Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Urease Enzyme
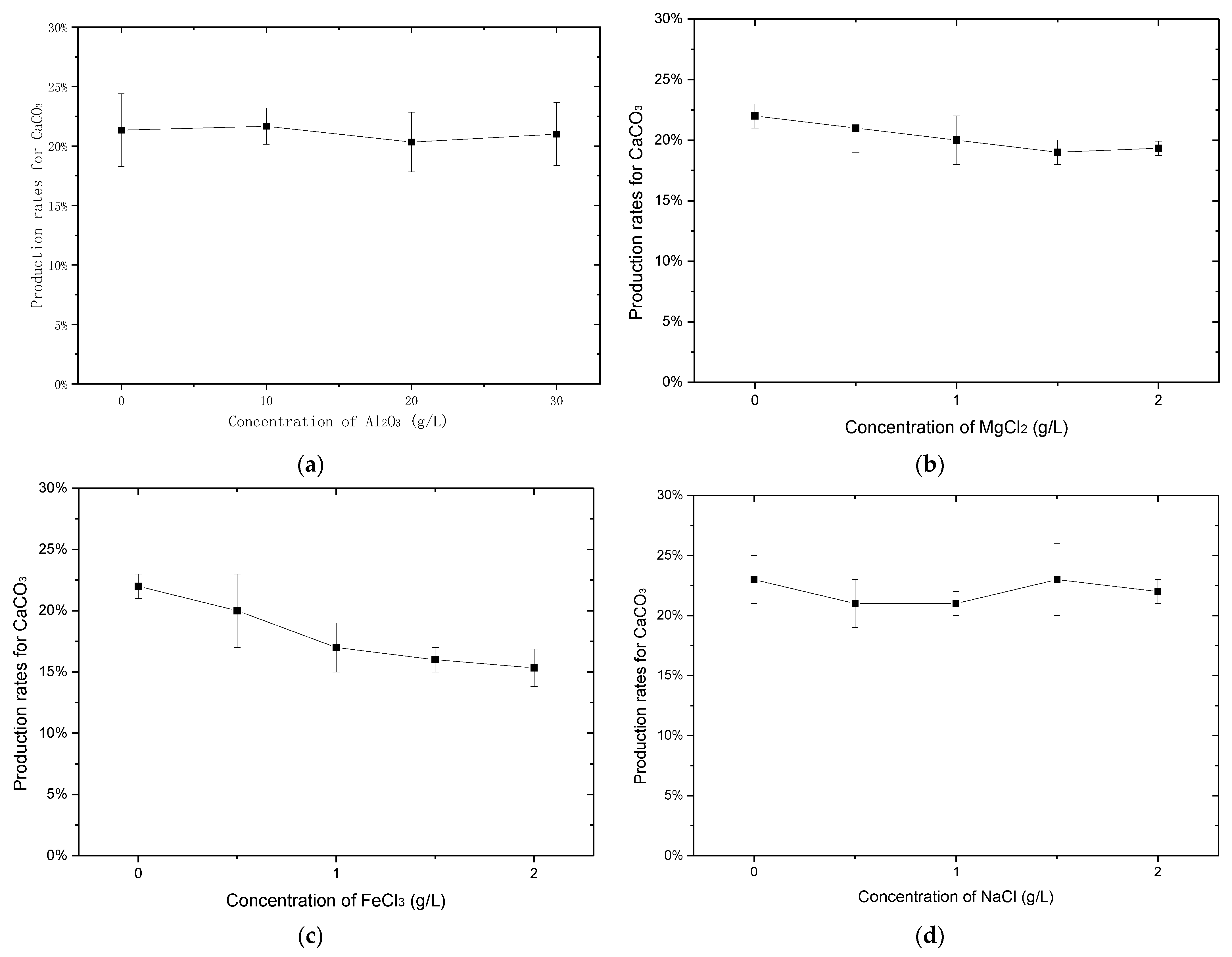
2.2. Effect of the Ions in Kaolin Clay on Production of CaCO3
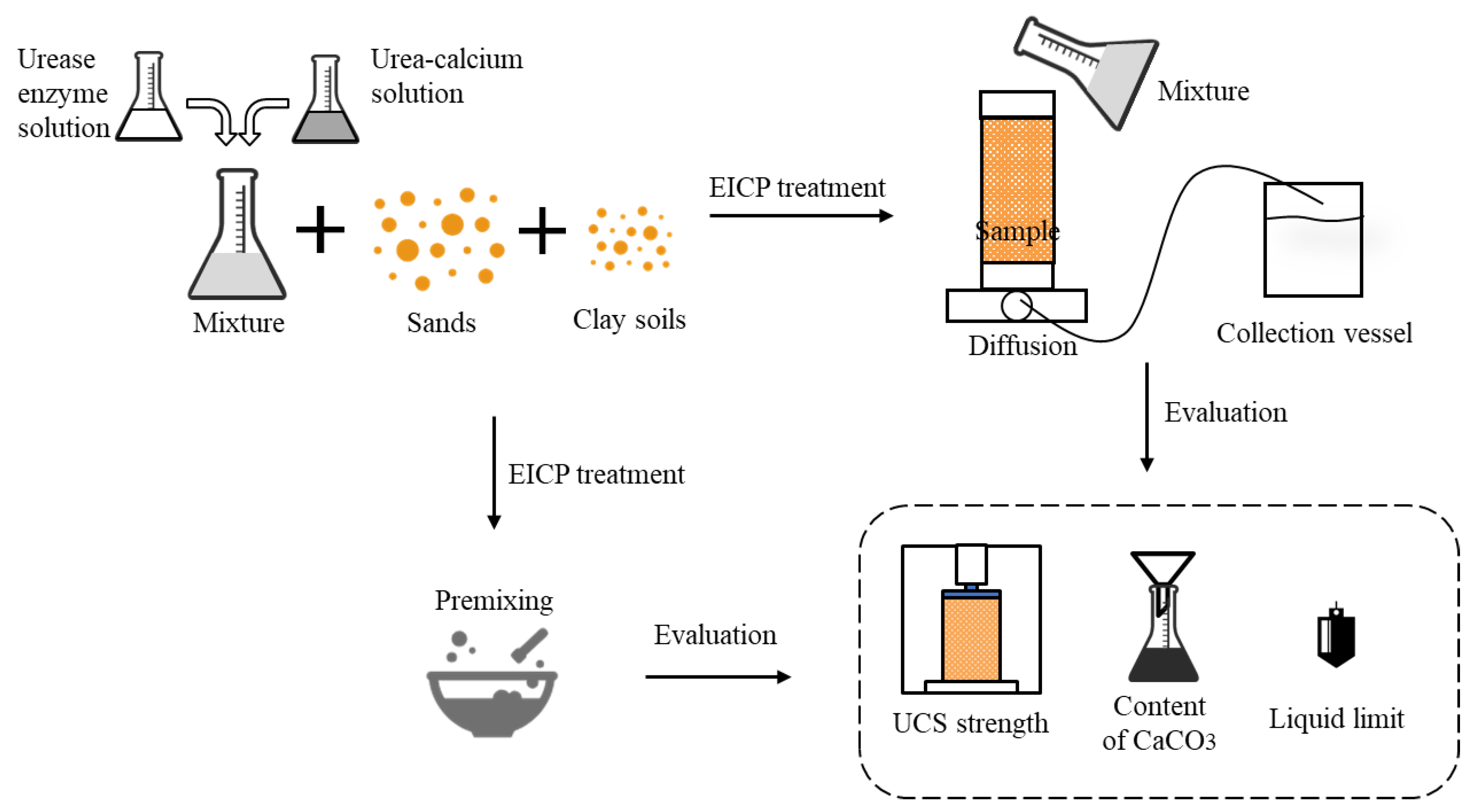
2.3. EICP Sand–Clay Mixtures Solidification in PVC Cylinders
2.3.1. Sand–Clay Mixture Preparations
2.3.2. EICP Treatment
2.3.3. UCS and Content of CaCO3
2.3.4. Liquid Limit
2.3.5. Scanning Electron Microscope Test
3. Results and Discussions
3.1. Effect of the Ions in Kaolin Clay on Production of CaCO3
3.1.1. Effect of Al2O3 on the Production Rate for CaCO3
3.1.2. Effect of NaCl, MgCl2 and FeCl3 on the Production Rate for Calcium Carbonate
3.2. EICP Sand–Clay Mixtures Solidification in PVC Cylinders
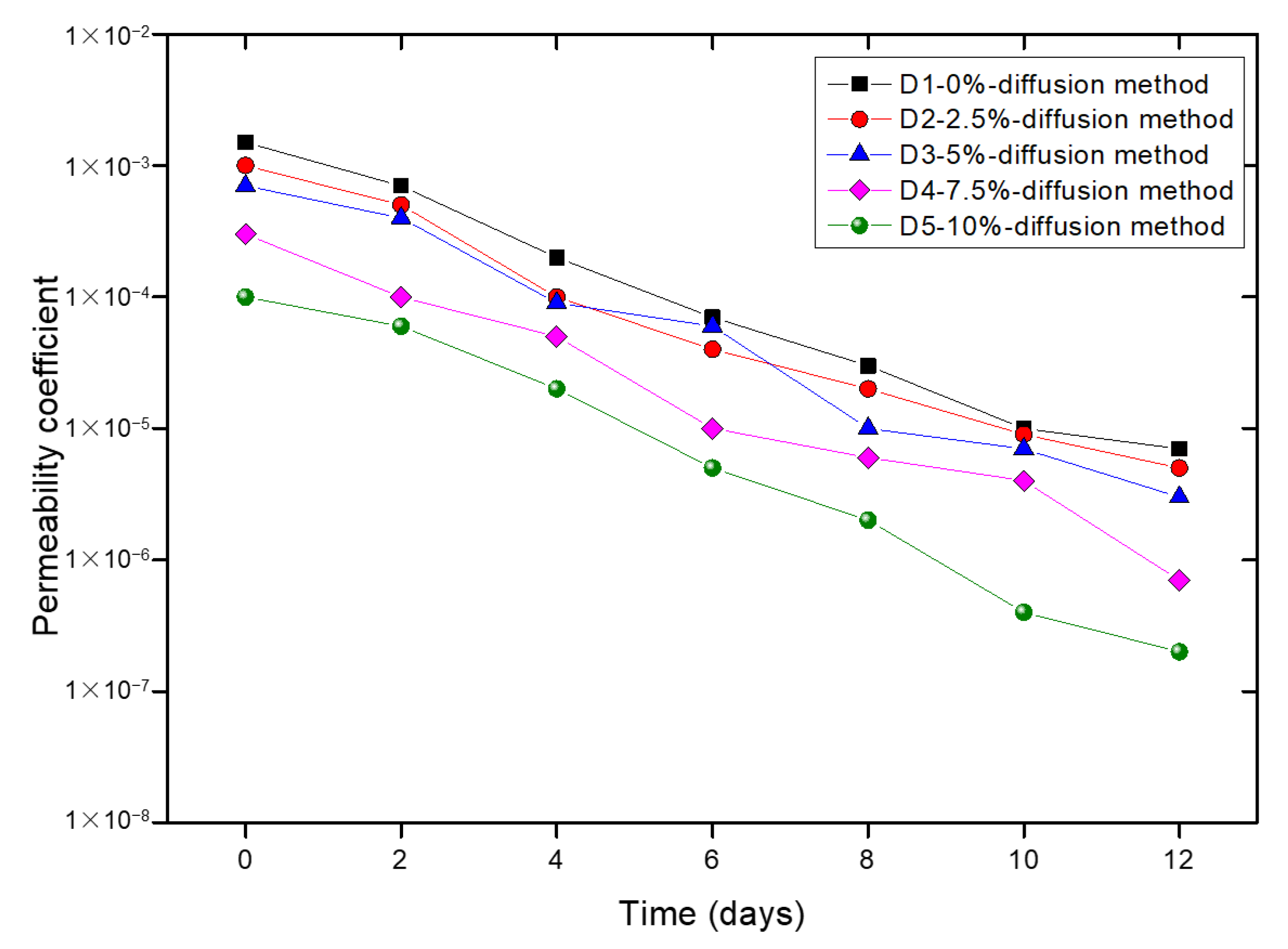
3.2.1. Permeability
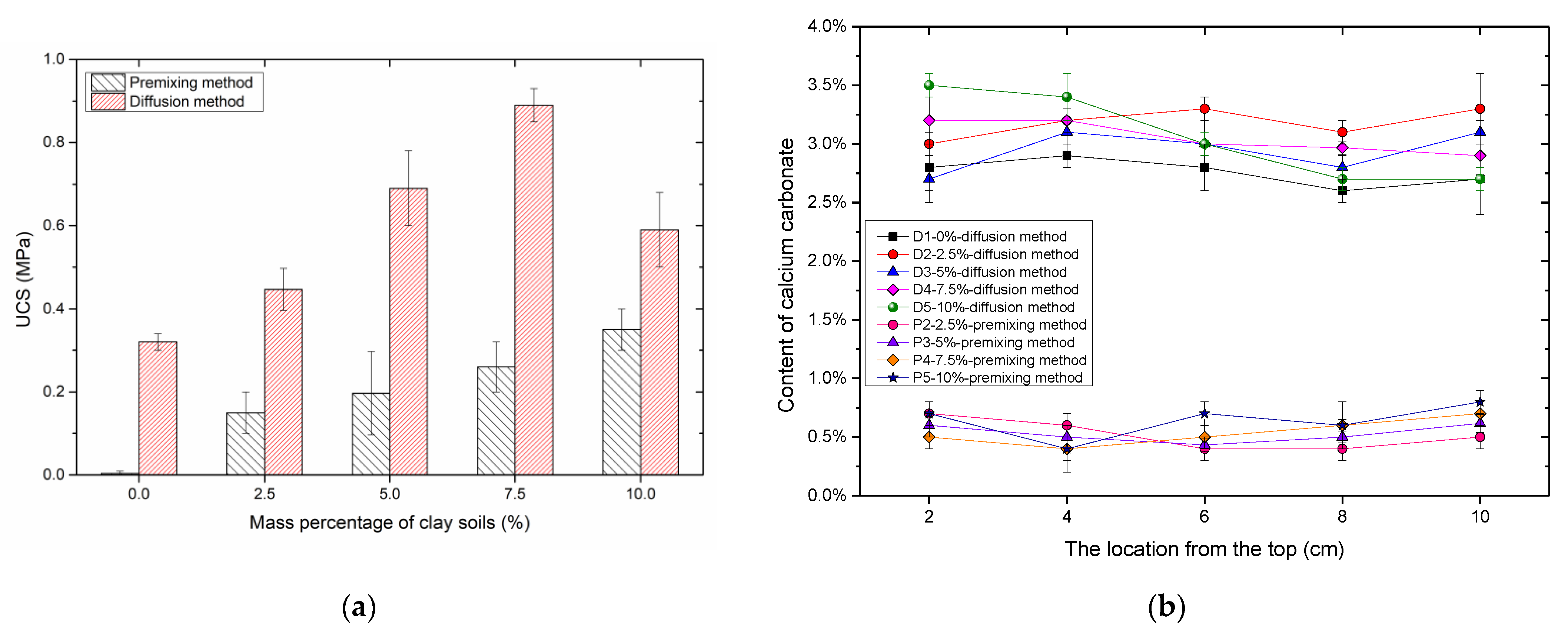
3.2.2. UCS and Content of Precipitation
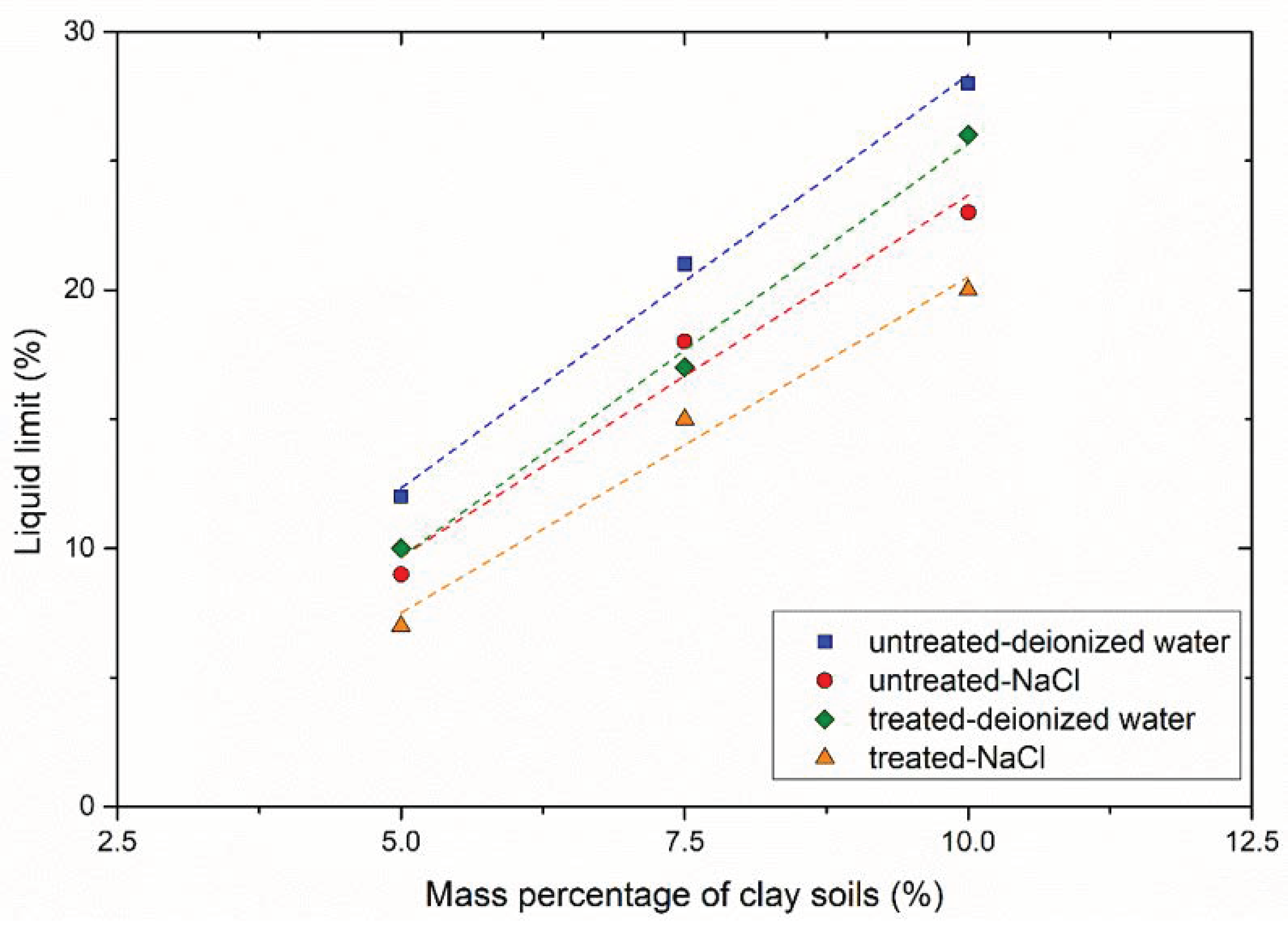
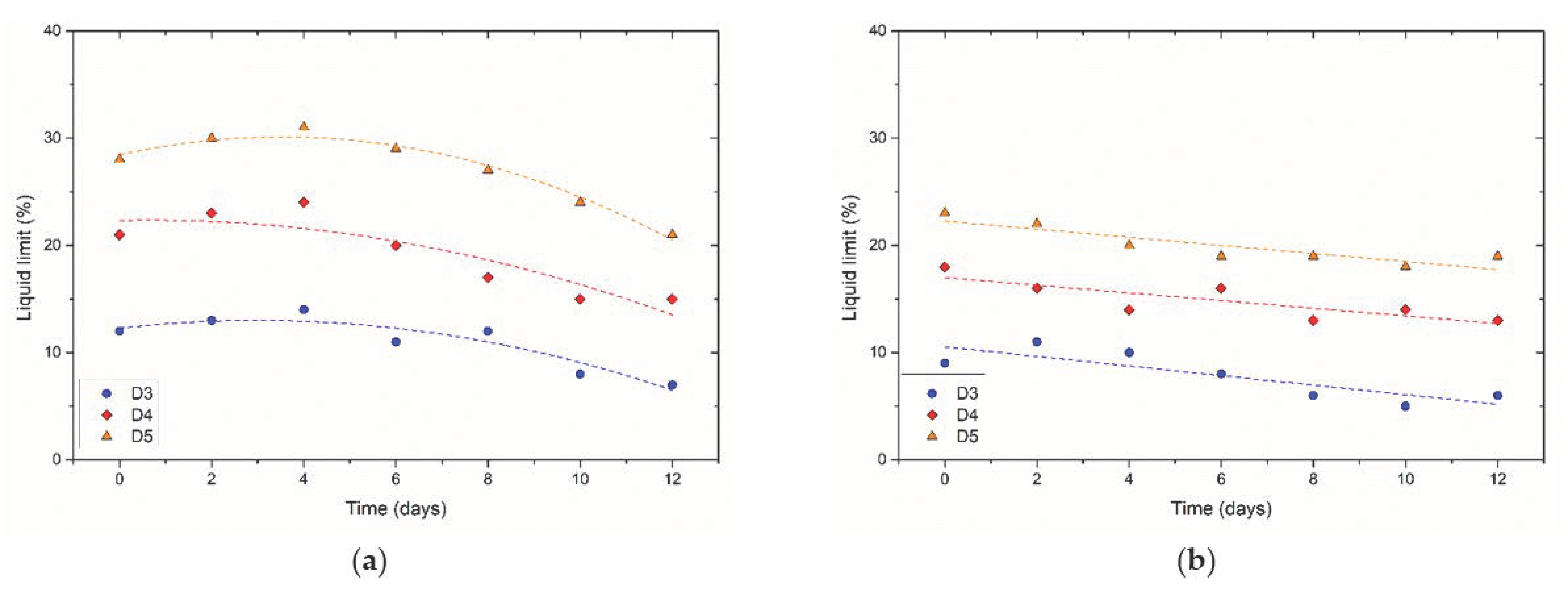
3.2.3. Soil Consistency
3.2.4. Scanning Electron Microscope Test
3.2.5. Applications and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dejong, J. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Soon, N.; Lee, L.; Khun, T.; Ling, H. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of Enzymatic Calcification for dust control and rainfall-erosion resistance improvement. Sci. Total Environ. 2020, 759, 143468. [Google Scholar] [CrossRef]
- Whiffin, V.; van Paassen, L.; Harkes, M. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Improvement of Microbial-Induced Calcium Carbonate Precipitation Technology for Sand Solidification. J. Mater. Civ. Eng. 2018, 30, 04018301. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Wang, H. Theoretical quantification for cracks repair based on microbially induced carbonate precipitation (micp) method. Cem. Concr. Compos. 2021, 1, 103950. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Yin, W.; Wu, L. Mineralization crust field experiment for desert sand solidification based on enzymatic calcification. J. Environ. Manag. 2021, 287, 112315. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Unno, T. Distribution of mineralized carbonate and its quantification method in enzyme mediated calcite precipitation technique. Soils Found. 2015, 55, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, C.; Zanetti, S.; Grunewald, E.; Walsh, D.; Codd, S.; Phillips, A. Detecting microbially induced calcite precipitation in a model well-bore using downhole low-field NMR. Environ. Sci. Technol. 2017, 51, 1537–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Suleiman, M.; Brown, D.; Kavazanjian, E. Mechanical behavior of sands treated by microbially induced carbonate precipitation. J. Geotech. Geoenviron. Eng. 2015, 142, 04015066. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Zamani, A.; Montoya, B. Permeability Reduction Due to Microbial Induced Calcite Precipitation in Sand. Geo-Chicago 2016, 2016, 94–103. [Google Scholar]
- Morales, L.; Garzón, E.; Romero, E.; Sánchez-Soto, P. Microbiological induced carbonate (CaCO3) precipitation using clay phyllites to replace chemical stabilizers (cement or lime). Appl. Clay Sci. 2019, 174, 15–28. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M. Assessment of different treatment methods by microbialinduced calcite precipitation for clayey soil improvement. In Proceedings of the 68th Canadian Geotechnical Conference, GeoQuebec, Quebec, QC, Canada, 21–23 September 2015. [Google Scholar]
- Cardoso, R.; Pires, I.; Duarte, S.; Monteiro, G. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. Effects of Different Clay’s Percentages on Improvement of Sand-Clay Mixtures with Microbially Induced Calcite Precipitation. Geomicrobiol. J. 2019, 36, 1–9. [Google Scholar] [CrossRef]
- Terzis, D.; Bernier-Latmani, R.; Laloui, L. Fabric characteristics and mechanical response of bio-improved sand to various treatment conditions. Geotech. Lett. 2016, 6, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, N. Applications of Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2015. [Google Scholar]
- Ismail, M.; Joer, H.; Randolph, M.; Merit, A. Cementation of porous materials using calcite. Géotechnique 2002, 52, 313–324. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wu, L.; Wang, C. Study of magnesium precipitation based on biocementation. Mar. Georesources Geotechnol. 2019, 37, 1257–1266. [Google Scholar] [CrossRef]
- Whiffin, V. Microbial CaCO3 Precipitation for the Production of Biocement; Murdoch University: Perth, Australia, 2004. [Google Scholar]
- Fidaleo, M.; Lavecchia, R. Kinetic study of enzymatic urea hydrolysis in the pH range 4–9. Chem. Biochem. Eng. Q 2003, 17, 311–318. [Google Scholar]
- Perry, D. Handbook of Inorganic Compounds, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2018, 14, 627–638. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Chen, R.; Guo, X. Improvement of characteristics and freeze-thaw durability of solidified loess based on microbially induced carbonate precipitation. Bulletin of Engineering Geology and the Environment. Sumner, J. (1926). The isolation and crystallization of the enzyme urease. J. Biol. Chem. 2021, 69, 435–441. [Google Scholar]
- Chang, I.; Kwon, Y.; Im, J.; Choi, G. Soil consistency and inter-particle characteristics of xanthan gum biopolymer containing soils with pore-fluid variation. Can. Geotech. J. 2019, 56, 1206–1213. [Google Scholar] [CrossRef]
- Jang, J.; Santamarina, J. Fines classification based on sensitivity to pore-fluid chemistry. J. Geotech. Geoenviron. Eng. 2016, 142. [Google Scholar] [CrossRef] [Green Version]
- Al-Thawadi, S. Ureolytic bacteria and calcium carbonate formation as a mechanism of strength enhancement of sand. J. Adv. Sci. Eng. Res. 2011, 1, 98–114. [Google Scholar]
- Sondi, I.; Matijevic, E. Homogeneous precipitation of calcium carbonates by enzyme catalyzed reaction. J. Colloid Interface Sci. 2001, 238, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Lara, E.; Mitchell, E. Checklist, diversity and distribution of testate amoebae in Chile. Eur. J. Protistol. 2015, 51, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Iler, R. The Chemistry of Silica; Plenum Press: New York, NY, USA, 1979; ISBN 978-0-471-02404-0. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.; Bang, S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. Adding Aluminum Oxide to Improve the Repairing Effect of Cracks Based on Bio-Remediation. J. Adv. Concr. Technol. 2019, 17, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concr. Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Gowthaman, S.; Chen, M.; Nakashima, K.; Kawasaki, S. Effect of Scallop Powder Addition on MICP Treatment of Amorphous Peat. Frontiers 2021, 9, 690376. [Google Scholar] [CrossRef]
- Islam, M.; Chittoori, B.; Burbank, M. Evaluating the applicability of biostimulated calcium carbonate precipitation to stabilize clayey soils. J. Mater. Civ. Eng. 2020, 32, 04019369. [Google Scholar] [CrossRef]
- Mahawish, A.; Bouazza, A.; Gates, W. Effect of particle size distribution on the bio-cementation of coarse aggregates. Acta Geotech. 2017, 13, 1019–1025. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Cheng, X. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Li, B. Geotechnical Properties of Biocement Treated Sand and Clay; Civil and Environmental Engineering, Nanyang Technological University: Singapore, 2015. [Google Scholar]
- Ural, N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: An overview. Open Geosci. 2021, 13, 197–218. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, Y.; Cai, H.; Wang, Z.; Yang, T.; Wang, Q.; Wu, S. Microbial-induced carbonate precipitation for strengthening soft clay. Adv. Mater. Sci. Eng. 2020, 2020, 8140724. [Google Scholar] [CrossRef] [Green Version]

| Sample No. | Mass Percentages Of Kaolin Clay | Mass of Sands (g) | Mass of Clay Soils (g) | Treatment Cycle | Treatment State |
|---|---|---|---|---|---|
| U1 | 0% | 300 | 0 | / | Untreated |
| P1 | 300 | 0 | 1 | Premixing treated | |
| D1 | 300 | 0 | 6 | Diffusion treated | |
| U2 | 2.5% | 292.5 | 7.5 | / | Untreated |
| P2 | 292.5 | 7.5 | 1 | Premixing treated | |
| D2 | 292.5 | 7.5 | 6 | Diffusion treated | |
| U3 | 5% | 285 | 15 | / | Untreated |
| P3 | 285 | 15 | 1 | Premixing treated | |
| D3 | 285 | 15 | 6 | Diffusion treated | |
| U4 | 7.5% | 277.5 | 22.5 | / | Untreated |
| P4 | 277.5 | 22.5 | 1 | Premixing treated | |
| D4 | 277.5 | 22.5 | 6 | Diffusion treated | |
| U5 | 10% | 270 | 30 | / | Untreated |
| P5 | 270 | 30 | 1 | Premixing treated | |
| D5 | 270 | 30 | 6 | Diffusion treated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Y.; Yue, S.; Zhou, Q.; Liu, X. Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation. Materials 2021, 14, 5140. https://doi.org/10.3390/ma14185140
Mo Y, Yue S, Zhou Q, Liu X. Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation. Materials. 2021; 14(18):5140. https://doi.org/10.3390/ma14185140
Chicago/Turabian StyleMo, Yixin, Songlin Yue, Qizhen Zhou, and Xiao Liu. 2021. "Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation" Materials 14, no. 18: 5140. https://doi.org/10.3390/ma14185140
APA StyleMo, Y., Yue, S., Zhou, Q., & Liu, X. (2021). Improvement and Soil Consistency of Sand–Clay Mixtures Treated with Enzymatic-Induced Carbonate Precipitation. Materials, 14(18), 5140. https://doi.org/10.3390/ma14185140






