Oxidation of MBE-Grown ZnTe and ZnTe/Zn Nanowires and Their Structural Properties
Abstract
:1. Introduction
2. Materials and Methods
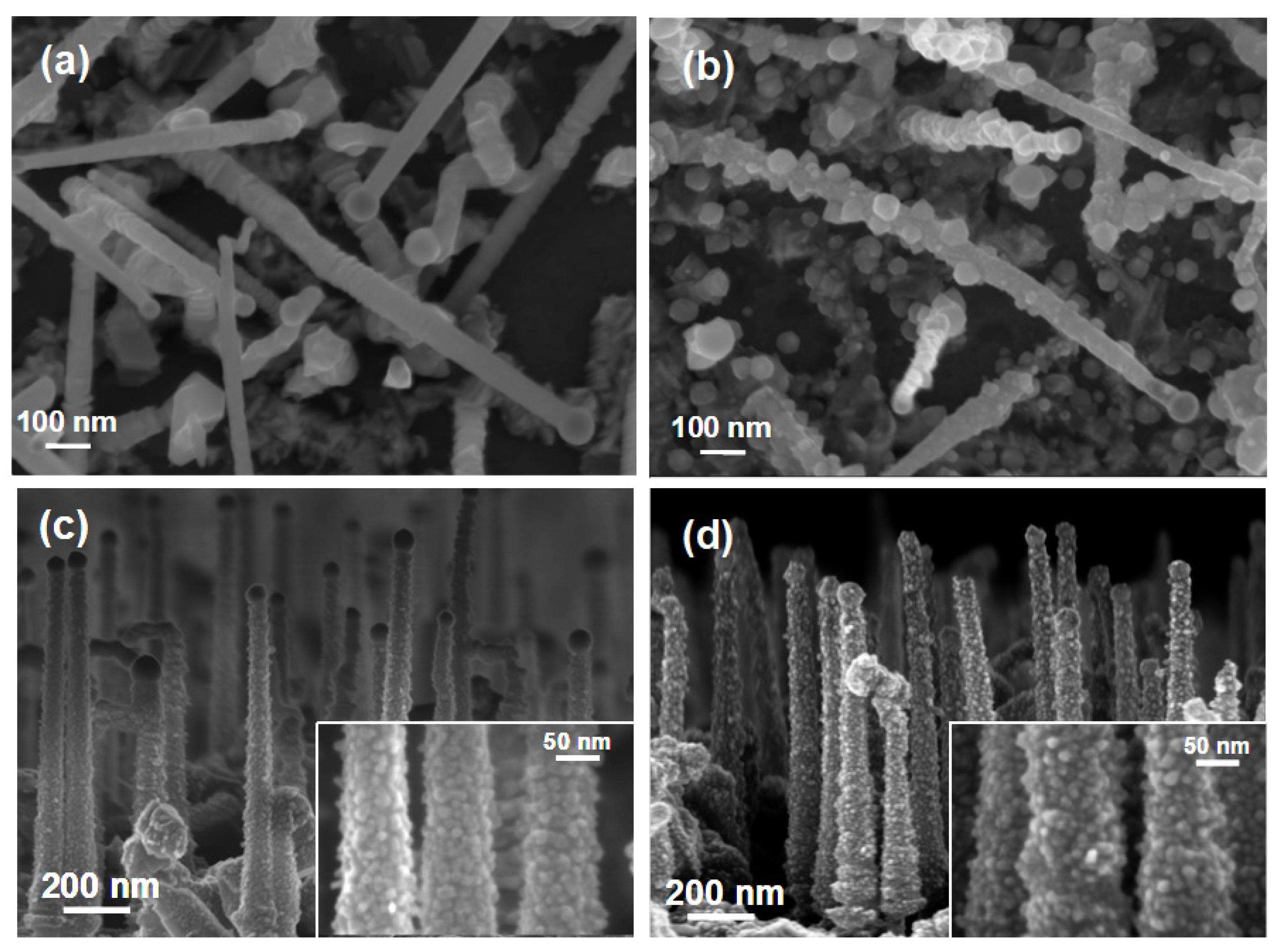
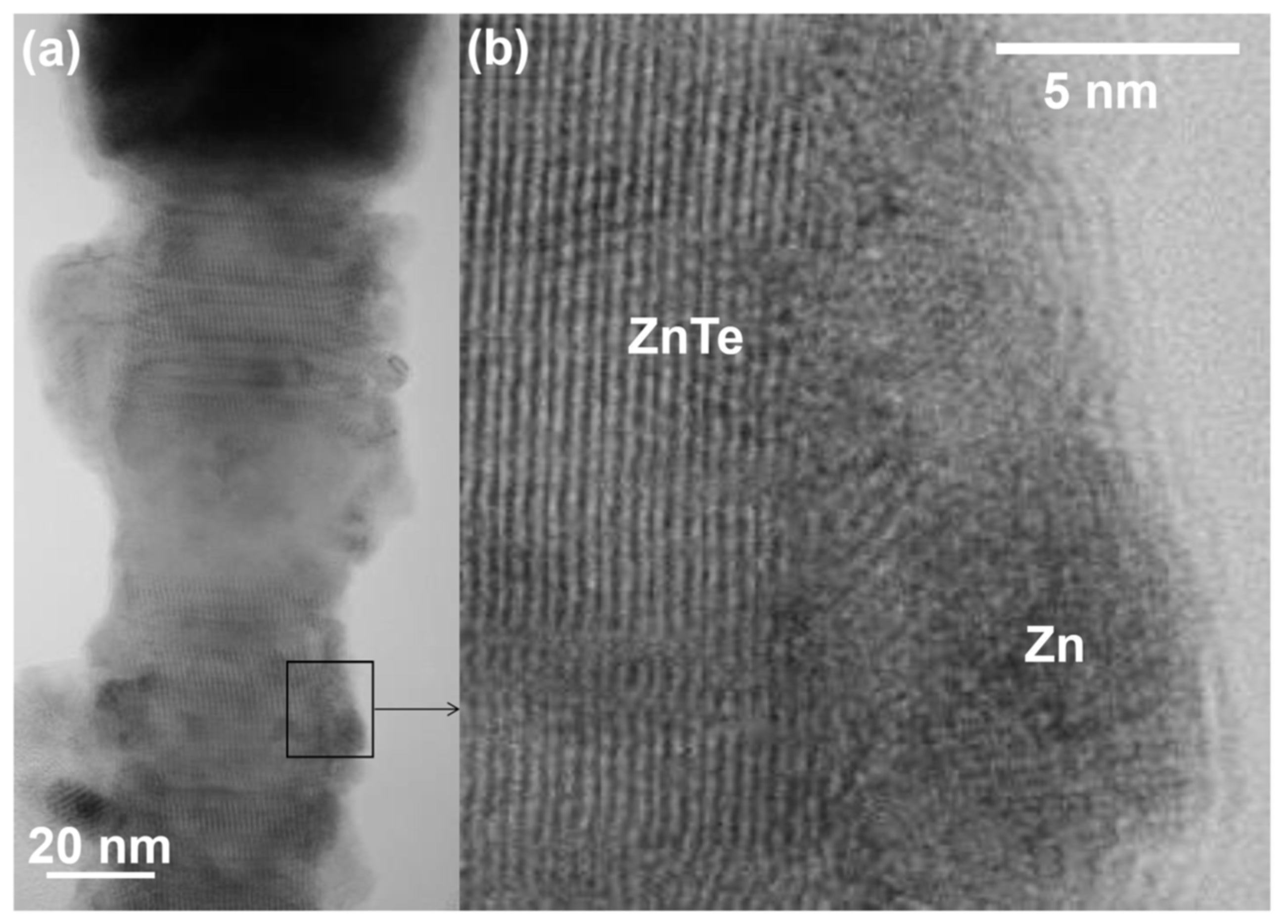
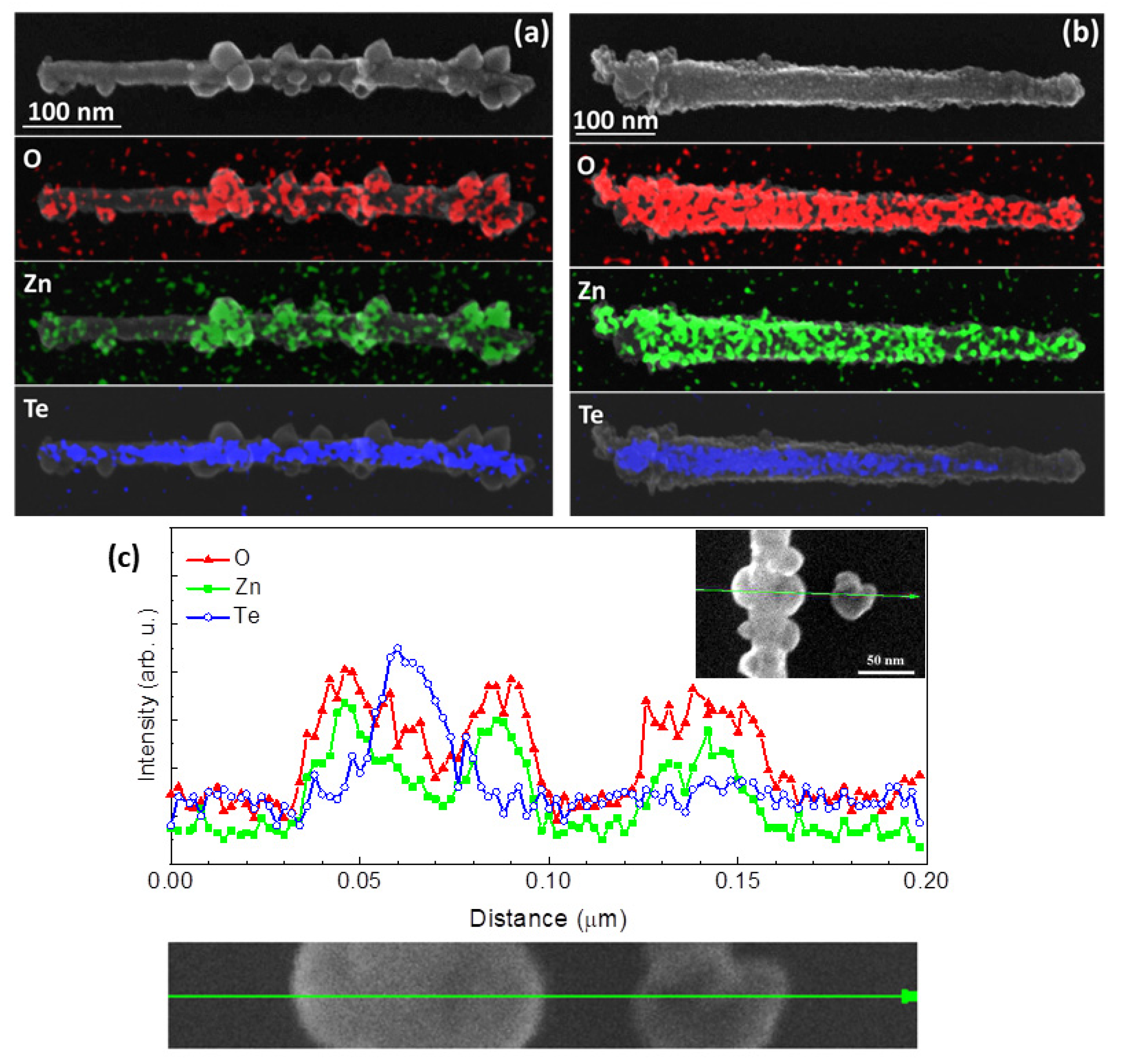
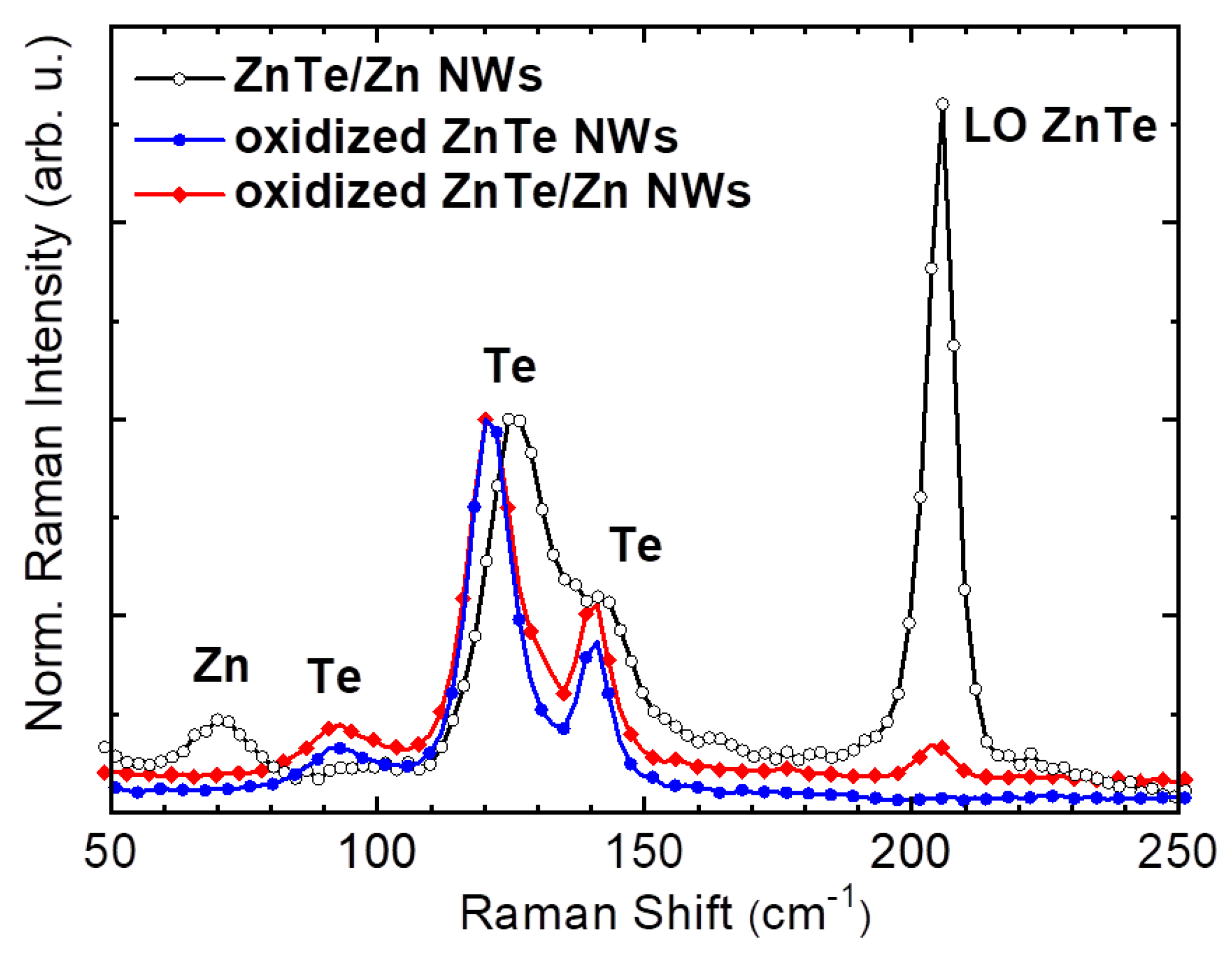
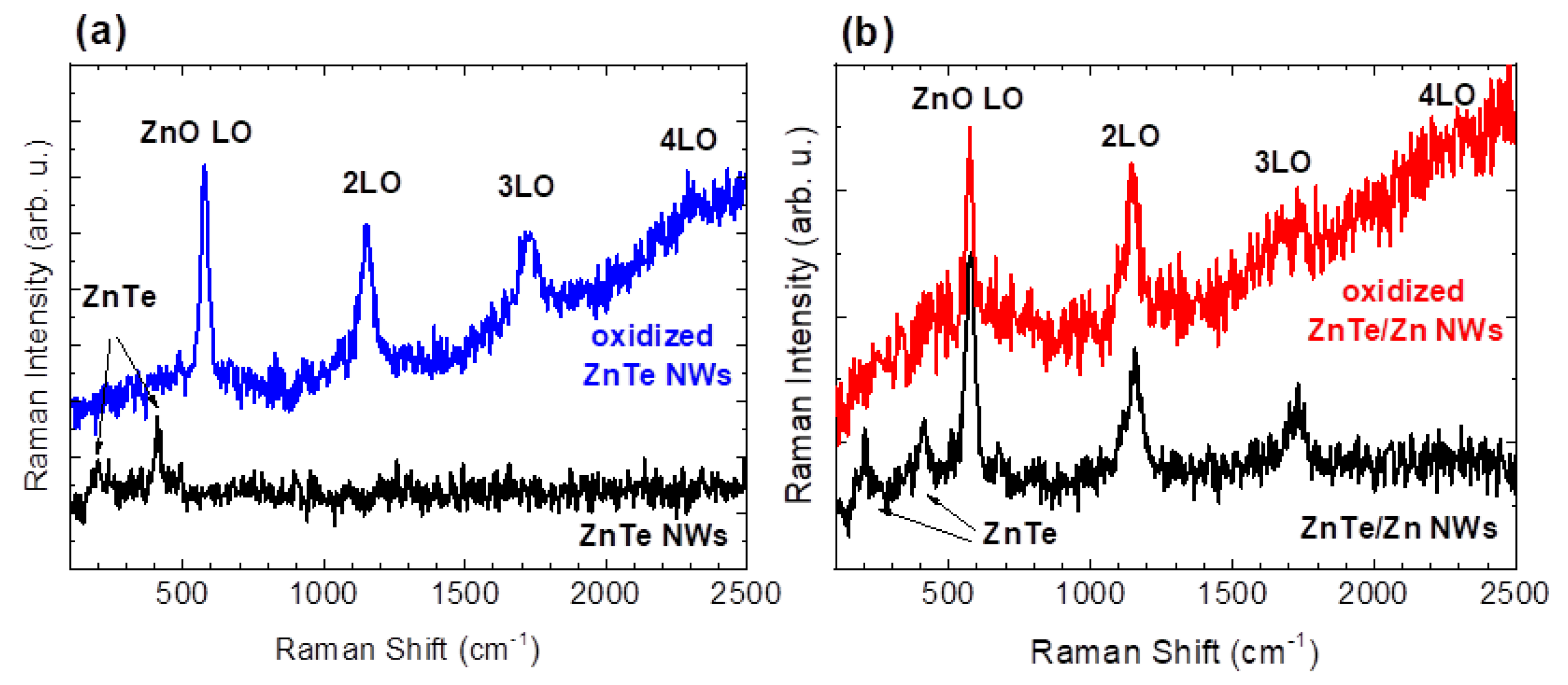
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, A.; Zheng, G.; Lieber, C. Nanowires: Building Blocks for Nanoscience and Nanotechnology; NanoScience and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-41979-4. [Google Scholar]
- Sobanska, M.; Zytkiewicz, Z.R.; Ekielski, M.; Klosek, K.; Sokolovskii, A.S.; Dubrovskii, V.G. Surface Diffusion of Gallium as the Origin of Inhomogeneity in Selective Area Growth of GaN Nanowires on AlxOy Nucleation Stripes. Cryst. Growth Des. 2020, 20, 4770–4778. [Google Scholar] [CrossRef]
- Calleja, E.; Ristić, J.; Fernández-Garrido, S.; Cerutti, L.; Sánchez-García, M.A.; Grandal, J.; Trampert, A.; Jahn, U.; Sánchez, G.; Griol, A.; et al. Growth, Morphology, and Structural Properties of Group-III-Nitride Nanocolumns and Nanodisks. Phys. Status Solidi 2007, 244, 2816–2837. [Google Scholar] [CrossRef]
- Gas, K.; Sadowski, J.; Kasama, T.; Siusys, A.; Zaleszczyk, W.; Wojciechowski, T.; Morhange, J.-F.; Altintaş, A.; Xu, H.Q.; Szuszkiewicz, W. Structural and Optical Properties of Self-Catalytic GaAs:Mn Nanowires Grown by Molecular Beam Epitaxy on Silicon Substrates. Nanoscale 2013, 5, 7410–7418. [Google Scholar] [CrossRef] [Green Version]
- Sadowski, J.; Kret, S.; Šiušys, A.; Wojciechowski, T.; Gas, K.; Islam, M.F.; Canali, C.M.; Sawicki, M. Wurtzite (Ga,Mn)As Nanowire Shells with Ferromagnetic Properties. Nanoscale 2017, 9, 2129–2137. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, A.; Soda, M.; Kiessling, M.; Wojtowicz, T.; Schuh, D.; Wegscheider, W.; Zweck, J.; Back, C.; Reiger, E. Ferromagnetic GaAs/GaMnAs Core−Shell Nanowires Grown by Molecular Beam Epitaxy. Nano Lett. 2009, 9, 3860–3866. [Google Scholar] [CrossRef] [Green Version]
- Šiušys, A.; Sadowski, J.; Sawicki, M.; Kret, S.; Wojciechowski, T.; Gas, K.; Szuszkiewicz, W.; Kaminska, A.; Story, T. All-Wurtzite (In,Ga)As-(Ga,Mn)As Core–Shell Nanowires Grown by Molecular Beam Epitaxy. Nano Lett. 2014, 14, 4263–4272. [Google Scholar] [CrossRef] [Green Version]
- Gas, K.; Sadowski, J.; Sawicki, M. Magnetic Properties of Wurtzite (Ga,Mn)As. J. Magn. Magn. Mater. 2021, 533, 168012. [Google Scholar] [CrossRef]
- Wojnar, P.; Szymura, M.; Zaleszczyk, W.; Kłopotowski, Ł.; Janik, E.; Wiater, M.; Baczewski, L.T.; Kret, S.; Karczewski, G.; Kossut, J.; et al. Activation of an Intense near Band Edge Emission from ZnTe/ZnMgTe Core/Shell Nanowires Grown on Silicon. Nanotechnology 2013, 24, 365201. [Google Scholar] [CrossRef] [PubMed]
- Szymura, M.; Wojnar, P.; Kłopotowski, Ł.; Suffczyński, J.; Goryca, M.; Smoleński, T.; Kossacki, P.; Zaleszczyk, W.; Wojciechowski, T.; Karczewski, G.; et al. Spin Splitting Anisotropy in Single Diluted Magnetic Nanowire Heterostructures. Nano Lett. 2015, 15, 1972–1978. [Google Scholar] [CrossRef]
- Kaleta, A.; Kret, S.; Gas, K.; Kurowska, B.; Kryvyi, S.B.; Rutkowski, B.; Szwacki, N.G.; Sawicki, M.; Sadowski, J. Enhanced Ferromagnetism in Cylindrically Confined MnAs Nanocrystals Embedded in Wurtzite GaAs Nanowire Shells. Nano Lett. 2019, 19, 7324–7333. [Google Scholar] [CrossRef] [PubMed]
- Schrier, J.; Demchenko, D.O.; Wang, L.; Alivisatos, A.P. Optical Properties of ZnO/ZnS and ZnO/ZnTe Heterostructures for Photovoltaic Applications. Nano Lett. 2007, 7, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Flores, E.M.; Gouvea, R.A.; Piotrowski, M.J.; Moreira, M.L. Band Alignment and Charge Transfer Predictions of ZnO/ZnX (X = S, Se or Te) Interfaces Applied to Solar Cells: A PBE+U Theoretical Study. Phys. Chem. Chem. Phys. 2018, 20, 4953–4961. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.Y.; Cheng, J.H.; Lu, J.Y.; Chang, Y.H.; Cheng, C.L.; Chen, Y.F. Growth and Characterization of Type-II ZnO/ZnTe Core-Shell Nanowire Arrays for Solar Cell Applications. Superlattices Microstruct. 2010, 47, 160–164. [Google Scholar] [CrossRef]
- Luo, S.; He, X.; Shen, H.; Li, J.; Yin, X.; Oron, D.; Lin, H. Vertically Aligned ZnO/ZnTe Core/Shell Heterostructures on an AZO Substrate for Improved Photovoltaic Performance. RSC Adv. 2017, 7, 14837–14845. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhao, Q.; Gao, J.; Ye, Y.; Wang, W.; Zhu, R.; Xu, J.; Chen, L.; Yang, J.; Dai, L.; et al. In Situ Growth, Structure Characterization, and Enhanced Photocatalysis of High-Quality, Single-Crystalline ZnTe/ZnO Branched Nanoheterostructures. Nanoscale 2011, 3, 4418–4426. [Google Scholar] [CrossRef]
- Iqbal, M.; Wang, Y.; Hu, H.; He, M.; Shah, A.H.; Li, P.; Lin, L.; Woldu, A.R.; He, T. Interfacial Charge Kinetics of ZnO/ZnTe Heterostructured Nanorod Arrays for CO2 Photoreduction. Electrochim. Acta 2018, 272, 203–211. [Google Scholar] [CrossRef]
- Shaygan, M.; Davami, K.; Jin, B.; Gemming, T.; Lee, J.-S.; Meyyappan, M. Highly Sensitive Photodetectors Using ZnTe/ZnO Core/Shell Nanowire Field Effect Transistors with a Tunable Core/Shell Ratio. J. Mater. Chem. C 2016, 4, 2040–2046. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Zhang, W.; Zhao, J.; Qin, F.; Shi, Z. Photovoltaic-Pyroelectric Effect Coupled Broadband Photodetector in Self-Powered ZnO/ZnTe Core/Shell Nanorod Arrays. Nano Energy 2019, 62, 310–318. [Google Scholar] [CrossRef]
- Janik, E.; Wachnicka, A.; Guziewicz, E.; Godlewski, M.; Kret, S.; Zaleszczyk, W.; Dynowska, E.; Presz, A.; Karczewski, G.; Wojtowicz, T. ZnTe–ZnO Core–Shell Radial Heterostructures Grown by the Combination of Molecular Beam Epitaxy and Atomic Layer Deposition. Nanotechnology 2009, 21, 015302. [Google Scholar] [CrossRef]
- Gas, K.; Janik, E.; Zaleszczyk, W.; Dynowska, E.; Kutrowski, M.; Kamińska, A.; Morhange, J.-F.; Wachnicki, Ł.; Wojciechowski, T.; Hołyst, R.; et al. Selected Optical Properties of Core/Shell ZnMnTe/ZnO Nanowire Structures. Phys. Status Solidi 2011, 248, 1592–1595. [Google Scholar] [CrossRef]
- Gas, K.; Janik, E.; Zaleszczyk, W.; Pasternak, I.; Dynowska, E.; Fronc, K.; Kolkovsky, V.; Kret, S.; Morhange, J.F.; Reszka, A.; et al. Morphology and Selected Properties of Core/Shell ZnTe-Based Nanowire Structures Containing ZnO. Acta Phys. Pol. A 2011, 119, 612–614. [Google Scholar] [CrossRef]
- Przeździecka, E.; Kamińska, E.; Dynowska, E.; Dobrowolski, W.; Jakieła, R.; Kłopotowski, Ł.; Sawicki, M.; Kiecana, M.; Kossut, J. P-Type ZnO and ZnMnO by Oxidation of Zn(Mn)Te Films. Phys. Status Solidi 2006, 3, 988–991. [Google Scholar] [CrossRef]
- Przeździecka, E.; Kamińska, E.; Korona, K.P.; Dynowska, E.; Dobrowolski, W.; Jakieła, R.; Kłopotowski, Ł.; Kossut, J. Photoluminescence Study and Structural Characterization of P-Type ZnO Doped by N and/or As Acceptors. Semicond. Sci. Technol. 2006, 22, 10–14. [Google Scholar] [CrossRef]
- Lu, P.; Smith, D.J. The Observation of in Situ and Ex Situ Oxidation Processes for ZnTe Surfaces by High-Resolution Electron Microscopy. Phys. Status Solidi 1988, 107, 681–691. [Google Scholar] [CrossRef]
- Janik, E.; Sadowski, J.; Dłużewski, P.; Kret, S.; Baczewski, L.T.; Petroutchik, A.; Łusakowska, E.; Wróbel, J.; Zaleszczyk, W.; Karczewski, G.; et al. ZnTe Nanowires Grown on GaAs(100) Substrates by Molecular Beam Epitaxy. Appl. Phys. Lett. 2006, 89, 133114. [Google Scholar] [CrossRef]
- Janik, E.; Dłużewski, P.; Kret, S.; Presz, A.; Kirmse, H.; Neumann, W.; Zaleszczyk, W.; Baczewski, L.T.; Petroutchik, A.; Dynowska, E.; et al. Catalytic Growth of ZnTe Nanowires by Molecular Beam Epitaxy: Structural Studies. Nanotechnology 2007, 18, 475606. [Google Scholar] [CrossRef]
- Dynowska, E.; Szuszkiewicz, W.; Domagala, J.Z.; Janik, E.; Presz, A.; Wojtowicz, T.; Karczewski, G.; Caliebe, W. X-Ray Characterization of Catalytically Grown ZnTe and ZnMgTe Nanowires. Radiat. Phys. Chem. 2009, 78, S120–S124. [Google Scholar] [CrossRef] [Green Version]
- Irwin, J.C.; LaCombe, J. Raman Scattering in ZnTe. J. Appl. Phys. 1970, 41, 1444–1450. [Google Scholar] [CrossRef]
- Szuszkiewicz, W.; Morhange, J.-F.; Janik, E.; Zaleszczyk, W.; Karczewski, G.; Wojtowicz, T. Raman Spectroscopy of MBE-Grown ZnTe-Based Nanowires. Phys. Status Solidi 2009, 6, 2047–2052. [Google Scholar] [CrossRef]
- Szuszkiewicz, W.; Jouanne, M.; Morhange, J.-F.; Kanehisa, M.; Dynowska, E.; Gas, K.; Janik, E.; Karczewski, G.; Kuna, R.; Wojtowicz, T. Raman Scattering as a Tool to Characterize Semiconductor Crystals, Thin Layers, and Low-Dimensional Structures Containing Transition Metals. Phys. Status Solidi 2014, 251, 1133–1143. [Google Scholar] [CrossRef]
- Grant, W.B.; Schulz, H.; Hüfner, S.; Pelzl, J. Investigation of k = 0 Phonons in Bi, Cd, Mg, and Zn by Raman Scattering. Phys. Status Solidi 1973, 60, 331–340. [Google Scholar] [CrossRef]
- Pine, A.S.; Dresselhaus, G. Raman Spectra and Lattice Dynamics of Tellurium. Phys. Rev. B 1971, 4, 356–371. [Google Scholar] [CrossRef]
- Brodsky, M.H.; Gambino, R.J.; Smith, J.E.; Yacoby, Y. The Raman Spectrum of Amorphous Tellurium. Phys. Status Solidi 1972, 52, 609–614. [Google Scholar] [CrossRef]
- Calleja, J.M.; Cardona, M. Resonant Raman Scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Stevenson, D.A. Self-Diffusion of Zinc and Tellurium in Zinc Telluride. J. Phys. Chem. Solids 1969, 30, 139–147. [Google Scholar] [CrossRef]
- Wojnar, P.; Janik, E.; Baczewski, L.T.; Kret, S.; Dynowska, E.; Wojciechowski, T.; Suffczyński, J.; Papierska, J.; Kossacki, P.; Karczewski, G.; et al. Giant Spin Splitting in Optically Active ZnMnTe/ZnMgTe Core/Shell Nanowires. Nano Lett. 2012, 12, 3404–3409. [Google Scholar] [CrossRef]
- Sköld, N.; Karlsson, L.S.; Larsson, M.W.; Pistol, M.-E.; Seifert, W.; Trägårdh, J.; Samuelson, L. Growth and Optical Properties of Strained GaAs−GaxIn1-XP Core−Shell Nanowires. Nano Lett. 2005, 5, 1943–1947. [Google Scholar] [CrossRef]
- Proselkov, O.; Sztenkiel, D.; Stefanowicz, W.; Aleszkiewicz, M.; Sadowski, J.; Dietl, T.; Sawicki, M. Thickness Dependent Magnetic Properties of (Ga,Mn)As Ultrathin Films. Appl. Phys. Lett. 2012, 100, 262405. [Google Scholar] [CrossRef]
- Yamaguchi, K.Y.K.; Yujobo, K.Y.K.; Kaizu, T.K.T. Stranski-Krastanov Growth of InAs Quantum Dots with Narrow Size Distribution. Jpn. J. Appl. Phys. 2000, 39, L1245. [Google Scholar] [CrossRef]
- Karczewski, G.; Maćkowski, S.; Kutrowski, M.; Wojtowicz, T.; Kossut, J. Photoluminescence Study of CdTe/ZnTe Self-Assembled Quantum Dots. Appl. Phys. Lett. 1999, 74, 3011–3013. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Liu, J.-W.; Yu, S.-H. Emerging Tellurium Nanostructures: Controllable Synthesis and Their Applications. Chem. Soc. Rev. 2017, 46, 2732–2753. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gas, K.; Kret, S.; Zaleszczyk, W.; Kamińska, E.; Sawicki, M.; Wojtowicz, T.; Szuszkiewicz, W. Oxidation of MBE-Grown ZnTe and ZnTe/Zn Nanowires and Their Structural Properties. Materials 2021, 14, 5252. https://doi.org/10.3390/ma14185252
Gas K, Kret S, Zaleszczyk W, Kamińska E, Sawicki M, Wojtowicz T, Szuszkiewicz W. Oxidation of MBE-Grown ZnTe and ZnTe/Zn Nanowires and Their Structural Properties. Materials. 2021; 14(18):5252. https://doi.org/10.3390/ma14185252
Chicago/Turabian StyleGas, Katarzyna, Slawomir Kret, Wojciech Zaleszczyk, Eliana Kamińska, Maciej Sawicki, Tomasz Wojtowicz, and Wojciech Szuszkiewicz. 2021. "Oxidation of MBE-Grown ZnTe and ZnTe/Zn Nanowires and Their Structural Properties" Materials 14, no. 18: 5252. https://doi.org/10.3390/ma14185252
APA StyleGas, K., Kret, S., Zaleszczyk, W., Kamińska, E., Sawicki, M., Wojtowicz, T., & Szuszkiewicz, W. (2021). Oxidation of MBE-Grown ZnTe and ZnTe/Zn Nanowires and Their Structural Properties. Materials, 14(18), 5252. https://doi.org/10.3390/ma14185252





