Review of Aluminum Alloy Development for Wire Arc Additive Manufacturing
Abstract
:1. Wire Arc Additive Manufacturing
2. Aluminum Alloys
3. Challenges Related to Aluminium WAAM
3.1. Porosity
3.2. Residual Stresses and Distortion
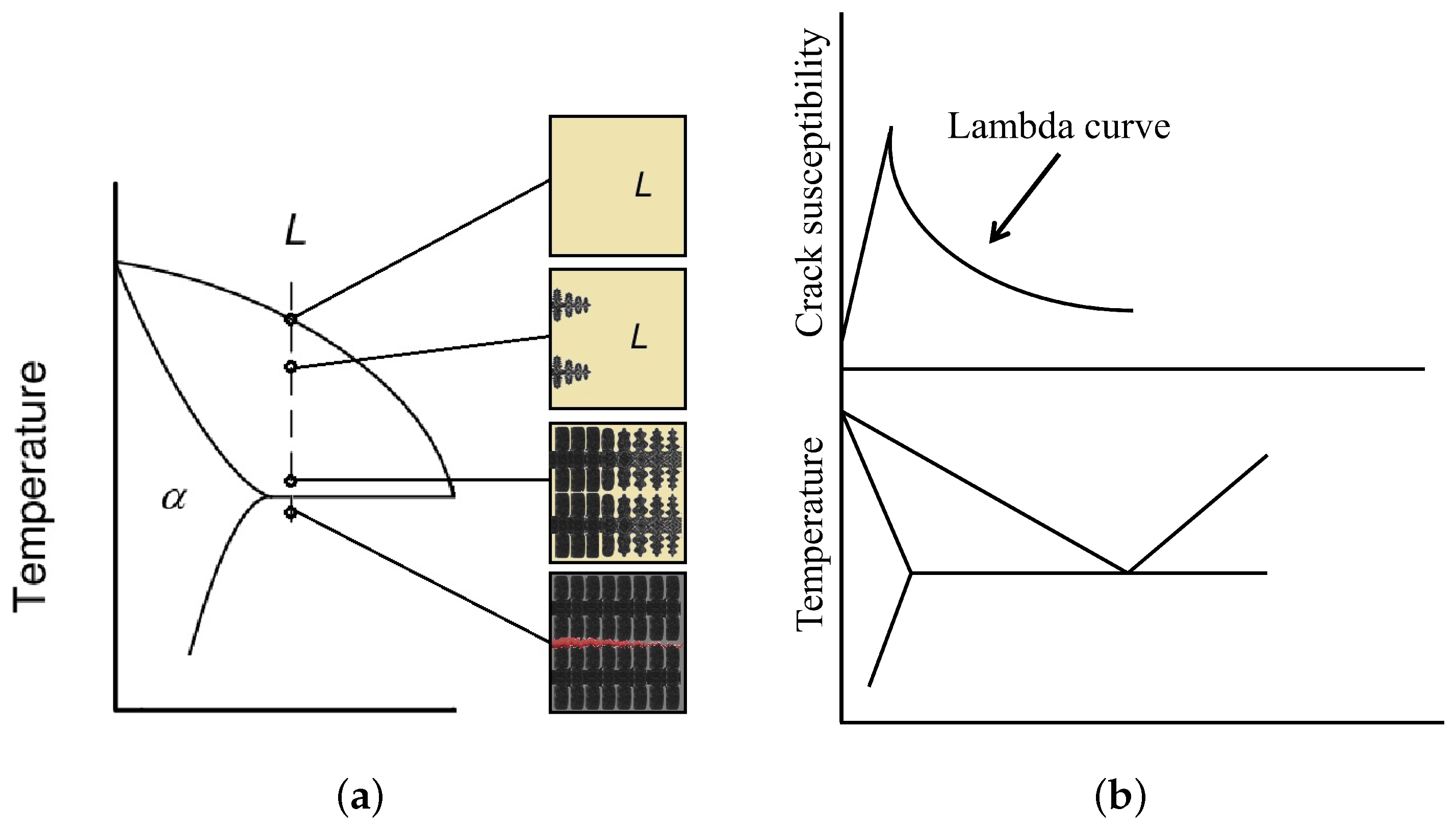
3.3. Cracking
4. Strategies to Mitigate Defects in WAAM
4.1. Mechanical Impact
4.2. Hardware
4.3. Microstructure Control
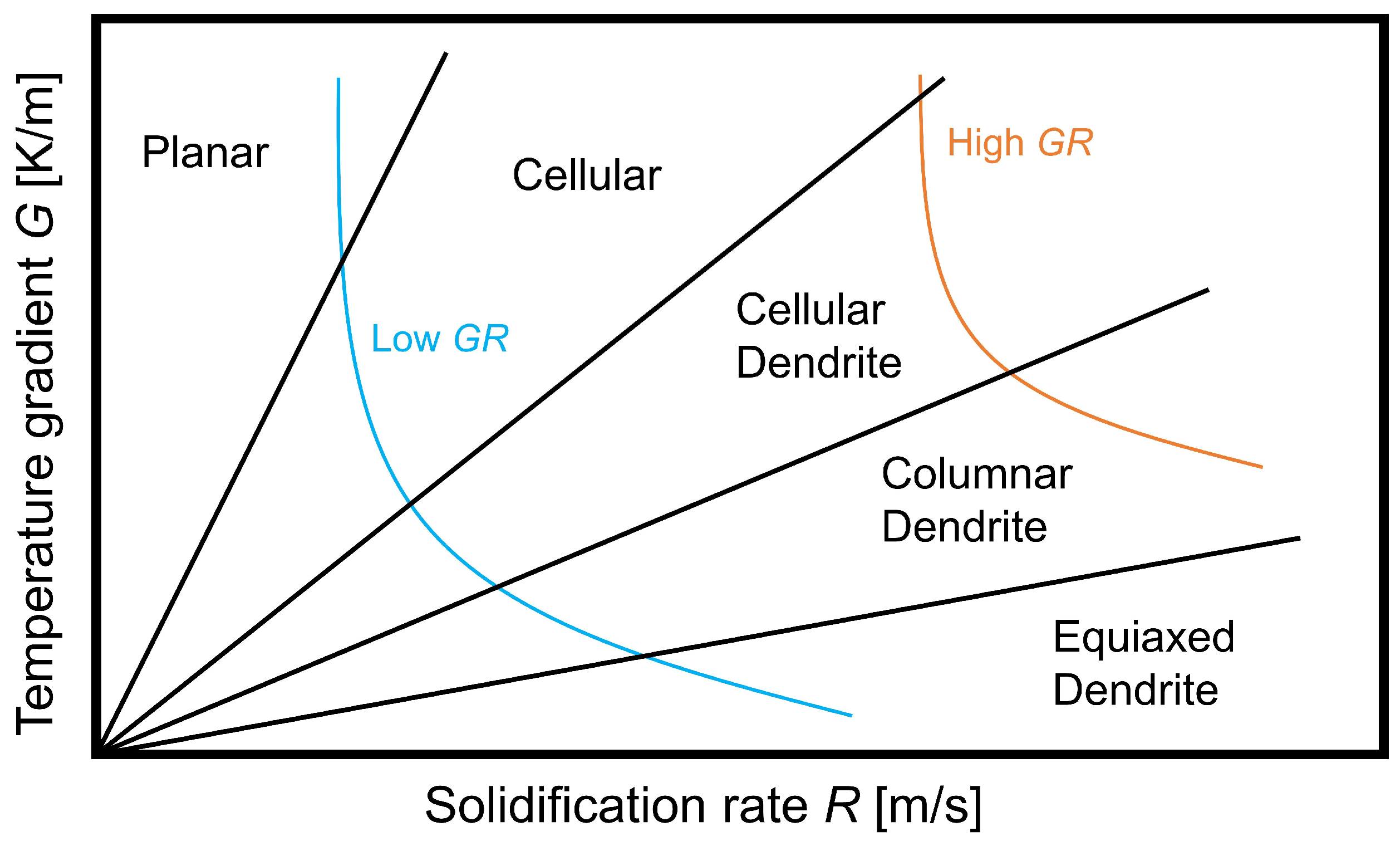
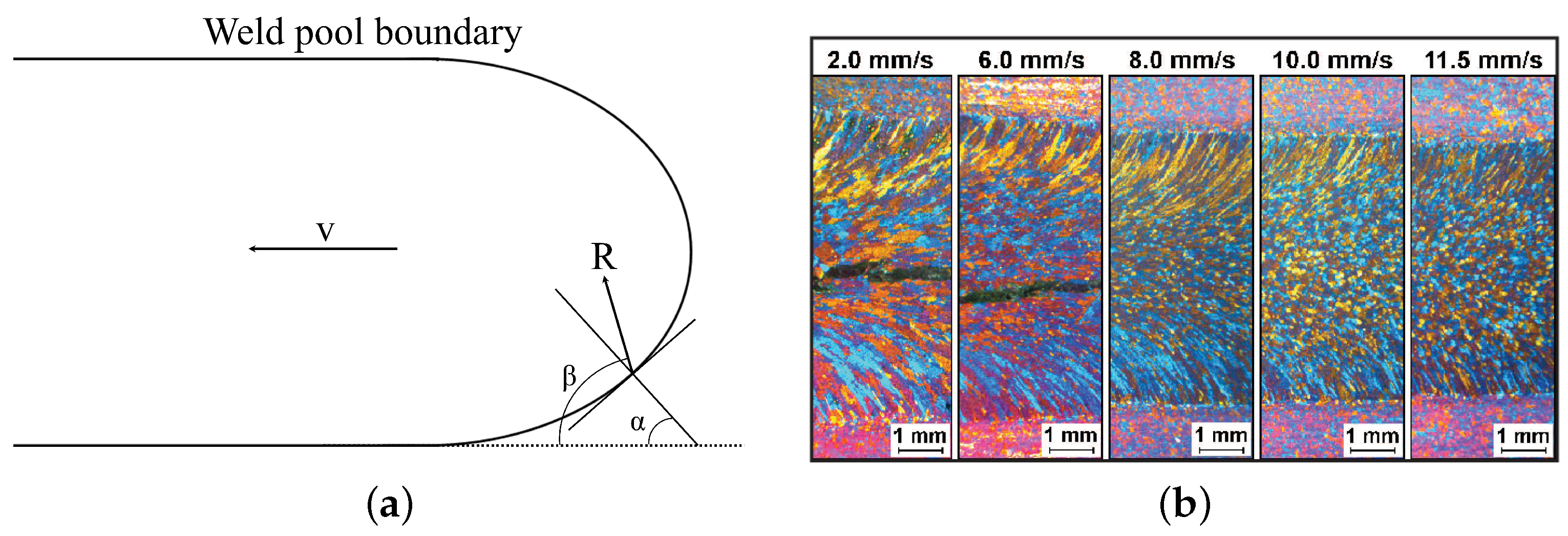
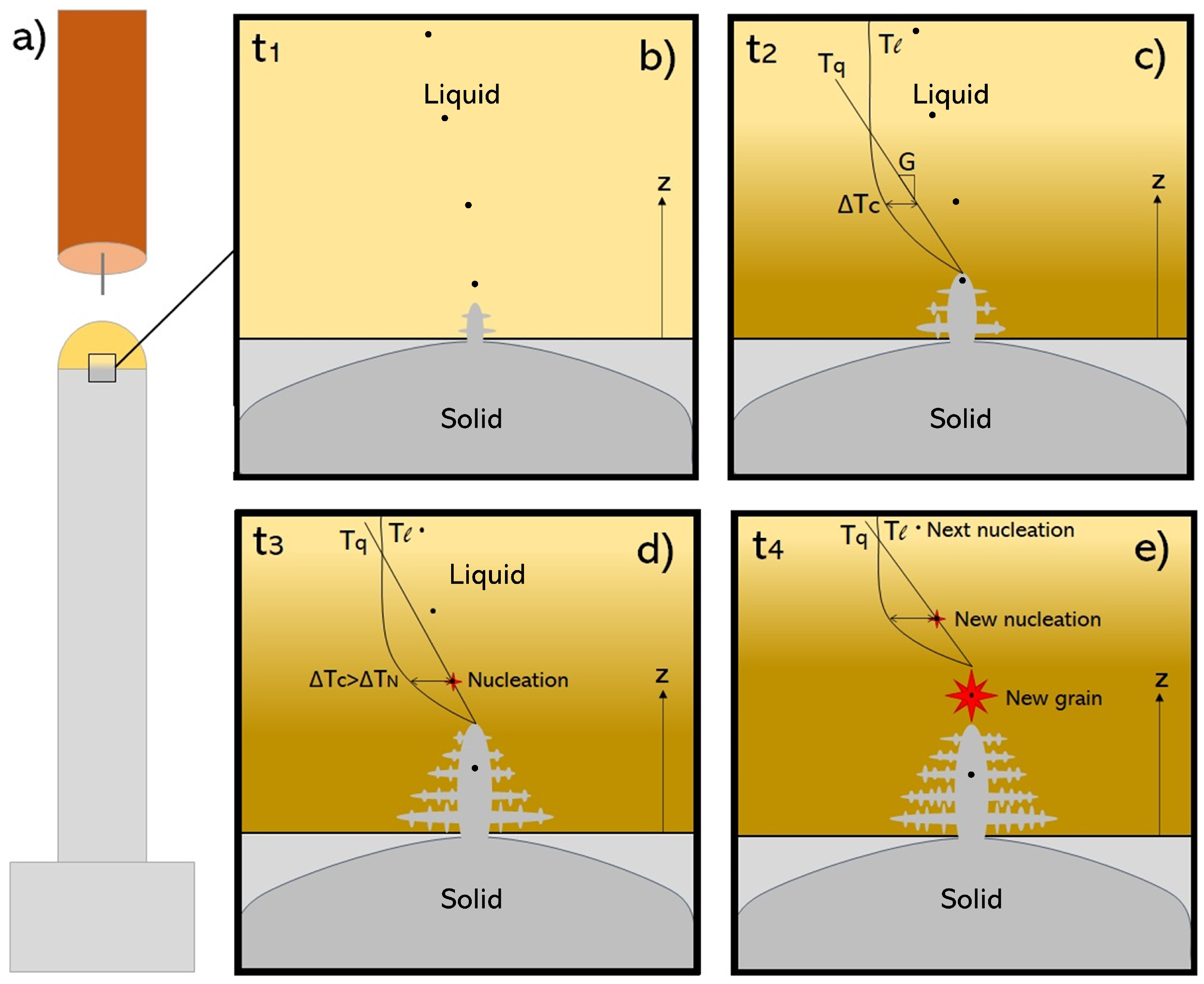
4.4. Solidification in WAAM and CET
5. Aluminum Alloys for WAAM
5.1. Commercial Selection
5.2. Other Alloys for WAAM
5.3. Alloy Modifications
5.4. Microalloying
5.5. Ceramic Particle Additions
6. Future Developments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| ASTM | American Society for Testing and Materials |
| CCD | Charge-coupled device |
| CET | Columnar-equiaxed-transition |
| CNC | Computer numerical control |
| CMT | Cold Metal Transfer |
| CMT-ADV | Cold Metal Transfer Advanced |
| CMT-P | Cold Metal Transfer Pulse |
| CMT-PADV | Cold Metal Transfer Pulse Advanced |
| CTE | Coefficient of Thermal Expansion |
| FCC | Face-centered cubic |
| GMAW | Gas metal arc welding |
| GTAW | Gas tungsten arc welding |
| LCA | Life-cycle assessment |
| RDG | Rappaz–Drezet–Gremaud |
| SLM | Selective Laser Melting |
| WAAM | Wire arc additive manufacturing |
References
- ASTM. ASTM F2792-12a, Standard Terminology for Additive Manufacturing Technologies; Technical Report 2; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.; Zuback, J.; Mukherjee, T.; Elmer, J.; Milewski, J.; Beese, A.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Frazier, W. Metal additive manufacturing: A review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horizons 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Williams, S.; Martina, F.; Addison, A.; Ding, J.; Pardal, G.; Colegrove, P. Wire + arc additive manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Alonso, U.; Veiga, F.; Suárez, A.; Artaza, T. Experimental investigation of the influence of wire arc additive manufacturing on the machinability of titanium parts. Metals 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liang, E. Metal additive manufacturing in aircraft: Current application, opportunities and challenges. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 493, p. 012032. [Google Scholar] [CrossRef]
- Liu, J.; Gaynor, A.; Chen, S.; Kang, Z.; Suresh, K.; Takezawa, A.; Li, L.; Kato, J.; Tang, J.; Wang, C.; et al. Current and future trends in topology optimization for additive manufacturing. Struct. Multidiscip. Optim. 2018, 57, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, M. The Future for WAAM. AMazing. 2019. Available online: http://additivemanufacturing.com/2019/08/27/the-future-for-waam/ (accessed on 31 August 2021).
- Doppler, L. Wire ARC Additive Manufacturing: Economical 3D Printing for Metal. Fronius.com. 2019. Available online: https://www.fronius.com/en/welding-technology/info-centre/press/waam (accessed on 31 August 2021).
- Taşdemir, A.; Nohut, S. An overview of wire arc additive manufacturing (WAAM) in shipbuilding industry. Ships Offshore Struct. 2020, 16, 797–814. [Google Scholar] [CrossRef]
- Tsurumaki, T.; Tsukamoto, S.; Chibahara, H.; Sasahara, H. Precise additive fabrication of wall structure on thin plate end with interlayer temperature monitoring. J. Adv. Mech. Des. Syst. Manuf. 2019, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Equinor. He’s Just Passed His Exams. But Weldar Is No Ordinary Apprentice. Equinor Magazine. 2020. Available online: https://www.equinor.com/en/magazine/weldar-the-welding-robot.html (accessed on 31 August 2021).
- Tabernero, I.; Paskual, A.; Álvarez, P.; Suárez, A. Study on arc welding processes for high deposition rate additive manufacturing. Procedia Cirp 2018, 68, 358–362. [Google Scholar] [CrossRef]
- Gu, J.; Bai, J.; Ding, J.; Williams, S.; Wang, L.; Liu, K. Design and cracking susceptibility of additively manufactured Al-Cu-Mg alloys with tandem wires and pulsed arc. J. Mater. Process. Technol. 2018, 262, 210–220. [Google Scholar] [CrossRef]
- DuPont, J.; Marder, A. Thermal efficiency of arc welding processes. Weld. J.-Incl. Weld. Res. Suppl. 1995, 74, 406–416. [Google Scholar]
- Unocic, R.; DuPont, J. Process efficiency measurements in the laser engineered net shaping process. Metall. Mater. Trans. B 2004, 35, 143–152. [Google Scholar] [CrossRef]
- Rännar, L.E.; Glad, A.; Gustafson, C.G. Efficient cooling with tool inserts manufactured by electron beam melting. Rapid Prototyp. J. 2007, 13, 128–135. [Google Scholar] [CrossRef]
- Bekker, A.; Verlinden, J. Life cycle assessment of wire+ arc additive manufacturing compared to green sand casting and CNC milling in stainless steel. J. Clean. Prod. 2018, 177, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Guo, J. Feature Based Cost and Carbon Emission Modelling for Wire and Arc Additive Manufacturing. Master’s Thesis, Cranfield University, Cranfield, UK, 2012. Available online: http://dspace.lib.cranfield.ac.uk/handle/1826/7923 (accessed on 1 September 2021).
- Zhang, Z.; Ma, Z.; He, S.; Song, G.; Liu, L. Effect of Laser Power on the Microstructure and Mechanical Properties of 2319-Al Fabricated by Wire-Based Additive Manufacturing. J. Mater. Eng. Perform. 2021, 1–10. [Google Scholar] [CrossRef]
- Dong, M.; Zhao, Y.; Li, Q.; Fei, Y.; Zhao, T.; Wang, F.; Wu, A. Microstructure Evolution and Mechanical Property Anisotropy of Wire and Arc-Additive-Manufactured Wall Structure Using ER2319 Welding Wires. J. Mater. Eng. Perform. 2021, 30, 258–268. [Google Scholar] [CrossRef]
- Gu, J.; Gao, M.; Yang, S.; Bai, J.; Ding, J.; Fang, X. Pore formation and evolution in wire+ arc additively manufactured 2319 Al alloy. Addit. Manuf. 2019, 30, 100900. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, J.; Chen, S. Comparison of Microstructure and Mechanical Properties of Aluminum Components Manufactured by CMT. Mater. Sci. Forum 2017, 898, 1318–1324. [Google Scholar] [CrossRef]
- Gu, J.; Ding, J.; Williams, S.; Gu, H.; Ma, P.; Zhai, Y. The effect of inter-layer cold working and post-deposition heat treatment on porosity in additively manufactured aluminum alloys. J. Mater. Process. Technol. 2016, 230, 26–34. [Google Scholar] [CrossRef]
- Cong, B.; Ding, J.; Williams, S. Effect of arc mode in cold metal transfer process on porosity of additively manufactured Al-6.3% Cu alloy. Int. J. Adv. Manuf. Technol. 2015, 76, 1593–1606. [Google Scholar] [CrossRef]
- Cong, B.; Qi, Z.; Qi, B.; Sun, H.; Zhao, G.; Ding, J. A comparative study of additively manufactured thin wall and block structure with Al-6.3% Cu alloy using cold metal transfer process. Appl. Sci. 2017, 7, 275. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X.; Kang, N.; Huang, W.; Wang, J.; Wang, Z. Influence of travel speed on microstructure and mechanical properties of wire+ arc additively manufactured 2219 aluminum alloy. J. Mater. Sci. Technol. 2020, 37, 143–153. [Google Scholar] [CrossRef]
- Fu, R.; Tang, S.; Lu, J.; Cui, Y.; Li, Z.; Zhang, H.; Xu, T.; Chen, Z.; Liu, C. Hot-wire arc additive manufacturing of aluminum alloy with reduced porosity and high deposition rate. Mater. Des. 2021, 199, 109370. [Google Scholar] [CrossRef]
- Fixter, J.; Gu, J.; Ding, J.; Williams, S.; Prangnell, P. Preliminary investigation into the suitability of 2xxx alloys for wire-arc additive manufacturing. Mater. Sci. Forum 2017, 877, 611–616. [Google Scholar] [CrossRef]
- Lincoln, E. Al Mn Stick Electrodes. 2021. Available online: https://www.lincolnelectric.com/en-gb/consumables/Pages/product.aspx?product=Products_ConsumableEU_StickElectrodes-Al-AlMn(LincolnElectric_EU_Base)&detail=809718(LincolnElectric_EU_Base) (accessed on 14 August 2021).
- Gomez Ortega, A.; Corona Galvan, L.; Deschaux-Beaume, F.; Mezrag, B.; Rouquette, S. Effect of process parameters on the quality of aluminum alloy Al5Si deposits in wire and arc additive manufacturing using a cold metal transfer process. Sci. Technol. Weld. Join. 2018, 23, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Q.; Zhang, P.L.; Li, S.W.; Wu, D.; Yu, Z.S. Wire and arc additive manufacturing of 4043 Al alloy using a cold metal transfer method. Int. J. Miner. Metall. Mater. 2020, 27, 783–791. [Google Scholar] [CrossRef]
- Miao, Q.; Wu, D.; Chai, D.; Zhan, Y.; Bi, G.; Niu, F.; Ma, G. Comparative study of microstructure evaluation and mechanical properties of 4043 aluminum alloy fabricated by wire-based additive manufacturing. Mater. Des. 2020, 186, 108205. [Google Scholar] [CrossRef]
- Ortega, A.; Corona Galvan, L.; Salem, M.; Moussaoui, K.; Segonds, S.; Rouquette, S.; Deschaux-Beaume, F. Characterisation of 4043 aluminum alloy deposits obtained by wire and arc additive manufacturing using a Cold Metal Transfer process. Sci. Technol. Weld. Join. 2019, 24, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lu, J.; Tang, S.; Yu, L.; Fan, H.; Ji, L.; Liu, C. Reducing porosity and refining grains for arc additive manufacturing aluminum alloy by adjusting arc pulse frequency and current. Materials 2018, 11, 1344. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Chen, X.; Konovalov, S.; Singh, R.A.; Jayalakshmi, S.; Huang, L. Effect of Deposition Strategies on the Microstructure and Tensile Properties of Wire Arc Additive Manufactured Al-5Si Alloys. J. Mater. Eng. Perform. 2021, 30, 2136–2146. [Google Scholar] [CrossRef]
- Langelandsvik, G.; Horgar, A.; Furu, T.; Roven, H.J.; Akselsen, O.M. Comparative study of eutectic Al-Si alloys manufactured by WAAM and casting. Int. J. Adv. Manuf. Technol. 2020, 110, 935–947. [Google Scholar] [CrossRef]
- Köhler, M.; Fiebig, S.; Hensel, J.; Dilger, K. Wire and Arc Additive Manufacturing of Aluminum Components. Metals 2019, 9, 608. [Google Scholar] [CrossRef] [Green Version]
- Heard, D.; Brophy, S.; Brochu, M. Solid freeform fabrication of Al–Si components via the CSC-MIG process. Can. Metall. Q. 2012, 51, 302–312. [Google Scholar] [CrossRef]
- Yilmaz, A. The Portevin–Le Chatelier effect: A review of experimental findings. Sci. Technol. Adv. Mater. 2011, 12, 063001. [Google Scholar] [CrossRef]
- Gu, J.; Wang, X.; Bai, J.; Ding, J.; Williams, S.; Zhai, Y.; Liu, K. Deformation microstructures and strengthening mechanisms for the wire+ arc additively manufactured Al-Mg4.5Mn alloy with inter-layer rolling. Mater. Sci. Eng. A 2018, 712, 292–301. [Google Scholar] [CrossRef]
- Horgar, A.; Fostervoll, H.; Nyhus, B.; Ren, X.; Eriksson, M.; Akselsen, O. Additive manufacturing using WAAM with AA5183 wire. J. Mater. Process. Technol. 2018, 259, 68–74. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, L.; Chen, G.; Dang, X.; Huang, K.; Wang, L.; Lu, B. Correlations between Microstructure Characteristics and Mechanical Properties in 5183 Aluminium Alloy Fabricated by Wire-Arc Additive Manufacturing with Different Arc Modes. Materials 2018, 11, 2075. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Bacelar, R.; Pires, I.; Santos, T.; Sousa, J.; Quintino, L. Non-destructive testing application of radiography and ultrasound for wire and arc additive manufacturing. Addit. Manuf. 2018, 21, 298–306. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Wang, Z.; Zhang, L.; Gao, Q. Microstructure and properties of Al alloy ER5183 deposited by variable polarity cold metal transfer. J. Mater. Process. Technol. 2019, 267, 167–176. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.J.; Ning, J.; Wang, X.; Zhang, G.F.; Zhang, J.X.; Na, S.J.; Fatemeh, B. Comparative study on the microstructures and properties of wire+ arc additively manufactured 5356 aluminum alloy with argon and nitrogen as the shielding gas. Addit. Manuf. 2020, 34, 101206. [Google Scholar] [CrossRef]
- Köhler, M.; Hensel, J.; Dilger, K. Effects of thermal cycling on wire and arc additive manufacturing of Al-5356 components. Metals 2020, 10, 952. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Q.; Kong, X.; Chen, X. Arc Additively Manufactured 5356 Aluminum Alloy with Cable-Type Welding Wire: Microstructure and Mechanical Properties. J. Mater. Eng. Perform. 2021, 1–7. [Google Scholar] [CrossRef]
- Uddin, S.Z.; Murr, L.E.; Terrazas, C.A.; Morton, P.; Roberson, D.A.; Wicker, R.B. Processing and characterization of crack-free aluminum 6061 using high-temperature heating in laser powder bed fusion additive manufacturing. Addit. Manuf. 2018, 22, 405–415. [Google Scholar] [CrossRef]
- Migalco. MA-6063 AlMgSi0,5. 2021. Available online: https://migal.co/en/products/aluminium-welding-wire/ma-6063-almgsi05 (accessed on 26 June 2021).
- Danylenko, M. Aluminum alloys in aerospace. Alum. Int. Today 2018, 31, 35. [Google Scholar]
- Derekar, K. A review of wire arc additive manufacturing and advances in wire arc additive manufacturing of aluminium. Mater. Sci. Technol. 2018, 34, 895–916. [Google Scholar] [CrossRef]
- Gudeljevic, M.; Klein, T. Investigation of material characteristics of intersections built by wire and arc additive manufacturing using locally varying deposition parameters. Int. J. Adv. Manuf. Technol. 2021, 116, 2021–2029. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg–Zn–Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Liu, D.; Yürekli, B.; Ullsperger, T.; Matthäus, G.; Schade, L.; Nolte, S.; Rettenmayr, M. Microstructural aspects of additive manufacturing of AlLi alloys with high Li content. Mater. Des. 2021, 198, 109323. [Google Scholar] [CrossRef]
- Yuan, T.; Yu, Z.; Chen, S.; Xu, M.; Jiang, X. Loss of elemental Mg during wire+ arc additive manufacturing of Al-Mg alloy and its effect on mechanical properties. J. Manuf. Process. 2020, 49, 456–462. [Google Scholar] [CrossRef]
- Ransley, C. The solubility of hydrogen in liquid and solid aluminium. J. Inst. Met. 1948, 74, 599–620. [Google Scholar]
- Scully, J.; Young, G., Jr.; Smith, S. Hydrogen solubility, diffusion and trapping in high purity aluminum and selected Al-base alloys. Mater. Sci. Forum 2000, 331, 1583–1600. [Google Scholar] [CrossRef]
- Lee, P.; Chirazi, A.; See, D. Modeling microporosity in aluminum–Silicon alloys: A review. J. Light Met. 2001, 1, 15–30. [Google Scholar] [CrossRef]
- Poirier, D.; Yeum, K.; Maples, A. A thermodynamic prediction for microporosity formation in aluminum-rich Al-Cu alloys. Metall. Trans. A 1987, 18, 1979–1987. [Google Scholar] [CrossRef]
- Li, K.D.; Chang, E. Mechanism of nucleation and growth of hydrogen porosity in solidifying A356 aluminum alloy: An analytical solution. Acta Mater. 2004, 52, 219–231. [Google Scholar] [CrossRef]
- Derekar, K.; Addison, A.; Joshi, S.; Zhang, X.; Lawrence, J.; Xu, L.; Melton, G.; Griffiths, D. Effect of pulsed metal inert gas (pulsed-MIG) and cold metal transfer (CMT) techniques on hydrogen dissolution in wire arc additive manufacturing (WAAM) of aluminium. Int. J. Adv. Manuf. Technol. 2020, 107, 311–331. [Google Scholar] [CrossRef]
- Bai, J.; Ding, H.; Gu, J.; Wang, X.; Qiu, H. Porosity evolution in additively manufactured aluminum alloy during high temperature exposure. In IOP Conference Series: Materials Science and Engineering; IOP Publishing, IOP: Bristol, UK, 2017; Volume 167, p. 012045. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.; Sabin, T.; Watts, J.; Whiting, M. The influence of build parameters and wire batch on porosity of wire and arc additive manufactured aluminum alloy 2319. J. Mater. Process. Technol. 2018, 262, 577–584. [Google Scholar] [CrossRef]
- Johansen, I. 1995:32 Properties, Microstructure and Modelling of an RS Aluminum Alloy. Ph.D. Thesis, Norwegian University of Science and Technology, Hogskoleringen, Norway, 1995. [Google Scholar]
- Qiang, W.; Yingchun, G.; Baoqiang, C.; Bojin, Q. Laser cleaning of commercial Al alloy surface for tungsten inert gas welding. J. Laser Appl. 2016, 28, 022507. [Google Scholar] [CrossRef]
- Sun, J.; Hensel, J.; Köhler, M.; Dilger, K. Residual stress in wire and arc additively manufactured aluminum components. J. Manuf. Process. 2021, 65, 97–111. [Google Scholar] [CrossRef]
- Colegrove, P.; Coules, H.; Fairman, J.; Martina, F.; Kashoob, T.; Mamash, H.; Cozzolino, L. Microstructure and residual stress improvement in wire and arc additively manufactured parts through high-pressure rolling. J. Mater. Process. Technol. 2013, 213, 1782–1791. [Google Scholar] [CrossRef]
- Sealy, M.; Madireddy, G.; Williams, R.; Rao, P.; Toursangsaraki, M. Hybrid processes in additive manufacturing. J. Manuf. Sci. Eng. 2018, 140, 060801. [Google Scholar] [CrossRef]
- Robinson, J.S.; Redington, W. The influence of alloy composition on residual stresses in heat treated aluminum alloys. Mater. Charact. 2015, 105, 47–55. [Google Scholar] [CrossRef]
- Chang, C.; Chen, C.; Wen, J.; Cheng, C.; Chou, C. Characterization of hot cracking due to welding of high-strength aluminum alloys. Mater. Manuf. Process. 2012, 27, 658–663. [Google Scholar] [CrossRef]
- Eskin, D.; Suyitno, M.; Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminum alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Li, S.; Apelian, D. Hot tearing of aluminum alloys. Int. J. Met. 2011, 5, 23–40. [Google Scholar] [CrossRef]
- Song, J.; Pan, F.; Jiang, B.; Atrens, A.; Zhang, M.X.; Lu, Y. A review on hot tearing of magnesium alloys. J. Magnes. Alloy. 2016, 4, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Clyne, G.; TW, C. The influence of composition on solidification cracking susceptibility in binary alloy systems. Br. Foundrym. 1981, 74, 65–73. [Google Scholar]
- Rappaz, M.; Drezet, J.M.; Gremaud, M. A new hot-tearing criterion. Metall. Mater. Trans. A 1999, 30, 449–455. [Google Scholar] [CrossRef]
- Gu, J.; Ding, J.; Williams, S.; Gu, H.; Bai, J.; Zhai, Y.; Ma, P. The strengthening effect of inter-layer cold working and post-deposition heat treatment on the additively manufactured Al–6.3 Cu alloy. Mater. Sci. Eng. A 2016, 651, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Zhang, L.; Chen, G.; Huang, K.; Xue, F.; Wang, L.; Zhao, J.; Lu, B. Microstructure evolution of wire-arc additively manufactured 2319 aluminum alloy with interlayer hammering. Mater. Sci. Eng. A 2021, 800, 140168. [Google Scholar] [CrossRef]
- Gussev, M.N.; Sridharan, N.; Thompson, Z.; Terrani, K.A.; Babu, S. Influence of hot isostatic pressing on the performance of aluminum alloy fabricated by ultrasonic additive manufacturing. Scr. Mater. 2018, 145, 33–36. [Google Scholar] [CrossRef]
- Sun, R.; Li, L.; Zhu, Y.; Guo, W.; Peng, P.; Cong, B.; Sun, J.; Che, Z.; Li, B.; Guo, C.; et al. Microstructure, residual stress and tensile properties control of wire-arc additive manufactured 2319 aluminum alloy with laser shock peening. J. Alloys Compd. 2018, 747, 255–265. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, J.; Hu, S.; Han, J.; Wang, Q.; Cai, Y. Effects of ultrasonic peening treatment layer by layer on microstructure of components fabricated by wire and arc additive manufacturing. Mater. Lett. 2021, 284, 128917. [Google Scholar] [CrossRef]
- Geng, H.; Li, J.; Xiong, J.; Lin, X.; Zhang, F. Geometric limitation and tensile properties of wire and arc additive manufacturing 5A06 aluminum alloy parts. J. Mater. Eng. Perform. 2017, 26, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Kou, S. Welding Metallurgy; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 431–446. [Google Scholar]
- Xiong, J.; Lei, Y.; Chen, H.; Zhang, G. Fabrication of inclined thin-walled parts in multi-layer single-pass GMAW-based additive manufacturing with flat position deposition. J. Mater. Process. Technol. 2017, 240, 397–403. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Duarte, V.R.; Miranda, R.; Santos, T.G.; Oliveira, J. Ultracold-Wire and arc additive manufacturing (UC-WAAM). J. Mater. Process. Technol. 2021, 296, 117196. [Google Scholar] [CrossRef]
- Selvi, S.; Vishvaksenan, A.; Rajasekar, E. Cold metal transfer (CMT) technology—An overview. Def. Technol. 2018, 14, 28–44. [Google Scholar] [CrossRef]
- Su, C.; Chen, X.; Gao, C.; Wang, Y. Effect of heat input on microstructure and mechanical properties of Al-Mg alloys fabricated by WAAM. Appl. Surf. Sci. 2019, 486, 431–440. [Google Scholar] [CrossRef]
- Pang, J.; Hu, S.; Shen, J.; Wang, P.; Liang, Y. Arc characteristics and metal transfer behavior of CMT+ P welding process. J. Mater. Process. Technol. 2016, 238, 212–217. [Google Scholar] [CrossRef]
- Kang, B.Y.; Prasad, Y.K.; Kang, M.J.; Kim, H.; Kim, I.S. Characteristics of alternate supply of shielding gases in aluminum GMA welding. J. Mater. Process. Technol. 2009, 209, 4716–4721. [Google Scholar] [CrossRef]
- Da Silva, L.J.; Scotti, F.M.; Fernandes, D.B.; Reis, R.P.; Scotti, A. Effect of O2 content in argon-based shielding gas on arc wandering in WAAM of aluminum thin walls. CIRP J. Manuf. Sci. Technol. 2021, 32, 338–345. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.; Zeng, X. Workpiece vibration augmented wire arc additive manufacturing of high strength aluminum alloy. J. Mater. Process. Technol. 2019, 271, 85–92. [Google Scholar] [CrossRef]
- Eimer, E.; Williams, S.; Ding, J.; Ganguly, S.; Chehab, B. Effect of Substrate Alloy Type on the Microstructure of the Substrate and Deposited Material Interface in aluminum Wire Arc Additive Manufacturing. Metals 2021, 11, 916. [Google Scholar] [CrossRef]
- Xiong, Y.; Park, S.I.; Padmanathan, S.; Dharmawan, A.; Foong, S.; Rosen, D.; Soh, G. Process planning for adaptive contour parallel toolpath in additive manufacturing with variable bead width. Int. J. Adv. Manuf. Technol. 2019, 105, 4159–4170. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Sharma, S.; Rathod, D.W. A review on process planning strategies and challenges of WAAM. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Dai, F.; Huang, C.; Wang, G. End lateral extension path strategy for intersection in wire and arc additive manufactured 2319 aluminum alloy. Rapid Prototyp. J. 2019, 26, 360–369. [Google Scholar] [CrossRef]
- Hackenhaar, W.; Mazzaferro, J.A.; Montevecchi, F.; Campatelli, G. An experimental-numerical study of active cooling in wire arc additive manufacturing. J. Manuf. Process. 2020, 52, 58–65. [Google Scholar] [CrossRef]
- Teixeira, F.R.; Scotti, F.M.; Reis, R.P.; Scotti, A. Effect of the CMT advanced process combined with an active cooling technique on macro and microstructural aspects of aluminum WAAM. Rapid Prototyp. J. 2021, 27, 1209–1219. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Zhou, S.; Cui, C.; Qin, L. Effects of On-Line Vortex Cooling on the Microstructure and Mechanical Properties of Wire Arc Additively Manufactured Al-Mg Alloy. Metals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Wu, B.; Pan, Z.; Ding, D.; Cuiuri, D.; Li, H.; Xu, J.; Norrish, J. A review of the wire arc additive manufacturing of metals: Properties, defects and quality improvement. J. Manuf. Process. 2018, 35, 127–139. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, Z.; Tang, Y.; Ma, S.; Chu, Y.; Li, X.; Luo, W.; Guo, L.; Zeng, X.; Lu, Y. Laser opto-ultrasonic dual detection for simultaneous compositional, structural, and stress analyses for wire+ arc additive manufacturing. Addit. Manuf. 2020, 31, 100956. [Google Scholar] [CrossRef]
- Hauser, T.; Reisch, R.; Breese, P.; Nalam, Y.; Joshi, K.; Bela, K.; Kamps, T.; Volpp, J.; Kaplan, A. Oxidation in wire arc additive manufacturing of aluminum alloys. Add. Manufactur. 2021, 101958. [Google Scholar] [CrossRef]
- Campatelli, G.; Montevecchi, F.; Venturini, G.; Ingarao, G.; Priarone, P. Integrated WAAM-subtractive versus pure subtractive manufacturing approaches: An energy efficiency comparison. Int. J. Precis. Eng. Manuf.-Green Technol. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Song, Y.A.; Park, S. Experimental investigations into rapid prototyping of composites by novel hybrid deposition process. J. Mater. Process. Technol. 2006, 171, 35–40. [Google Scholar] [CrossRef]
- Pragana, J.; Sampaio, R.; Bragança, I.; Silva, C.; Martins, P. Hybrid metal additive manufacturing: A state-of-the-art review. Adv. Ind. Manuf. Eng. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Yehorov, Y.; da Silva, L.; Scotti, A. Exploring the use of switchback for mitigating homoepitaxial unidirectional grain growth and porosity in WAAM of aluminum alloys. Int. J. Adv. Manuf. Technol. 2019, 104, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Kurz, W.; Fisher, D. Fundamentals of Solidification; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 1989. [Google Scholar]
- Lee, Y.; Nordin, M.; Babu, S.; Farson, D. Effect of fluid convection on dendrite arm spacing in laser deposition. Metall. Mater. Trans. B 2014, 45, 1520–1529. [Google Scholar] [CrossRef]
- Lippold, J. Welding Metallurgy and Weldability; Wiley Online Library: Hoboken, NJ, USA, 2015. [Google Scholar]
- Dehoff, R.; Kirka, M.; Sames, W.; Bilheux, H.; Tremsin, A.; Lowe, L.; Babu, S. Site specific control of crystallographic grain orientation through electron beam additive manufacturing. Mater. Sci. Technol. 2015, 31, 931–938. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kato, M.; Matsuda, F.; Senda, T. X-ray investigations on solidification structures in weld metal. Trans. Jpn. Weld. Soc. 1970, 39, 94–103. [Google Scholar]
- Xiong, J.; Lei, Y.; Li, R. Finite element analysis and experimental validation of thermal behavior for thin-walled parts in GMAW-based additive manufacturing with various substrate preheating temperatures. Appl. Therm. Eng. 2017, 126, 43–52. [Google Scholar] [CrossRef]
- Schempp, P.; Cross, C.; Pittner, A.; Oder, G.; Neumann, R.; Rooch, H.; Dörfel, I.; Österle, W.; Rethmeier, M. Solidification of GTA aluminum weld metal: Part I–grain morphology dependent upon alloy composition and grain refiner content. Weld. J. 2014, 93, 53–59. [Google Scholar]
- Bermingham, M.; StJohn, D.; Krynen, J.; Tedman-Jones, S.; Dargusch, M. Promoting the columnar to equiaxed transition and grain refinement of titanium alloys during additive manufacturing. Acta Mater. 2019, 168, 261–274. [Google Scholar] [CrossRef]
- Desnain, P.; Fautrelle, Y.; Meyer, J.L.; Riquet, J.P.; Durand, F. Prediction of equiaxed grain density in multicomponent alloys, stirred electromagnetically. Acta Metall. Mater. 1990, 38, 1513–1523. [Google Scholar] [CrossRef]
- Mitrašinović, A.; Hernández, F. Determination of the growth restriction factor and grain size for aluminum alloys by a quasi-binary equivalent method. Mater. Sci. Eng. A 2012, 540, 63–69. [Google Scholar] [CrossRef]
- Chandrashekar, T.; Muralidhara, M.; Kashyap, K.; Rao, P. Effect of growth restricting factor on grain refinement of aluminum alloys. Int. J. Adv. Manuf. Technol. 2009, 40, 234–241. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.; Easton, M.; Taylor, J. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Yan, F.; Xiong, W.; Faierson, E. Grain structure control of additively manufactured metallic materials. Materials 2017, 10, 1260. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J. Steady state columnar and equiaxed growth of dendrites and eutectic. Mater. Sci. Eng. 1984, 65, 75–83. [Google Scholar] [CrossRef]
- Wintwire. Precision Wire for Precision Deposition. Wintwire.co.uk. 2021. Available online: https://wintwire.co.uk/additive-manufacturing/ (accessed on 29 August 2021).
- Lincoln. Large Format Metal Additive Manufacturing Parts. Additive.Lincolnelectric.com. 2021. Available online: https://additive.lincolnelectric.com/ (accessed on 29 August 2021).
- WAAM3D. WAAM3D-Wires. WAAM3D.com. 2020. Available online: https://waam3d.com/wires (accessed on 29 August 2021).
- Voestalpine Böhler Welding. A New Range of Solid Wires Designed for Wire Arc Additive Manufacturing. voestalpine.com. 2018. Available online: https://www.voestalpine.com/welding/Company/News/A-new-range-of-solid-wires-designed-for-Wire-Arc-Additive-Manufacturing (accessed on 29 August 2021).
- Safra. Aluminum Alloys Technical Sheets. safraspa.it. 2021. Available online: http://www.safraspa.it/en/aluminum-alloys-technical-sheets/ (accessed on 29 August 2021).
- Haselhuhn, A.; Buhr, M.; Wijnen, B.; Sanders, P.; Pearce, J. Structure-property relationships of common aluminum weld alloys utilized as feedstock for GMAW-based 3-D metal printing. Mater. Sci. Eng. A 2016, 673, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.C.; Gil, E.; Solaberrieta, L.; San Sebastián, M.; Bilbao, Y.; Rodríguez, P.P. Comparison of AlSi7Mg0.6 alloy obtained by selective laser melting and investment casting processes: Microstructure and mechanical properties in as-built/as-cast and heat-treated conditions. Mater. Sci. Eng. A 2020, 778, 139124. [Google Scholar] [CrossRef]
- Li, C.; Gu, H.; Wang, W.; Wang, S.; Ren, L.; Ming, Z.; Zhai, Y.; Wang, Z. Investigation on high-temperature mechanical properties of Al–7Si–0.6 Mg alloy by wire+ arc additive manufacturing. Mater. Sci. Technol. 2020, 36, 1516–1522. [Google Scholar] [CrossRef]
- Li, C.; Gu, H.; Wang, W.; Wang, S.; Ren, L.; Wang, Z.; Ming, Z.; Zhai, Y. Effect of heat input on formability, microstructure, and properties of Al–7Si–0.6 Mg alloys deposited by CMT-WAAM process. Appl. Sci. 2020, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Xia, C.; Deng, Y.; Li, X.; Wang, H. Microstructure and mechanical properties of AlSi7Mg0.6 aluminum alloy fabricated by wire and arc additive manufacturing based on cold metal transfer (WAAM-CMT). Materials 2019, 12, 2525. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Li, H.; Li, X.; Huang, K.; Zhang, L.; Lu, B. Effect of post heat treatment on the microstructure and mechanical properties of wire-arc additively manufactured A357 alloy components. Mater. Lett. 2020, 269, 127674. [Google Scholar] [CrossRef]
- Ünsal, I.; Hirtler, M.; Sviridov, A.; Bambach, M. Material Properties of Features Produced from EN AW 6016 by Wire-Arc Additive Manufacturing. Procedia Manuf. 2020, 47, 1129–1133. [Google Scholar] [CrossRef]
- Hauser, T.; Reisch, R.T.; Seebauer, S.; Parasar, A.; Kamps, T.; Casati, R.; Volpp, J.; Kaplan, A.F. Multi-Material Wire Arc Additive Manufacturing of low and high alloyed aluminum alloys with in-situ material analysis. J. Manuf. Process. 2021, 69, 378–390. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Tan, S.; Dong, Z.; Zhou, X. Microstructure and corrosion behaviour of wire arc additive manufactured AA2024 alloy thin wall structure. Corros. Sci. 2021, 186, 109453. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Y.; Mao, P.; Liu, C.; Guo, Y.; Qian, F.; Zhang, C.; Liu, K.; Zhang, M.; Tang, S.; et al. Improving mechanical properties of wire arc additively manufactured AA2196 Al–Li alloy by controlling solidification defects. Addit. Manuf. 2021, 43, 102019. [Google Scholar] [CrossRef]
- Zhong, H.; Qi, B.; Cong, B.; Qi, Z.; Sun, H. Microstructure and Mechanical Properties of Wire+ Arc Additively Manufactured 2050 Al–Li Alloy Wall Deposits. Chin. J. Mech. Eng. 2019, 32, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, L.J.; Ning, J.; Wang, X.; Zhang, G.F.; Zhang, J.X.; Na, S.J. Microstructures and mechanical properties of Al–Zn–Mg aluminum alloy samples produced by wire+ arc additive manufacturing. J. Mater. Res. Technol. 2020, 9, 13770–13780. [Google Scholar] [CrossRef]
- Li, S.; Ning, J.; Zhang, G.F.; Zhang, L.J.; Wu, J.; Zhang, L.X. Microstructural and mechanical properties of wire-arc additively manufactured Al–Zn–Mg aluminum alloy: The comparison of as-deposited and heat-treated samples. Vacuum 2021, 184, 109860. [Google Scholar] [CrossRef]
- Morais, P.; Gomes, B.; Santos, P.; Gomes, M.; Gradinger, R.; Schnall, M.; Bozorgi, S.; Klein, T.; Fleischhacker, D.; Warczok, P.; et al. Characterisation of a High-Performance Al–Zn–Mg–Cu Alloy Designed for Wire Arc Additive Manufacturing. Materials 2020, 13, 1610. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Chen, G.; Yang, J.; Xie, Y.; Huang, K.; Lu, B. Wire and arc additive manufacturing of high strength Al-Zn-Mg aluminum alloy. Front. Mater. 2021, 8, 116. [Google Scholar] [CrossRef]
- Gu, J.; Gao, M.; Yang, S.; Bai, J.; Zhai, Y.; Ding, J. Microstructure, defects, and mechanical properties of wire+ arc additively manufactured AlCu4.3-Mg1.5 alloy. Mater. Des. 2020, 186, 108357. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Zeng, X. Effect of Process Parameters on Mechanical Properties of Wire and Arc Additive-Manufactured AlCu6Mn. JOM 2019, 71, 886–892. [Google Scholar] [CrossRef]
- Li, C.; Gu, H.; Wang, W.; Wang, S.; Ren, L.; Wang, Z.; Ming, Z.; Zhai, Y. Effects of magnesium on the microstructure and properties of Al–Si alloy deposited by wire and arc-based additive manufacturing. Mater. Technol. 2020, 1–6. [Google Scholar] [CrossRef]
- Qi, Z.; Qi, B.; Cong, B.; Sun, H.; Zhao, G.; Ding, J. Microstructure and mechanical properties of wire+ arc additively manufactured 2024 aluminum alloy components: As-deposited and post heat-treated. J. Manuf. Process. 2019, 40, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Yuan, T.; Xu, M.; Zhang, H.; Jiang, X.; Chen, S. Microstructure and mechanical properties of Al–Zn–Mg–Cu alloy fabricated by wire+ arc additive manufacturing. J. Manuf. Process. 2021, 62, 430–439. [Google Scholar] [CrossRef]
- Klein, T.; Birgmann, A.; Schnall, M. In situ alloying of aluminium-based alloys by (multi-) wire-arc additive manufacturing. In Proceedings of the MATEC Web of Conferences, EDP Sciences, 17th International Conference on Aluminium Alloys, Grenoble, France, 26–29 October 2020; Volume 326, p. 01003. [Google Scholar] [CrossRef]
- Qi, Z.; Cong, B.; Qi, B.; Sun, H.; Zhao, G.; Ding, J. Microstructure and mechanical properties of double-wire+ arc additively manufactured Al-Cu-Mg alloys. J. Mater. Process. Technol. 2018, 255, 347–353. [Google Scholar] [CrossRef]
- Qi, Z.; Qi, B.; Cong, B.; Zhang, R. Microstructure and mechanical properties of wire+ arc additively manufactured Al-Mg-Si aluminum alloy. Mater. Lett. 2018, 233, 348–350. [Google Scholar] [CrossRef]
- Eimer, E.; Suder, W.; Williams, S.W.; Ding, J. Wire laser arc additive manufacture of aluminum zinc alloys. Weld. World 2020, 64, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Morinaga, M.; Yukawa, N.; Ogawa, H.; Kato, M. Solidification structures of Inconel 718 with microalloying elements. Superalloys 1994, 718, 81–88. [Google Scholar]
- Khorev, A. Complex alloying and microalloying of titanium alloys. Weld. Int. 2011, 25, 56–63. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Z.h.; Wang, G.s.; Zhang, C.; Cui, J.z. Microstructure and mechanical properties of the welding joint filled with microalloying 5183 aluminum welding wires. Int. J. Miner. Metall. Mater. 2014, 21, 577–582. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminum alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Katsas, S.; Dashwood, R.; Grimes, R.; Jackson, M.; Todd, G.; Henein, H. Dynamic recrystallisation and superplasticity in pure aluminum with zirconium addition. Mater. Sci. Eng. A 2007, 444, 291–297. [Google Scholar] [CrossRef]
- Qian, F.; Jin, S.; Sha, G.; Li, Y. Enhanced dispersoid precipitation and dispersion strengthening in an Al alloy by microalloying with Cd. Acta Mater. 2018, 157, 114–125. [Google Scholar] [CrossRef]
- Clyne, T.; Nazar, A.; Prates, M.; Davies, G. Grain refinement of aluminum using niobium additions. Met. Technol. 1978, 5, 302–308. [Google Scholar] [CrossRef]
- Ding, W.; Xia, T.; Zhao, W. Performance comparison of Al–Ti master alloys with different microstructures in grain refinement of commercial purity aluminum. Materials 2014, 7, 3663–3676. [Google Scholar] [CrossRef] [Green Version]
- Czerwinski, F. Cerium in aluminum alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, D.W.; Zhu, Z.X.; Chen, B.A.; Xin, C.; Yang, C.L.; Zhang, H.Y.; Wei, K. Grain refinement mechanism of as-cast aluminum by hafnium. Trans. Nonferrous Met. Soc. China 2016, 26, 3059–3069. [Google Scholar] [CrossRef]
- Xiao, D.; Song, M.; Chen, K.; Huang, B. Effect of rare earth Yb addition on mechanical properties of Al–5.3Cu–0.8Mg–0.6Ag alloy. Mater. Sci. Technol. 2007, 23, 1156–1160. [Google Scholar] [CrossRef]
- Wu, H.; Wen, S.; Gao, K.; Huang, H.; Wang, W.; Nie, Z. Effect of Er additions on the precipitation strengthening of Al–Hf alloys. Scr. Mater. 2014, 87, 5–8. [Google Scholar] [CrossRef]
- Souza, P.; de Oliveira, C.; do Vale Quaresma, J. Precipitation hardening in dilute Al–Zr alloys. J. Mater. Res. Technol. 2018, 7, 66–72. [Google Scholar] [CrossRef]
- Knipling, K.E.; Karnesky, R.A.; Lee, C.P.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–0.1 Sc, Al–0.1 Zr and Al–0.1 Sc–0.1 Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184–5195. [Google Scholar] [CrossRef]
- Clouet, E.; Barbu, A.; Laé, L.; Martin, G. Precipitation kinetics of Al3Zr and Al3Sc in aluminum alloys modeled with cluster dynamics. Acta Mater. 2005, 53, 2313–2325. [Google Scholar] [CrossRef] [Green Version]
- Ram, G.J.; Mitra, T.; Shankar, V.; Sundaresan, S. Microstructural refinement through inoculation of type 7020 Al–Zn–Mg alloy welds and its effect on hot cracking and tensile properties. J. Mater. Process. Technol. 2003, 142, 174–181. [Google Scholar] [CrossRef]
- Norman, A.; Birley, S.; Prangnell, P. Development of new high strength Al–Sc filler wires for fusion welding 7000 series aluminum aerospace alloys. Sci. Technol. Weld. Join. 2003, 8, 235–245. [Google Scholar] [CrossRef]
- Sales, A.; Ricketts, N. Effect of Scandium on Wire Arc Additive Manufacturing of 5 Series Aluminium Alloys. In Light Metals 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1455–1461. [Google Scholar] [CrossRef]
- Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. Effect of Sc Content on the Microstructure and Properties of Al–Mg–Sc Alloys Deposited by Wire Arc Additive Manufacturing. Met. Mater. Int. 2021, 27, 68–77. [Google Scholar] [CrossRef]
- Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. Microstructure and Properties of Al-6.0 Mg-0.3 Sc Alloy Deposited by Double-Wire Arc Additive Manufacturing. 3D Print. Addit. Manuf. 2021. [Google Scholar] [CrossRef]
- Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. The microstructure and properties of an Al-Mg-0.3 Sc alloy deposited by wire arc additive manufacturing. Metals 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Ponomareva, T.; Ponomarev, M.; Kisarev, A.; Ivanov, M. Wire Arc Additive Manufacturing of Al-Mg Alloy with the Addition of Scandium and Zirconium. Materials 2021, 14, 3665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Suo, Y.; Liang, Z.; Wang, D.; Wang, Q. Effect of titanium powder on microstructure and mechanical properties of wire+ arc additively manufactured Al-Mg alloy. Mater. Lett. 2019, 241, 231–234. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, K.; Yang, G.; Deng, F.; Hou, N.; Qin, L.; Wei, W. Grain-refining of wire arc additive manufactured aluminum alloy with Nb powder addition. Mater. Res. Express 2021, 8, 026520. [Google Scholar] [CrossRef]
- Nowak, M.; Yeoh, W.; Bolzoni, L.; Babu, N.H. Development of Al–Nb–B master alloys using Nb and KBF4 Powders. Mater. Des. 2015, 75, 40–46. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Q.; Li, X.; Meng, C.; Ye, B.; Gou, B. Low-temperature synthesis of Ti3Al(Sn)C2 solid solution using replacement reaction. J. Mater. Sci. Mater. Electron. 2020, 31, 20601–20610. [Google Scholar] [CrossRef]
- Wang, S.; Gu, H.; Wang, W.; Li, C.; Ren, L.; Wang, Z.; Zhai, Y.; Ma, P. The Influence of Heat Input on the Microstructure and Properties of Wire-Arc-Additive-Manufactured Al-Cu-Sn Alloy Deposits. Metals 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gu, H.; Wang, W.; Li, C.; Ren, L.; Wang, Z.; Zhai, Y.; Ma, P. Study on Microstructural and Mechanical Properties of an Al–Cu–Sn Alloy Wall Deposited by Double-Wire Arc Additive Manufacturing Process. Materials 2020, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, X.; Wu, L.; Yang, Q.; Chen, Y. Microstructure and Performance of WAAM TiB2-Reinforced Al–Si-Based Composites. In Chinese Materials Conference; Springer: Berlin, Germany, 2018; pp. 321–328. [Google Scholar] [CrossRef]
- Yang, Q.; Xia, C.; Deng, Y. Microstructure and Mechanical Properties of TiB2/Al-Si Composites Fabricated by TIG Wire and Arc Additive Manufacturing. Mater. Sci. Forum 2019, 944, 64–72. [Google Scholar] [CrossRef]
- Fattahi, M.; Mohammady, M.; Sajjadi, N.; Honarmand, M.; Fattahi, Y.; Akhavan, S. Effect of TiC nanoparticles on the microstructure and mechanical properties of gas tungsten arc welded aluminum joints. J. Mater. Process. Technol. 2015, 217, 21–29. [Google Scholar] [CrossRef]
- Jin, P.; Liu, Y.; Sun, Q. Evolution of crystallographic orientation, columnar to equiaxed transformation and mechanical properties realized by adding TiCps in wire and arc additive manufacturing 2219 aluminum alloy. Addit. Manuf. 2021, 39, 101878. [Google Scholar] [CrossRef]
- Winterkorn, R.; Pittner, A.; Rethmeier, M. Wire Arc Additive Manufacturing with Novel Al-Mg-Si Filler Wire—Assessment of Weld Quality and Mechanical Properties. Metals 2021, 11, 1243. [Google Scholar] [CrossRef]
- Oropeza, D.; Hofmann, D.; Williams, K.; Firdosy, S.; Bordeenithikasem, P.; Sokoluk, M.; Liese, M.; Liu, J.; Li, X. Welding and additive manufacturing with nanoparticle-enhanced aluminum 7075 wire. J. Alloys Compd. 2020, 834, 154987. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Aghaei, V.N.; Dabiri, A.; Amirkhanlou, S.; Akhavan, S.; Fattahi, Y. Novel manufacturing process of nanoparticle/Al composite filler metals of tungsten inert gas welding by accumulative roll bonding. Mater. Sci. Eng. A 2015, 648, 47–50. [Google Scholar] [CrossRef]
- Sokoluk, M.; Cao, C.; Pan, S.; Li, X. Nanoparticle-enabled phase control for arc welding of unweldable aluminum alloy 7075. Nat. Commun. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.; Yahata, B.; Hundley, J.; Mayer, J.; Schaedler, T.; Pollock, T. 3D printing of high-strength aluminum alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Li, X. Selective laser melting of aluminum and aluminum metal matrix composites. Mater. Technol. 2016, 31, 77–85. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Sun, Q.; Fan, Z.; Li, G.; Yin, Y.; Liu, Y.; Zhang, M.X. Inoculation treatment of an additively manufactured 2024 aluminium alloy with titanium nanoparticles. Acta Mater. 2020, 196, 98. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Z.; Xiao, Z.; You, D.; Wong, K.; Akbarzadeh, A. Selective laser melting of TiN nanoparticle-reinforced AlSi10Mg composite: Microstructural, interfacial, and mechanical properties. J. Mater. Process. Technol. 2020, 281, 116618. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M. Particle distribution in cast metal matrix composites—Part I. J. Mater. Process. Technol. 2002, 123, 251–257. [Google Scholar] [CrossRef]
- Liu, W.; Cao, C.; Xu, J.; Wang, X.; Li, X. Molten salt assisted solidification nanoprocessing of Al-TiC nanocomposites. Mater. Lett. 2016, 185, 392–395. [Google Scholar] [CrossRef]
- Gu, D.; Rao, X.; Dai, D.; Ma, C.; Xi, L.; Lin, K. Laser additive manufacturing of carbon nanotubes (CNTs) reinforced aluminum matrix nanocomposites: Processing optimization, microstructure evolution and mechanical properties. Addit. Manuf. 2019, 29, 100801. [Google Scholar] [CrossRef]
- Carvalho, O.; Miranda, G.; Soares, D.; Silva, F. CNT-reinforced aluminum composites: Processing and mechanical properties. Ciência Tecnol. Dos Mater. 2013, 25, 75–78. [Google Scholar] [CrossRef]
- Werenskiold, J.; Auran, L.; Roven, H.; Ryum, N.; Reiso, O. Screw Extruder for Continous Extrusion of Materials with High Viscosity. US Patent EP2086697B1 WO2008 06307, 20 November 2008. [Google Scholar]
- Langelandsvik, G.; Grandcolas, M.; Skorpen, K.; Furu, T.; Akselsen, O.; Roven, H. Development of Al-TiC Wire Feedstock for Additive Manufacturing by Metal Screw Extrusion. Metals 2020, 10, 1485. [Google Scholar] [CrossRef]
- Langelandsvik, G.; Ragnvaldsen, O.; Flåm, J.E.; Akselsen, O.M.; Roven, H.J. Wire and Arc Additive Manufacturing with TiC-Nanoparticle Reinforced AA5183 Alloy. In Proceedings of the MATEC Web of Conferences, EDP Sciences, 17th International Conference on Aluminium Alloys, Grenoble, France, 26–29 October 2020; Volume 326, p. 07002. [Google Scholar] [CrossRef]
- Jin, P.; Liu, Y.; Li, F.; Sun, Q. Realization of synergistic enhancement for fracture strength and ductility by adding TiC particles in wire and arc additive manufacturing 2219 aluminum alloy. Compos. Part B Eng. 2021, 219, 108921. [Google Scholar] [CrossRef]
| Element i | |||
|---|---|---|---|
| Si | 0.11 | −6.6 | 5.9 |
| Mg | 0.51 | −6.2 | 3.0 |
| Mn | 0.94 | −1.6 | 0.1 |
| Cu | 0.17 | −3.4 | 2.8 |
| Zn | 0.88 | −2.97 | 0.3 |
| Fe | 0.02 | −3.0 | 2.9 |
| Ti | 7.8 | 33.3 | 220 |
| V | 4.0 | 10.0 | 30 |
| Mo | 2.5 | 5.0 | 7.5 |
| Nb | 1.5 | 13.3 | 6.6 |
| Cr | 2.0 | 3.5 | 3.5 |
| B | 0.067 | 1.0 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langelandsvik, G.; Akselsen, O.M.; Furu, T.; Roven, H.J. Review of Aluminum Alloy Development for Wire Arc Additive Manufacturing. Materials 2021, 14, 5370. https://doi.org/10.3390/ma14185370
Langelandsvik G, Akselsen OM, Furu T, Roven HJ. Review of Aluminum Alloy Development for Wire Arc Additive Manufacturing. Materials. 2021; 14(18):5370. https://doi.org/10.3390/ma14185370
Chicago/Turabian StyleLangelandsvik, Geir, Odd M. Akselsen, Trond Furu, and Hans J. Roven. 2021. "Review of Aluminum Alloy Development for Wire Arc Additive Manufacturing" Materials 14, no. 18: 5370. https://doi.org/10.3390/ma14185370







