.; Schmidt, P.W.; Dorfman, K.D.; Lodge, T.P.; Bates, F.S. Thermodynamics of Aqueous Methylcellulose Solutions. Macromolecules 2015, 48, 7205–7215. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimazaki, M.; Yamamoto, J. Thermoreversible Gelation and Phase Separation in Aqueous Methyl Cellulose Solutions. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 91–100. [Google Scholar] [CrossRef]
- Hynninen, V.; Hietala, S.; McKee, J.R.; Murtomäki, L.; Rojas, O.J.; Ikkala, O. Inverse Thermoreversible Mechanical Stiffening and Birefringence in a Methylcellulose/Cellulose Nanocrystal Hydrogel. Biomacromolecules 2018, 19, 2795–2804. [Google Scholar] [CrossRef]
- Hirrien, M.; Desbrières, J.; Rinaudo, M. Physical Properties of Methylcelluloses in Relation with the Conditions for Cellulose Modification. Carbohydr. Polym. 1996, 31, 243–252. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Influence of Molar Mass and Concentration on the Thermogelation of Methylcelluloses. Int. J. Polym. Anal. Character. 2015, 20, 110–118. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D Bioprinting of Highly Thixotropic Alginate/Methylcellulose Hydrogel with Strong Interface Bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097. [Google Scholar] [CrossRef] [PubMed]
- Negrini, N.C.; Bonetti, L.; Contili, L.; Farè, S. 3D Printing of Methylcellulose-Based Hydrogels. Bioprinting 2018, 10, e00024. [Google Scholar] [CrossRef]
- Hynninen, V.; Chandra, S.; Das, S.; Amini, M.; Dai, Y.; Lepikko, S.; Mohammadi, P.; Hietala, S.; Ras, R.H.A.; Sun, Z.; et al. Luminescent Gold Nanocluster-Methylcellulose Composite Optical Fibers with Low Attenuation Coefficient and High Photostability. Small 2021, 17, 2005205. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic Modulus of Single Cellulose Microfibrils from Tunicate Measured by Atomic Force Microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding Nanocellulose Chirality and Structure-Properties Relationship at the Single Fibril Level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyström, G.; Arcari, M.; Adamcik, J.; Usov, I.; Mezzenga, R. Nanocellulose Fragmentation Mechanisms and Inversion of Chirality from the Single Particle to the Cholesteric Phase. ACS Nano 2018, 12, 5141–5148. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Hamad, W.Y. Critical Insights into the Reinforcement Potential of Cellulose Nanocrystals in Polymer Nanocomposites. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100761. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pääkkönen, T.; Spiliopoulos, P.; Nonappa; Kontturi, K.S.; Penttilä, P.; Viljanen, M.; Svedström, K.; Kontturi,, E. Sustainable High Yield Route to Cellulose Nanocrystals from Bacterial Cellulose. ACS Sust. Chem. Eng. 2019, 7, 14384–14388. [Google Scholar] [CrossRef] [Green Version]
- Malho, J.M.; Morits, M.; Löbling, T.I.; Majoinen, J.; Schacher, F.H.; Ikkala, O.; Gröschel, A.H. Rod-Like Nanoparticles with Striped and Helical Topography. ACS Macro Lett. 2016, 5, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Majoinen, J.; Kontturi, E.; Ikkala, O.; Gray, D.G. SEM Imaging of Chiral Nematic Films Cast from Cellulose Nanocrystal Suspensions. Cellulose 2012, 19, 1599–1605. [Google Scholar] [CrossRef]
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J.P.F. Macroscopic Control of Helix Orientation in Films Dried from Cholesteric Liquid-Crystalline Cellulose Nanocrystal Suspensions. Chem. Phys. Chem 2014, 15, 1477–1484. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Radavidson, H.; Jean, B.; Heux, L. Dynamically Controlled Iridescence of Cholesteric Cellulose Nanocrystal Suspensions Using Electric Fields. Adv. Mater. 2017, 29, 1606208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardy, B.L.; Mattos, B.D.; Greca, L.G.; Kämäräinen, T.; Klockars, K.W.; Rojas, O.J. Tessellation of Chiral-Nematic Cellulose Nanocrystal Films by Microtemplating. Adv. Funct. Mater. 2019, 29, 1808518. [Google Scholar] [CrossRef]
- McKee, J.R.; Hietala, S.; Seitsonen, J.; Laine, J.; Kontturi, E.; Ikkala, O. Thermoresponsive Nanocellulose Hydrogels with Tunable Mechanical Properties. ACS Macro Lett. 2014, 3, 266–270. [Google Scholar] [CrossRef]
- Hynninen, V.; Mohammadi, P.; Wagermaier, W.; Hietala, S.; Linder, M.B.; Ikkala, O.; Nonappa. Methyl Cellulose/Cellulose Nanocrystal Nanocomposite Fibers with High Ductility. Eur. Polym. J. 2019, 112, 334–345. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A Protocol for Rheological Characterization of Hydrogels for Tissue Engineering Strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Jung, H.-S.; Kim, H.C.; Park, W.H. Robust Methylcellulose Hydrogels Reinforced with Chitin Nanocrystals. Carbohydr. Polym. 2019, 213, 311–319. [Google Scholar] [CrossRef]
- McAllister, J.W.; Lott, J.R.; Schmidt, P.W.; Sammler, R.L.; Bates, F.S.; Lodge, T.P. Linear and Nonlinear Rheological Behavior of Fibrillar Methylcellulose Hydrogels. ACS Macro Lett. 2015, 4, 538–542. [Google Scholar] [CrossRef]
- Morozova, S.; Coughlin, M.L.; Early, J.T.; Ertem, S.P.; Reineke, T.M.; Bates, F.S.; Lodge, T.P. Properties of Chemically Cross-Linked Methylcellulose Gels. Macromolecules 2019, 52, 7740–7748. [Google Scholar] [CrossRef]
- Chevillard, C.; Axelos, M.A.V. Phase Separation of Aqueous Solution of Methylcellulose. Coll. Polym. Sci. 1997, 275, 537–545. [Google Scholar] [CrossRef]
- Heymann, E. Studies on Sol-Gel Transformations. I. The Inverse Sol-Gel Transformation of Methylcellulose in Water. Trans. Farad. Soc. 1935, 31, 846–864. [Google Scholar] [CrossRef]
- Lott, J.R.; Mcallister, J.W.; Arvidson, S.A.; Bates, F.S.; Lodge, T.P. Fibrillar Structure of Methylcellulose Hydrogels. Biomacromolecules 2013, 14, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M. Aggregation and Network Formation of Aqueous Methylcellulose and Hydroxypropylmethylcellulose Solutions. Coll. Surf. A Physicochem. Eng. Asp. 2010, 354, 162–171. [Google Scholar] [CrossRef]
- Lott, J.R.; Mcallister, J.W.; Wasbrough, M.; Sammler, R.L.; Bates, F.S.; Lodge, T.P. Fibrillar Structure in Aqueous Methylcellulose Solutions and Gels. Macromolecules 2013, 46, 9760–9771. [Google Scholar] [CrossRef]
- Huang, W.; Ramesh, R.; Jha, P.K.; Larson, R.G. A Systematic Coarse-Grained Model for Methylcellulose Polymers: Spontaneous Ring Formation at Elevated Temperature. Macromolecules 2016, 49, 1490–1503. [Google Scholar] [CrossRef]
- Ginzburg, V.V.; Sammler, R.L.; Huang, W.; Larson, R.G. Anisotropic Self-Assembly and Gelation in Aqueous Methylcellulose-Theory and Modeling. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1624–1636. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bates, F.S.; Dorfman, K.D. Rapid Conformational Fluctuations in a Model of Methylcellulose. Phys. Rev. Mater. 2017, 1, 025604. [Google Scholar] [CrossRef]
- Sethuraman, V.; Dorfman, K.D. Simulating Precursor Steps for Fibril Formation in Methylcellulose Solutions. Phys.Rev. Mater. 2019, 3, 055601. [Google Scholar] [CrossRef]
- Kundu, P.P.; Kundu, M. Effect of Salts and Surfactant and Their Doses on the Gelation of Extremely Dilute Solutions of Methyl Cellulose. Polymer 2001, 42, 2015–2020. [Google Scholar] [CrossRef]
- Morozova, S.; Schmidt, P.W.; Metaxas, A.; Bates, F.S.; Lodge, T.P.; Dutcher, C.S. Extensional Flow Behavior of Methylcellulose Solutions Containing Fibrils. ACS Macro Lett. 2018, 7, 347–352. [Google Scholar] [CrossRef]
- Schmidt, P.W.; Morozova, S.; Ertem, S.P.; Coughlin, M.L.; Davidovich, I.; Talmon, Y.; Reineke, T.M.; Bates, F.S.; Lodge, T.P. Internal Structure of Methylcellulose Fibrils. Macromolecules 2020, 53, 398–405. [Google Scholar] [CrossRef]
- Morozova, S. Methylcellulose Fibrils: A Mini Review. Polym. Int. 2020, 69, 125–130. [Google Scholar] [CrossRef]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl Cellulose Solutions and Gels: Fibril Formation and Gelation Properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Bertula, K.; Martikainen, L.; Munne, P.; Hietala, S.; Klefström, J.; Ikkala, O. Nonappa Strain-Stiffening of Agarose Gels. ACS Macro Lett. 2019, 8, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Storm, C.; Pastore, J.J.; MacKintosh, F.C.; Lubensky, T.C.; Janmey, P.A. Nonlinear Elasticity in Biological Gels. Nature 2005, 435, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Janmey, P.A. Effects of Non-Linearity on Cell-ECM Interactions. Exp. Cell Res. 2013, 319, 2481–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Nishinari, K.; Zhang, H.; Funami, T. A Novel Liquid-Crystalline Phase in Dilute Aqueous Solutions of Methylcellulose. Macromol. Rapid Commun. 2006, 27, 971–975. [Google Scholar] [CrossRef]
- Gullapalli, R.P.; Sheth, B.B. Effect of Methylcellulose on the Stability of Oil-in-Water Emulsions. Int. J. Pharm. 1996, 1, 97–109. [Google Scholar] [CrossRef]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic Stabilization of Emulsions and Emulsion Gels with Water-Soluble Polymers and Cellulose Nanocrystals. ACS Sust. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Dubé, M.A.; Cranston, E.D. Cellulose Nanocrystals and Methyl Cellulose as Costabilizers for Nanocomposite Latexes with Double Morphology. ACS Sust. Chem. Eng. 2017, 5, 10509–10517. [Google Scholar] [CrossRef]
- Thiel, A.E.; Hartel, R.W.; Spicer, P.T. Fat Crystals Influence Methylcellulose Stabilization of Lipid Emulsions. J. Am. Oil Chem. Soc. 2017, 94, 325–331. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose Nanocrystal-Based Materials: From Liquid Crystal Self-Assembly and Glass Formation to Multifunctional Thin Films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef] [Green Version]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase Separation Behavior in Aqueous Suspensions of Bacterial Cellulose Nanocrystals Prepared by Sulfuric Acid Treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the Surface Sulfur Content of Cellulose Nanocrystals Prepared by Sulfuric Acid Hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef]
- Yeh, W.Y.; Young, R.J. Molecular Deformation Processes in Aromatic High Modulus Polymer Fibres. Polymer 1999, 40, 857–870. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Hoeger, I.; Rojas, O.J.; Efimenko, K.; Velev, O.D.; Kelley, S.S. Ultrathin Film Coatings of Aligned Cellulose Nanocrystals from a Convective-Shear Assembly System and Their Surface Mechanical Properties. Soft Matter 2011, 7, 1957–1967. [Google Scholar] [CrossRef]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic Polymer Brush-Modified Cellulose Nanocrystals for High-Affinity Virus Binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Cousin, F.; Putaux, J.L.; Jean, B. Temperature-Controlled Star-Shaped Cellulose Nanocrystal Assemblies Resulting from Asymmetric Polymer Grafting. ACS Macro Lett. 2019, 8, 345–351. [Google Scholar] [CrossRef]
- Morits, M.; Hynninen, V.; Niederberger, A.; Ikkala, O.; Gröschel, A.H.; Müllner, M. Polymer Brush Guided Templating on Well-Defined Rod-like Cellulose Nanocrystals. Polym. Chem. 2018, 9, 1650–1657. [Google Scholar] [CrossRef]
- Zare, Y. Study of Nanoparticles Aggregation/Agglomeration in Polymer Particulate Nanocomposites by Mechanical Properties. Compos. Part A Appl. Sci. Manufact. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Structure and Transformation of Tactoids in Cellulose Nanocrystal Suspensions. Nat. Commun. 2016, 7, 11515. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y. Electron Microdiffraction Reveals the Nanoscale Twist Geometry of Cellulose Nanocrystals. Nanoscale 2019, 11, 21767–21774. [Google Scholar] [CrossRef] [PubMed]
- Cranston, E.D.; Gray, D.G. Formation of Cellulose-Based Electrostatic Layer-by-Layer Films in a Magnetic Field. Sci. Technol. Adv. Mater. 2006, 7, 319–321. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shopsowitz, K.E.; Ahn, J.M.; Hamad, W.Y.; Maclachlan, M.J. Chiral Nematic Stained Glass: Controlling the Optical Properties of Nanocrystalline Cellulose-Templated Materials. Langmuir 2012, 28, 17256–17262. [Google Scholar] [CrossRef] [PubMed]
- Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Vignolini, S. Controlling the Photonic Properties of Cholesteric Cellulose Nanocrystal Films with Magnets. Adv. Mater. 2017, 29, 1701469. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Pan, H.; Ma, J.; Li, Y.; Bokhari, S.W.; Jiang, X.; Zhu, S.; Zhang, D. Cellulose Nanocrystals/Polyacrylamide Composites of High Sensitivity and Cycling Performance to Gauge Humidity. ACS Appl. Mater. Interfaces 2017, 9, 18231–18237. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Dai, Y.; Nguyen, T.-D.; Hamad, W.Y.; Maclachlan, M.J. Aerogel Materials with Periodic Structures Imprinted with Cellulose Nanocrystals. Nanoscale 2018, 10, 3805–3812. [Google Scholar] [CrossRef]
- Baxter, S.C.; Robinson, C.T. Pseudo-Percolation: Critical Volume Fractions and Mechanical Percolation in Polymer Nanocomposites. Compos. Sci. Technol. 2011, 71, 1273–1279. [Google Scholar] [CrossRef]
- Pakzad, A.; Simonsen, J.; Yassar, R.S. Gradient of Nanomechanical Properties in the Interphase of Cellulose Nanocrystal Composites. Compos. Sci. Technol. 2012, 72, 314–319. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the Properties of Cellulose Nanocrystals and Cellulose Nanofibrils Isolated from Bacteria, Tunicate, and Wood Processed Using Acid, Enzymatic, Mechanical, and Oxidative Methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose Nanomaterials as Green Nanoreinforcements for Polymer Nanocomposites. Phil. Trans. Roy. Soc. A 2018, 376, 20170040. [Google Scholar] [CrossRef] [PubMed]
- Hassanabadi, H.M.; Rodrigue, D. Effect of Particle Size and Shape on the Reinforcing Efficiency of Nanoparticles in Polymer Nanocomposites. Macromol. Mater. Eng. 2014, 299, 1220–1231. [Google Scholar] [CrossRef]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to Optical Methods for Characterizing Liquid Crystals at Interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratani, T.; Kose, O.; Hamad, W.Y.; Maclachlan, M.J. Stable and Sensitive Stimuli-Responsive Anisotropic Hydrogels for Sensing Ionic Strength and Pressure. Mater. Horiz. 2018, 5, 1076–1081. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent Semi-crystalline Polymeric Materials and Their Nanocomposites: A Review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki-Suonio, S.; Wagermaier, W.; Hynninen, V.; Hietala, S.; Ikkala, O. Interfacial Polyelectrolyte Complex Spinning of Cellulose Nanofibrils for Advanced Bicomponent Fibers. Biomacromolecules 2017, 18, 1293–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashkour, M.; Kimura, T.; Mashkour, M.; Kimura, F.; Tajvidi, M. Printing Birefringent Figures by Surface Tension-Directed Self-Assembly of a Cellulose Nanocrystal/Polymer Ink Components. ACS Appl. Mater. Interfaces 2019, 11, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Toivonen, M.S.; Ikkala, O.; Wagermaier, W.; Linder, M.B. Aligning Cellulose Nanofibril Dispersions for Tougher Fibers. Sci. Rep. 2017, 7, 11860. [Google Scholar] [CrossRef]
- Mujica-Garcia, A.; Hooshmand, S.; Skrifvars, M.; Kenny, J.M.; Oksman, K.; Peponi, L. Poly(Lactic Acid) Melt-Spun Fibers Reinforced with Functionalized Cellulose Nanocrystals. RSC Adv. 2016, 6, 9221–9231. [Google Scholar] [CrossRef]
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Brown, P.J.; Kitchens, C.L. Effect of Jet Stretch and Particle Load on Cellulose Nanocrystal-Alginate Nanocomposite Fibers. Langmuir 2010, 26, 14263–14270. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Benavides, E.E.; Kitchens, C.L. Cellulose Nanocrystal Reinforced Alginate Fibers—Biomimicry Meets Polymer Processing. Mol. Cryst. Liq. Cryst. 2012, 556, 275–287. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Kitchens, C.L. Wide-Angle X-ray Diffraction of Cellulose Nanocrystal−Alginate Nanocomposite Fibers. Macromolecules 2011, 44, 3478–3484. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Sun, F.; Xiao, R.; Guo, Y.; Song, J. Liquid Crystal Microphase Separation of Cellulose Nanocrystals in Wet-Spun PVA Composite Fibers. RSC Adv. 2014, 4, 30784–30789. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Yu, H.; Ma, C.; Yao, J. Biomimicking the Structure of Silk Fibers via Cellulose Nanocrystal as β-Sheet Crystallite. RSC Adv. 2014, 4, 14304–14313. [Google Scholar] [CrossRef]
- Wang, L.; Lundahl, M.J.; Greca, L.G.; Papageorgiou, A.C.; Borghei, M.; Rojas, O.J. Effects of Non-Solvents and Electrolytes on the Formation and Properties of Cellulose I Filaments. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y. Fundamentals of Plastic Optical Fibers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527410064. [Google Scholar]
- Nazempour, R.; Zhang, Q.; Fu, R.; Sheng, X. Biocompatible and Implantable Optical Fibers and Waveguides for Biomedicine. Materials 2018, 11, 1283. [Google Scholar] [CrossRef] [Green Version]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical Cellulose Fiber Made from Regenerated Cellulose and Cellulose Acetate for Water Sensor Applications. Cellulose 2020, 27, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.T.; Domachuk, P.; Amsden, J.; Bressner, J.; Lewis, J.A.; Kaplan, D.L.; Omenetto, F.G. Biocompatible Silk Printed Optical Waveguides. Adv. Mater. 2009, 21, 2411–2415. [Google Scholar] [CrossRef]
- Jain, A.; Yang, A.H.J.; Erickson, D. Gel-Based Optical Waveguides with Live Cell Encapsulation and Integrated Microfluidics. Opt. Lett. 2012, 37, 1472. [Google Scholar] [CrossRef]
- Choi, M.; Choi, J.W.; Kim, S.; Nizamoglu, S.; Hahn, S.K.; Yun, S.H. Light-Guiding Hydrogels for Cell-Based Sensing and Optogenetic Synthesis In Vivo. Nat. Photonics 2013, 7, 987–994. [Google Scholar] [CrossRef]
- Applegate, M.B.; Perotto, G.; Kaplan, D.L.; Omenetto, F.G. Biocompatible Silk Step-Index Optical Waveguides. Biomed. Opt. Exp. 2015, 6, 4221–4227. [Google Scholar] [CrossRef] [Green Version]
- Kujala, S.; Mannila, A.; Karvonen, L.; Kieu, K.; Sun, Z. Natural Silk as a Photonics Component: A Study on Its Light Guiding and Nonlinear Optical Properties. Sci. Rep. 2016, 6, 22358. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Z.F.; Tam, H.-Y.; Tao, X. Multifunctional Smart Optical Fibers: Materials, Fabrication, and Sensing Applications. Photonics 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, A.; Guo, N.; Gao, Y.; Godbout, N.; Lacroix, S.; Dubois, C.; Skorobogatiy, M. Prospective for Biodegradable Microstructured Optical Fibers. Opt. Lett. 2007, 32, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Lokman, A.; Nodehi, S.; Batumalay, M.; Arof, H.; Ahmad, H.; Harun, S.W. Optical Fiber Humidity Sensor Based on a Tapered Fiber with Hydroxyethylcellulose/Polyvinylidenefluoride Composite. Micro. Opt. Technol. Lett. 2014, 56, 380–382. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Dick, C.; Meng, L.; Mahpeykar, S.M.; Ahvazi, B.; Wang, X. Cellulose Nanocrystals as Host Matrix and Waveguide Materials for Recyclable Luminescent Solar Concentrators. RSC Adv. 2017, 7, 32436–32441. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, J.; Sun, H.; Hong, D.; Li, L.; Yang, Y.; Yong, X.; Zhang, C.; Cui, J. An Optical Fiber Relative Humidity Sensor Based on Hollow-Core Fiber and Hydroxypropyl Methylcellulose Hydrogel Film. Optik 2019, 195, 163172. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Klar, V.; Wang, L.; Ago, M.; Rojas, O.J. Spinning of Cellulose Nanofibrils into Filaments: A Review. Indust. Eng. Chem. Res. 2017, 56, 8–19. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.-H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef]
- Fujiwara, E.; Cabral, T.D.; Sato, M.; Oku, H.; Cordeiro, C.M.B. Agarose-Based Structured Optical Fibre. Sci. Rep. 2020, 10, 7035. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. A New Route for Silk. Nat. Photonics 2008, 2, 641–643. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Li, B. Bacteria-Based Branched Structures for Bionanophotonics. Laser Photonics Rev. 2015, 9, 554–563. [Google Scholar] [CrossRef]
- Bezryadina, A.; Hansson, T.; Gautam, R.; Wetzel, B.; Siggins, G.; Kalmbach, A.; Lamstein, J.; Gallardo, D.; Carpenter, E.J.; Ichimura, A.; et al. Nonlinear Self-Action of Light through Biological Suspensions. Phys. Rev. Lett. 2017, 119, 058101. [Google Scholar] [CrossRef] [Green Version]
- Huby, N.; Vié, V.; Renault, A.; Beaufils, S.; Lefèvre, T.; Paquet-Mercier, F.; Pézolet, M.; Bêche, B. Native Spider Silk as a Biological Optical Fiber. Appl. Phys. Lett. 2013, 102, 123702. [Google Scholar] [CrossRef] [Green Version]
- Su, I.; Buehler, M.J. Spider Silk: Dynamic Mechanics. Nat. Mater. 2016, 15, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Qian, Z.; Li, J.; Sun, H.; Han, Y.; Xia, X.; Zhou, J.; Wang, C.; Wang, Y.; Wang, C. Synthetic Engineering of Spider Silk Fiber as Implantable Optical Waveguides for Low-Loss Light Guiding. ACS Appl. Mater. Interfaces 2017, 9, 14665–14676. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef] [PubMed]
- Nonappa, N. Luminescent Gold Nanoclusters for Bioimaging Applications. Beil. J. Nanotechnol. 2020, 11, 533–546. [Google Scholar] [CrossRef]
- Chandra, S.; Nonappa, N.; Beaune, G.; Som, A.; Zhou, S.; Lahtinen, J.; Jiang, H.; Timonen, J.V.I.; Ikkala, O.; Ras, R.H.A. Highly Luminescent Gold Nanocluster Frameworks. Adv. Opt. Mater. 2019, 7, 1900620. [Google Scholar] [CrossRef]
- Rival, J.V.; Mymoona, P.; Lakshmi, K.M.; Nonappa, N.; Pradeep, T.; Shibu, E.S. Self-Assembly of Precision Noble Metal Nanoclusters: Hierarchical Structural Complexity, Colloidal Superstructures, and Applications. Small 2021, 17, 2005718. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hynninen, V.; Patrakka, J.; Nonappa. Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers. Materials 2021, 14, 5137. https://doi.org/10.3390/ma14185137
Hynninen V, Patrakka J, Nonappa. Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers. Materials. 2021; 14(18):5137. https://doi.org/10.3390/ma14185137
Chicago/Turabian StyleHynninen, Ville, Jani Patrakka, and Nonappa. 2021. "Methylcellulose–Cellulose Nanocrystal Composites for Optomechanically Tunable Hydrogels and Fibers" Materials 14, no. 18: 5137. https://doi.org/10.3390/ma14185137




