The Preparation of Dense Materials in the MgO–ZrO2 System by the Application of Nanometric Powders
Abstract
:1. Introduction
2. Materials and Methods
3. Results
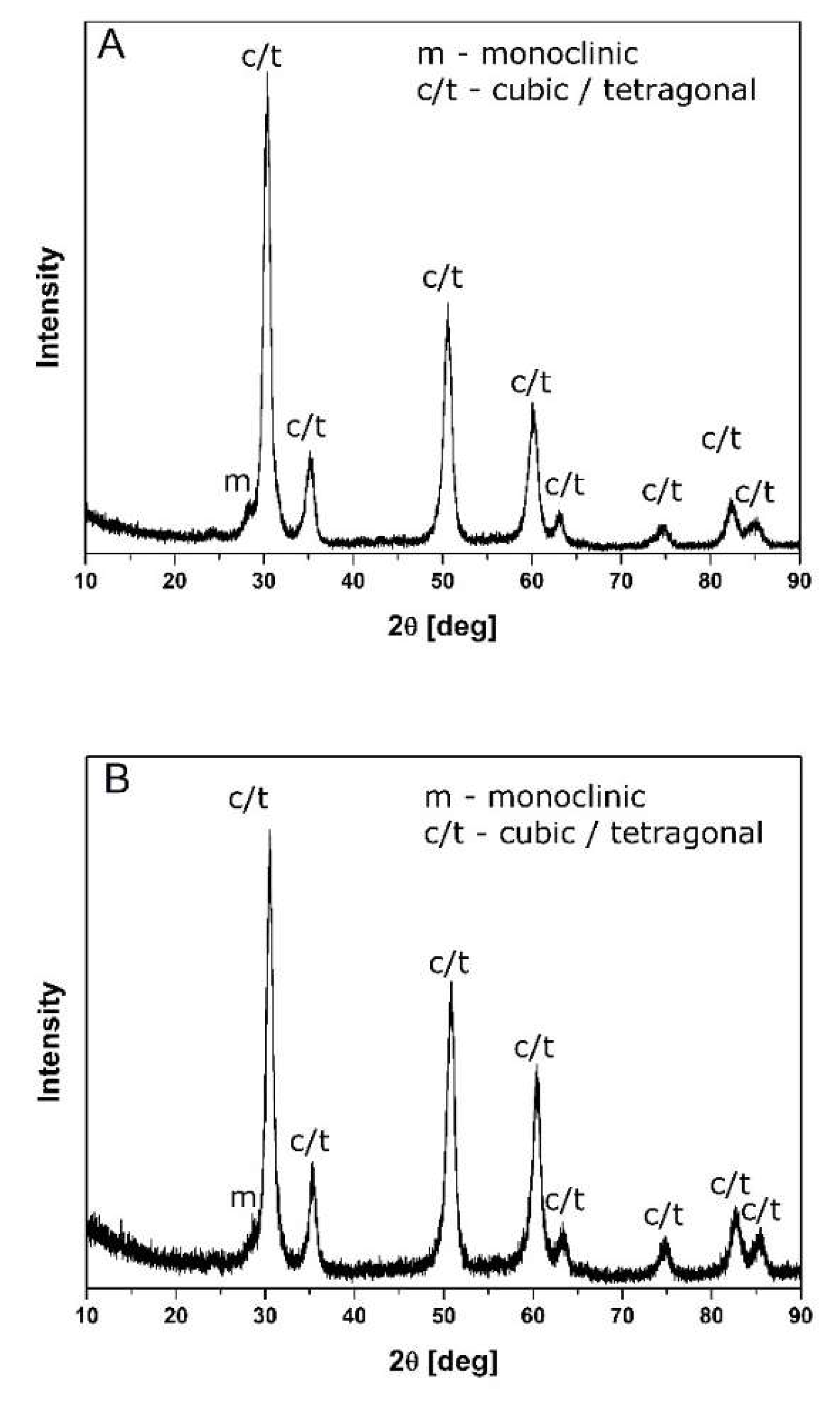
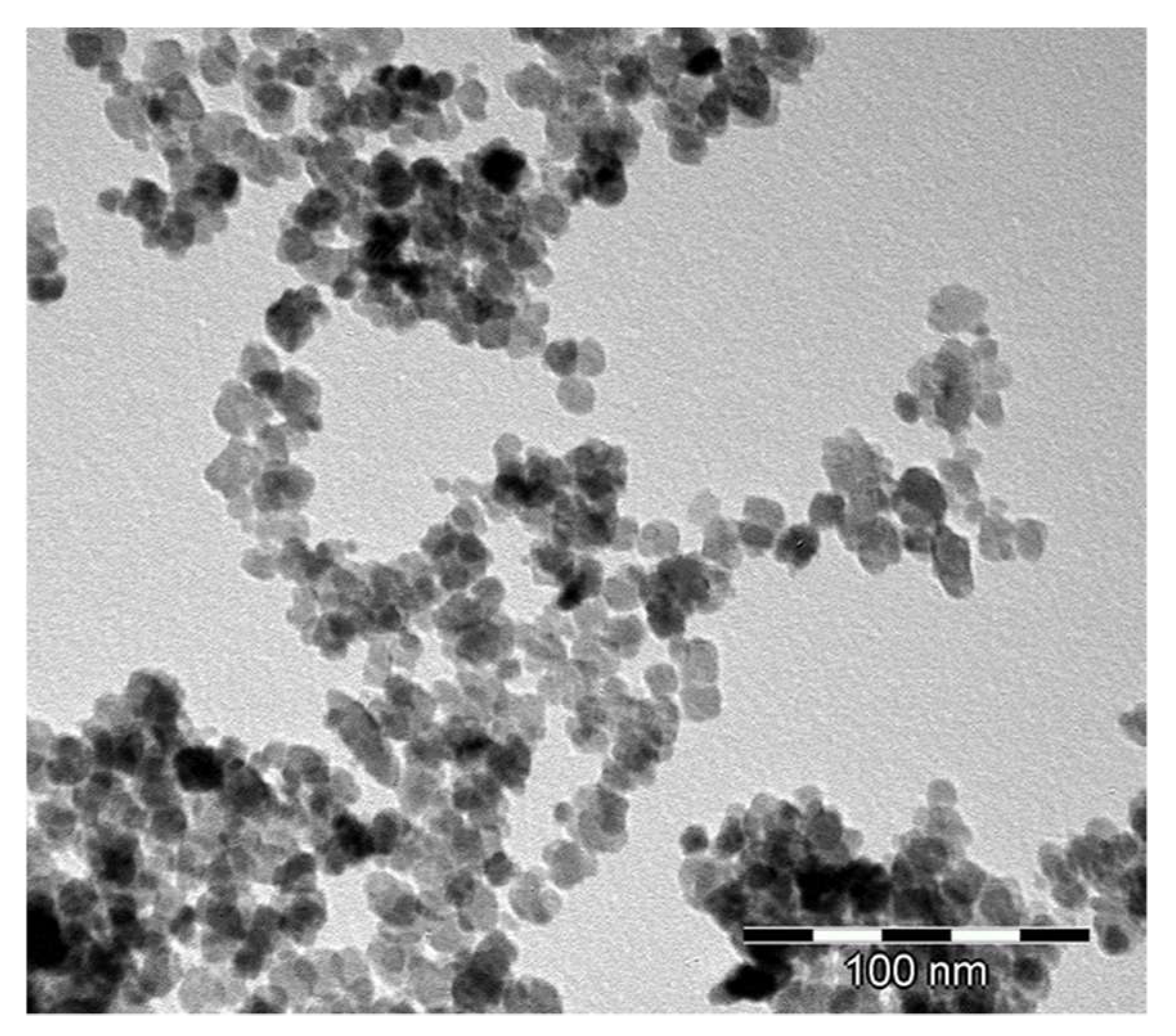
3.1. Powder Characteristics
3.2. Compact Characteristics
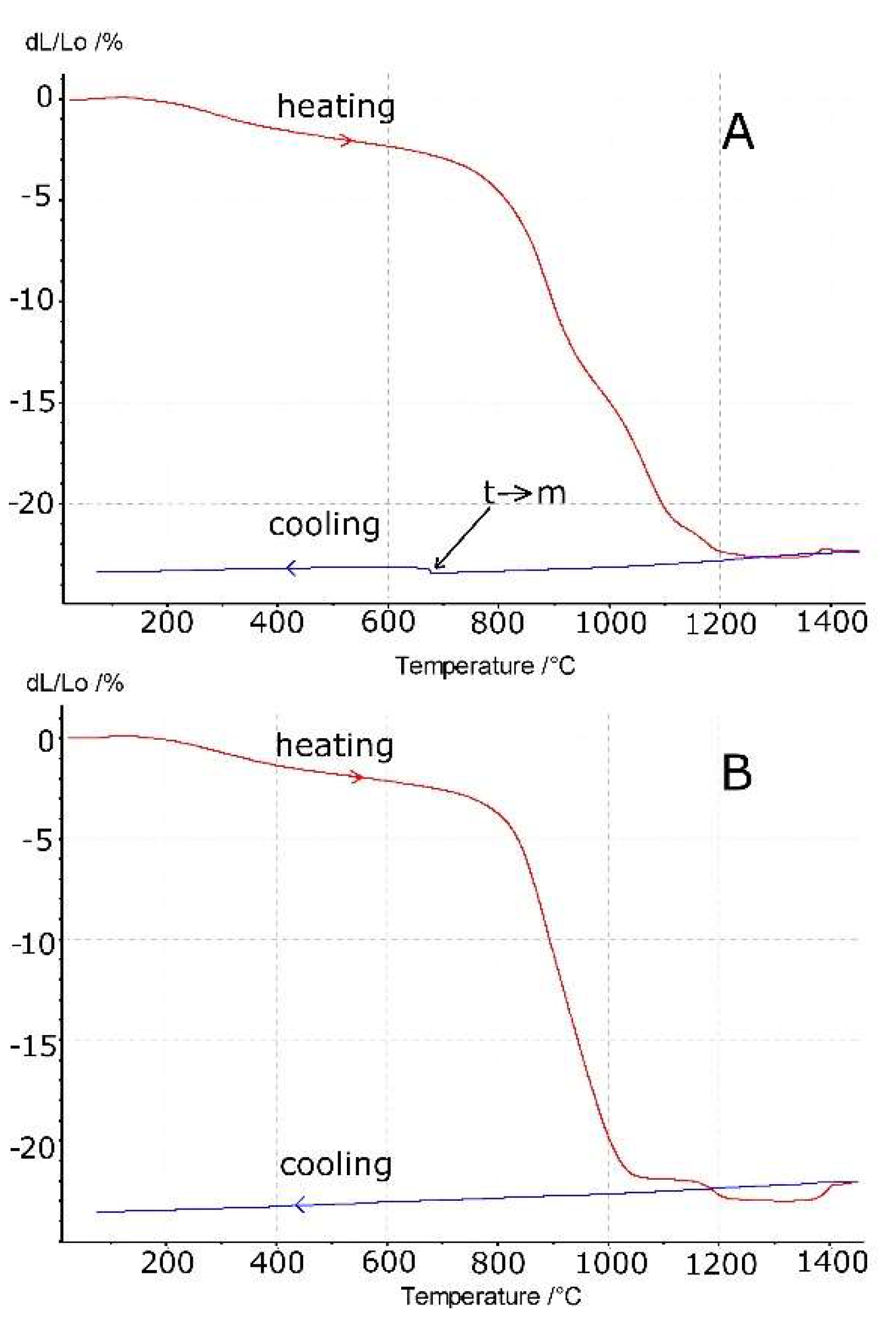
3.3. Sintering
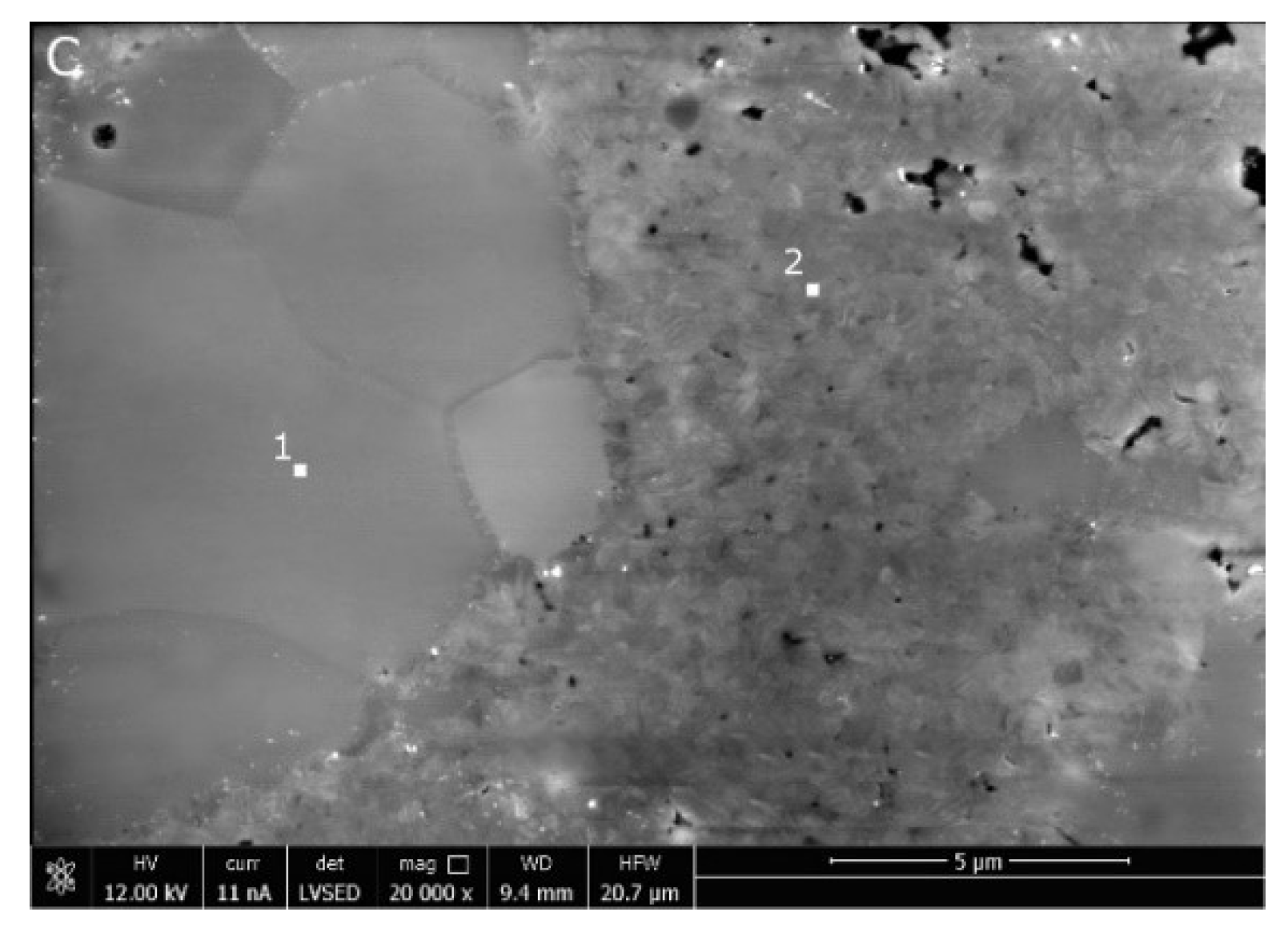
3.4. Material Characteristics
4. Conclusions
- The coprecipitation technique within the MgO–ZrO2 system is not quantitative when NH3∙aqa is used as the precipitating agent;
- Crystallization under hydrothermal conditions results in nanometric equi-axed particles with no strong contacts between them; this is due to the fact that the creation of new particles occurs in the water environment which wets and penetrates between them.
- The freeze drying of nanometric powder suspension leads to fluffy powders;
- The compacts of such powders show monomodal pore size distribution. The average pore sizes correspond well to their modal values, which indicates that agglomerates, if present in the starting powders, do not survive under compaction pressure;
- The dilatometric measurements show that the shrinkage of the compacts starts at a temperature as low as 200 °C. This was attributed to water desorption from the large surface area of the samples. Sintering shrinkage starts at about 800 °C and is completed at about 1300 °C;
- A monotonous cooling run occurs in the material with a higher MgO concentration. This is explained by its single-phase (cubic) composition. The material with a lower MgO content (7.4 mol%) shows discontinuity at about 680 °C, which can be attributed to the tetragonal- to monoclinic-phase transformation;
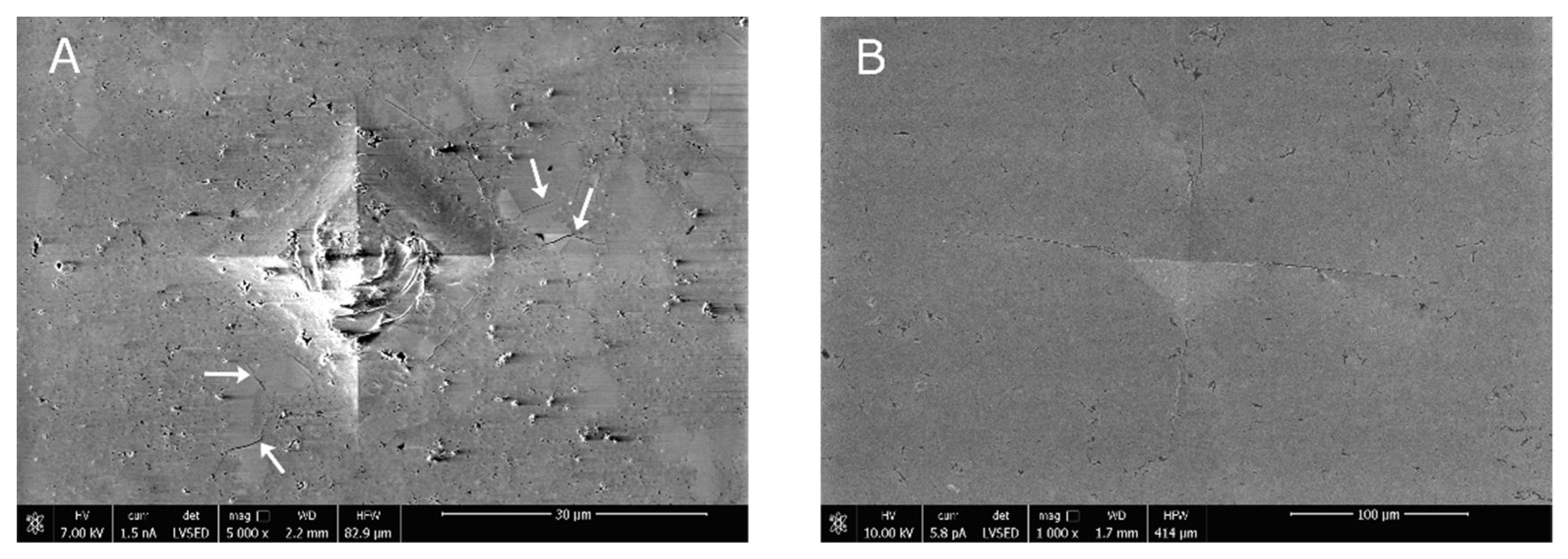
- The microstructure of the material with a higher MgO concentration is typical of single-phase polycrystals. However, the one with a lower MgO content shows larger cubic grains surrounded by particles of monoclinic and tetragonal symmetry. Such a microstructure differs substantially from the partially stabilized zirconia manufactured via “classical” technology. The relatively high fracture toughness of this material is explained by the formation of numerous cracks on the Vickers pyramid penetration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porter, D.L.; Heuer, A.H. Microstructural development in MgO-Partially Stabilized Zirconia. J. Am. Ceram. Soc. 1979, 62, 298–305. [Google Scholar] [CrossRef]
- Hannink, R.H.J. Microstructural development of sub-eutectoid aged MgO-ZrO2 alloys. Micron 1982, 13, 277–278. [Google Scholar]
- Muddle, B.C.; Hannink, R.H.J. Crystallography of the Tetragonal to monoclinic transformation in MgO-partially-stabilized zirconia. J. Am. Ceram. Soc. 1986, 69, 547–555. [Google Scholar] [CrossRef]
- Hughan, R.R.; Hannink, R.H.J. Precipitation during controlled cooling of magnesia-partially-stabilized zirconia. J. Am. Ceram. Soc. 1986, 69, 556–563. [Google Scholar] [CrossRef]
- Nairn, J.D.; St John, D.H. The relationship between the mechanical properties of Mg-PSZ and the formation of metastable tetragonal precipitates. Mater. Sci. Forum 1988, 34, 111–115. [Google Scholar] [CrossRef]
- Hay, S.; Nairn, J.D.; St John, D.H. Secondary precipitate growth in Mg-PSZ. Key Eng. Mater. 1991, 53, 744–749. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Chyad, F.A. SEM-assisted thermophysical and mechanical properties of sintered MgO-ZrO2 composite. Energy Procedia 2013, 36, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Argent, B.B. Phase diagrams and Thermodynamics of the systems ZrO2-CaO and ZrO2-MgO. J. Phase Equlibria 1993, 14, 439–450. [Google Scholar] [CrossRef]
- Garvie, R.; Hannink, R.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Becher, P.F. Subcritical crack growth in partially stabilized ZrO2 (MgO). J. Mater. Sci. 1986, 21, 297–300. [Google Scholar] [CrossRef]
- Becher, P.F.; Ferber, M.K. Mechanical behavior of MgO-partially stabilized ZrO2 Ceramics at elevated temperatures. J. Mater. Sci. 1987, 22, 973–980. [Google Scholar] [CrossRef]
- Hine, T.J.; Ferber, M.K.; Leigh, D.W. Time-Dependent Mechanical Behavior of MgO-PSZ Ceramics. Adv. Ceram. Mater. 1988, 3, 80–87. [Google Scholar] [CrossRef]
- Andrews, M.J.; Ferber, M.K.; Lara-Curzio, E. Mechanical properties of zirconia-based ceramics as functions of temperature. J. Eur. Ceram. Soc. 2002, 22, 2633–2639. [Google Scholar] [CrossRef]
- Muccillo, E.N.S.; Tadokoro, S.K.; Muccillo, R. Physical characteristics and sintering behavior of MgO-Doped ZrO2 nanoparticles. J. Nanoparticle Res. 2004, 6, 301–305. [Google Scholar] [CrossRef]
- Settu, T. Characterisation of MgO-ZrO2 precursor powders prepared by in-situ peptisation of coprecipitated oxalate gel. Ceram. Int. 2000, 26, 517–521. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, Y.; Li, X.; Li, Y.; Wang, K. Coprecipitation Synthesis of MgO-Doped ZrO2 Nano Powder. J. Am. Ceram. Soc. 2006, 89, 3577–3581. [Google Scholar] [CrossRef]
- Readey, M.J.; Lee, R.R.; Halloran, J.W.; Heuer, A.H. Processing and Sintering of Ultrafine MgO-ZrO2 and (MgO, Y2O3)-ZrO2 Powders. J. Am. Ceram. Soc. 1990, 73, 1499–1503. [Google Scholar] [CrossRef]
- Rumpf, H. Grundlagen und Methoden des Granulirens. Chem. Ing. Tech. 1958, 30, 144–158. [Google Scholar] [CrossRef]
- Bućko, M.M.; Haberko, K.; Faryna, M. Crystallization of Zirconia under Hydrothermal Conditions. J. Am. Ceram. Soc. 1995, 78, 3397–3400. [Google Scholar] [CrossRef]
- Lach, R.; Bućko, M.M.; Haberko, K.; Sitarz, M.; Cholewa-Kowalska, K. From nanometric zirconia powder to transparent polycrystal. J. Eur. Ceram. Soc. 2014, 34, 4321–4326. [Google Scholar] [CrossRef]
- Lach, R.; Wojciechowski, K.; Zientara, D.; Haberko, K.; Bućko, M.M. Zirconia nano-powder—A useful precursor to dense polycrystals. Ceram. Int. 2017, 43, 4470–4474. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of KIC of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1981, 2, 13–16. [Google Scholar]
- Niihara, K. A fracture mechanics analysis of indentation indentation induced Palmqvist crack in ceramics. J. Mater. Sci. Lett. 1983, 2, 221–223. [Google Scholar] [CrossRef]
- Collongues, R. La-Nonstoechiometrie; Masson et Cie Editeures: Paris, France, 1971; Chapter 4. [Google Scholar]
- Kingery, W.D. Kinetics of High Temperature Processes; MIT Press: Cambridge, MA, USA; J.Wiley and Sons, Inc.: New York, NY, USA; Chapman and Hall Ltd.: London, UK, 1959; Chapter 24. [Google Scholar]
- Zych, Ł.; Haberko, K. Filter pressing and sintering of a zirconia nanpowder. J. Eur. Ceram. Soc. 2006, 26, 373–378. [Google Scholar] [CrossRef]
- Zych, Ł.; Haberko, K. Some observations on filter-pressing and sintering of zirconia nanopowder. J. Eur. Ceram. Soc. 2007, 27, 867–871. [Google Scholar] [CrossRef]


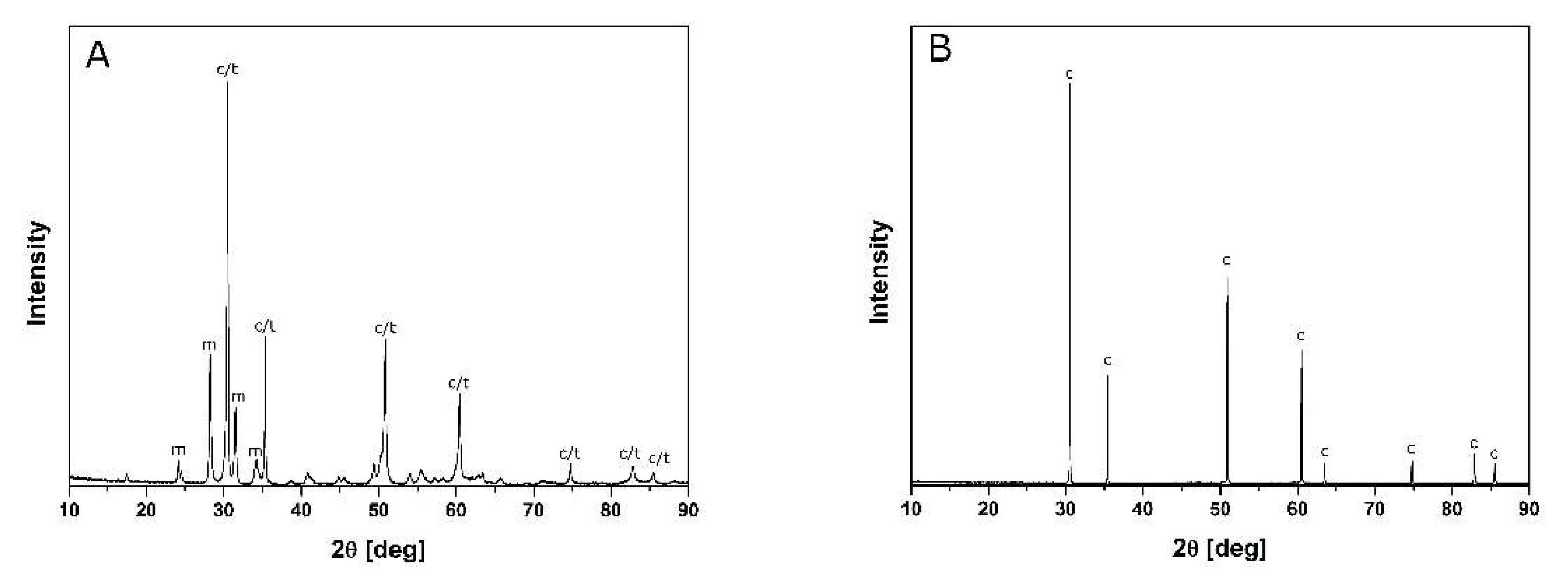
| MgO mol% | Sw m2/g | DBET nm | D111 nm | Monoclinic wt% | X-ray Density g/cm3 | Cubic/Tetragonal wt% |
|---|---|---|---|---|---|---|
| 7.4 | 124.5 | 8.1 | 8.5 | 9.1 | 5.91 | 90.9 |
| 11.5 | 128.3 | 7.9 | 8.3 | 9.5 | 5.92 | 90.5 |
| Powder | Density g/cm3 | Relative Density % | Average Pore Size dp nm | Mode, nm |
|---|---|---|---|---|
| 7.4 mol% MgO | 2.59 | 43.8 | 9.2 | 9.0 |
| 11.5 mol% MgO | 2.58 | 43.6 | 9.0 | 9.0 |
| Sample Sintering Conditions | Monoclinic wt% | Tetragonal w% | Cubic wt% | MgO wt% | HV, GPa | KIC, MPa∙m1/2 | σ, MPa | d, g/cm3 |
|---|---|---|---|---|---|---|---|---|
| 7.4 mol% MgO, 1450°C, (H-C) | 72.2 | 14.4 | 11.5 | 1.9 | 9.4 ± 0.8 | 9.48 ± 0.20 | - | 5.581 ± 0.003 |
| 7.4 mol% MgO, 1450°C, 2h | 41.6 | 13.8 | 44.6 | - | 10.5 ± 0.3 | 8.75 ± 0.18 | 424 ± 3 | 5.679 ± 0.003 |
| 11.5 mol% MgO, 1450 °C, (H-C) | - | - | 100 | - | 11.4 ± 0.6 | 5.77 ± 0.14 | - | 5.395 ± 0.002 |
| 11.5 mol% MgO 1450 °C, 2h | - | - | 100 | - | 10.9 ± 0.7 | 5.29 ± 0.11 | 198 ± 4 | 5.541 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojteczko, K.; Haberko, K.; Berent, K.; Rutkowski, P.; Bućko, M.M.; Pędzich, Z. The Preparation of Dense Materials in the MgO–ZrO2 System by the Application of Nanometric Powders. Materials 2021, 14, 5478. https://doi.org/10.3390/ma14195478
Wojteczko K, Haberko K, Berent K, Rutkowski P, Bućko MM, Pędzich Z. The Preparation of Dense Materials in the MgO–ZrO2 System by the Application of Nanometric Powders. Materials. 2021; 14(19):5478. https://doi.org/10.3390/ma14195478
Chicago/Turabian StyleWojteczko, Kamil, Krzysztof Haberko, Katarzyna Berent, Paweł Rutkowski, Mirosław M. Bućko, and Zbigniew Pędzich. 2021. "The Preparation of Dense Materials in the MgO–ZrO2 System by the Application of Nanometric Powders" Materials 14, no. 19: 5478. https://doi.org/10.3390/ma14195478
APA StyleWojteczko, K., Haberko, K., Berent, K., Rutkowski, P., Bućko, M. M., & Pędzich, Z. (2021). The Preparation of Dense Materials in the MgO–ZrO2 System by the Application of Nanometric Powders. Materials, 14(19), 5478. https://doi.org/10.3390/ma14195478







