AlN Production in Co-Flow Filtration Mode at Low Pressures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Components
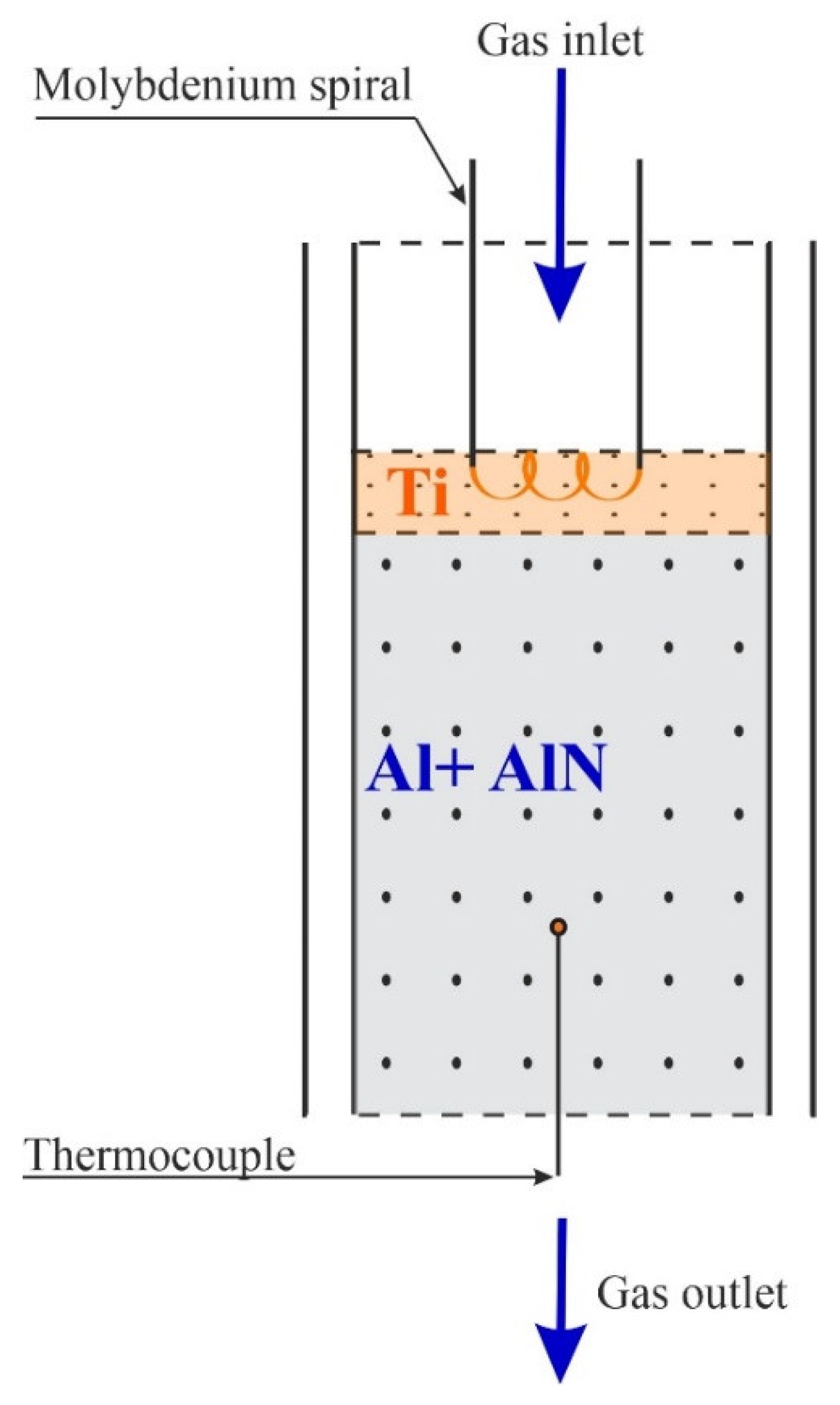
2.2. Experimental Part
2.3. Characterization
3. Results and Discussion
3.1. Determination of the Optimal Composition of the Initial Powder Mixture
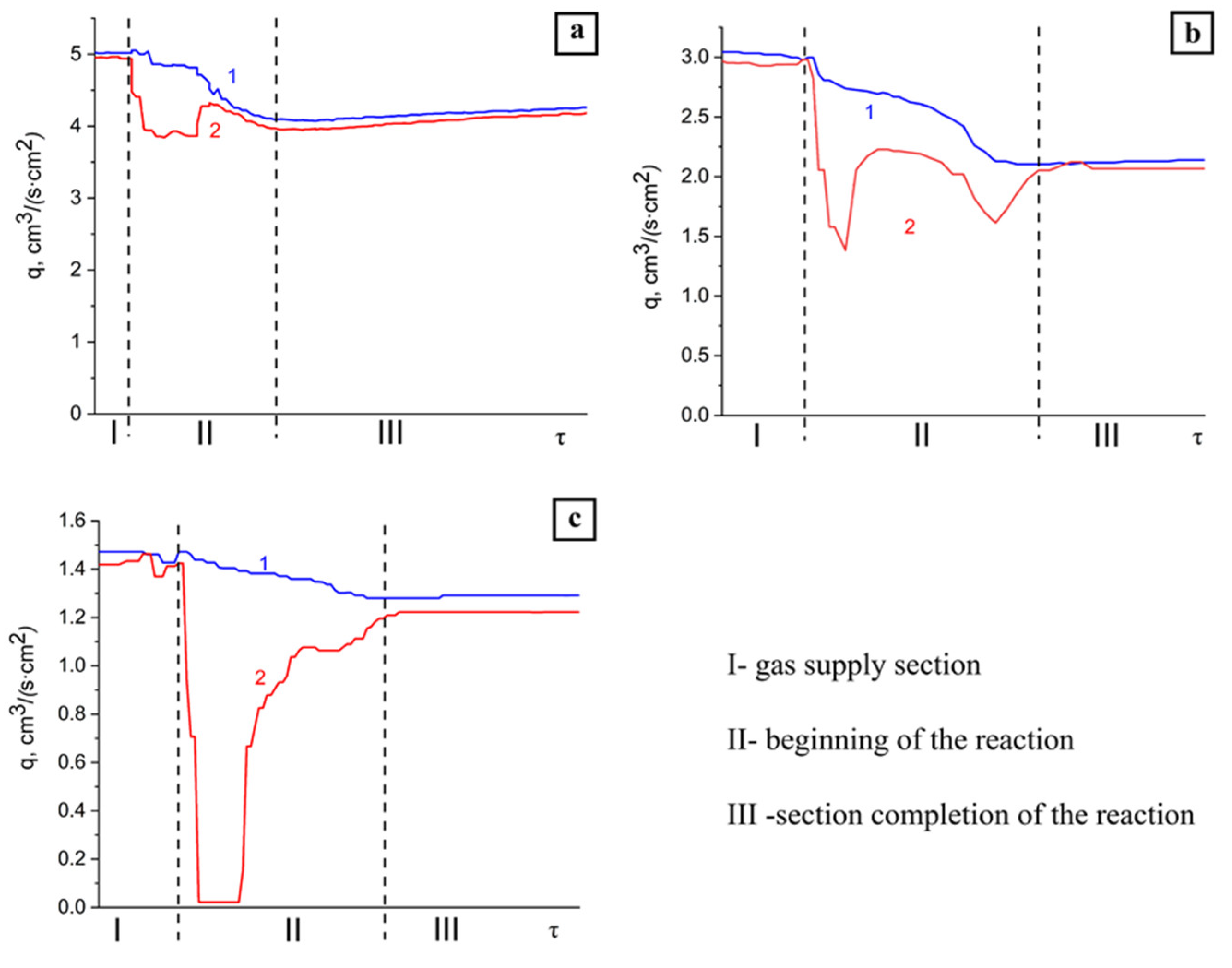
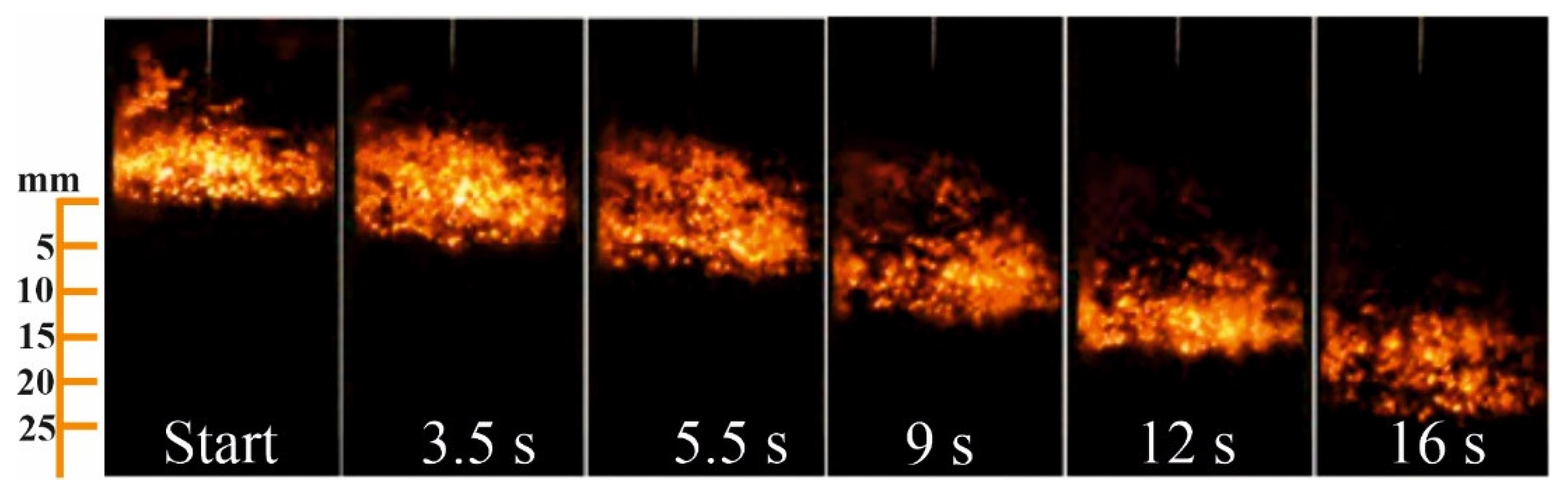
3.2. Influence of the Specific Nitrogen Flow Rate on the Combustion Rate of the Initial Composition
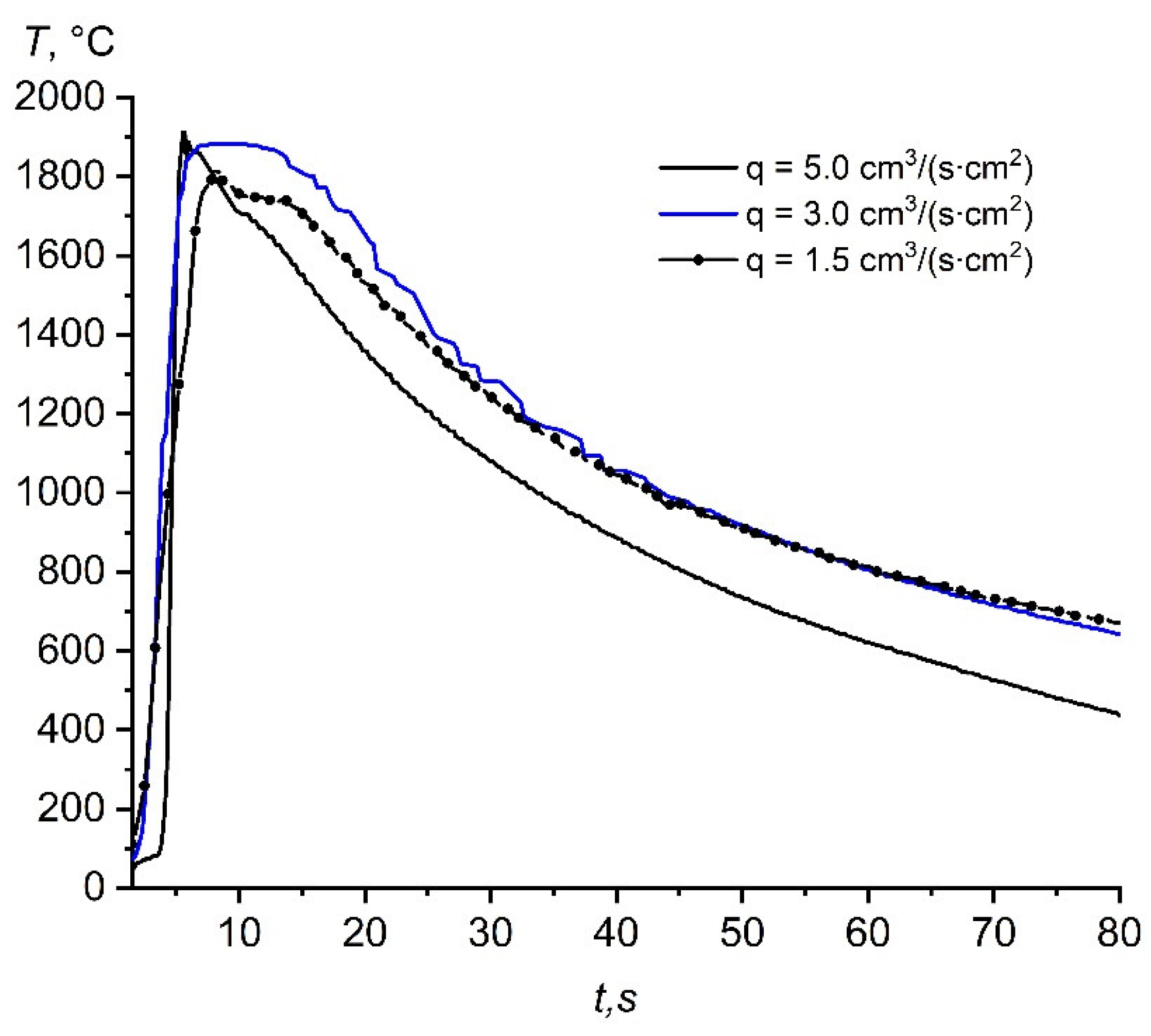
3.3. Influence of the Specific Flow Rate of Nitrogen on the Combustion Temperature of the Initial Composition
3.4. Influence of the Argon Addition to the Reaction Gas on the Combustion Characteristics of the Initial Composition
3.4.1. Influence of the Specific Flow Rate of the Nitrogen–Argon Mixture on the Combustion Rate of the Initial Composition
3.4.2. Influence of the Specific Flow Rate of the Nitrogen–Argon Mixture on the Combustion Temperature of the Initial Composition
3.5. Phase Composition of Combustion Products
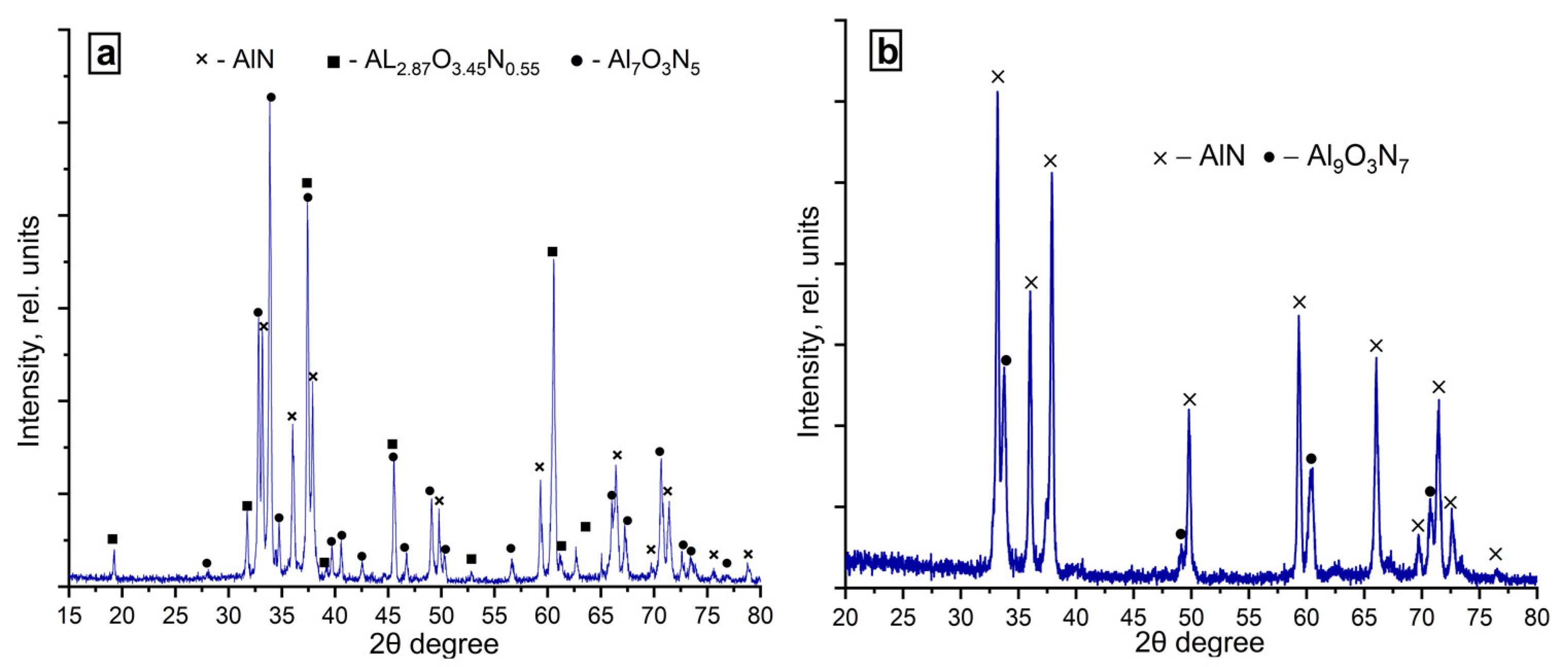
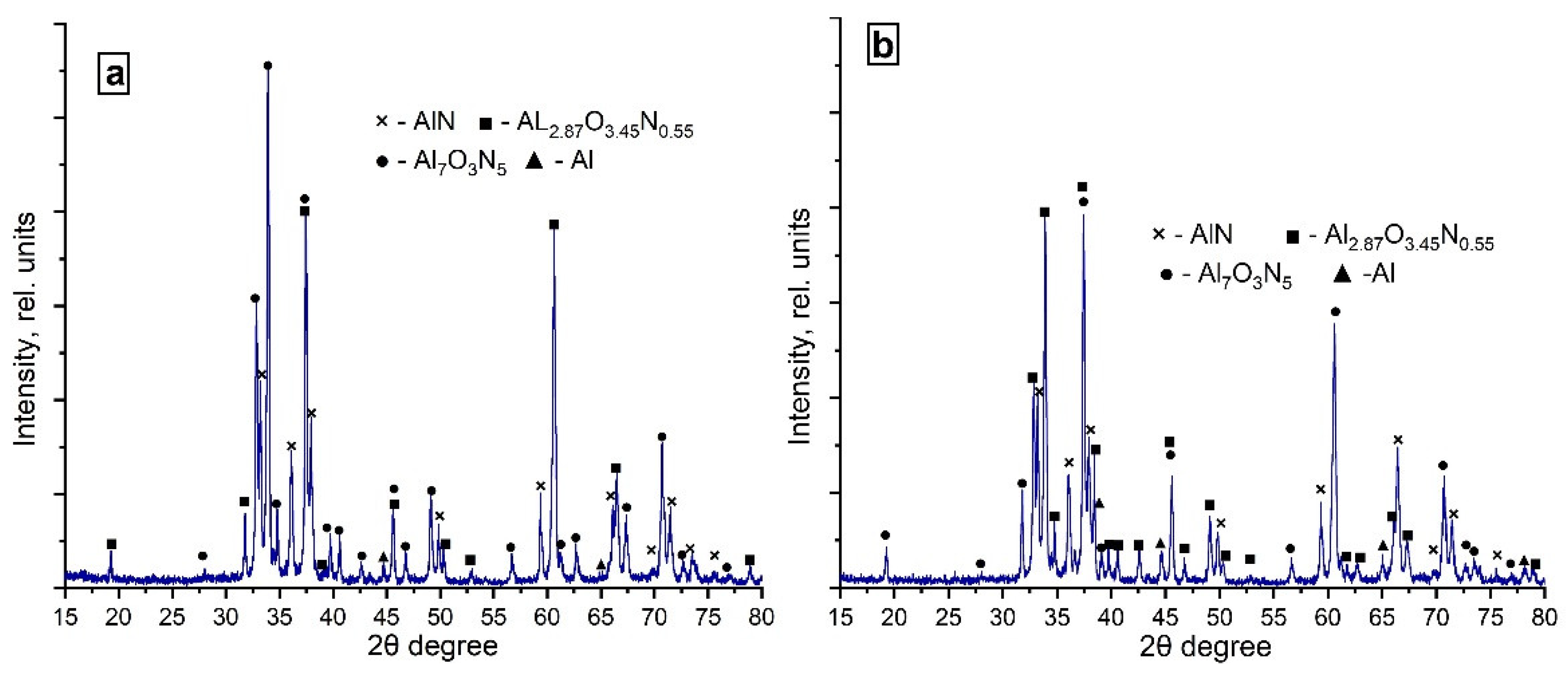
3.5.1. Phase Composition of the Combustion Products Obtained in the Nitrogen Flow
3.5.2. Phase Composition of the Combustion Products Obtained in the Nitrogen–Argon Flow
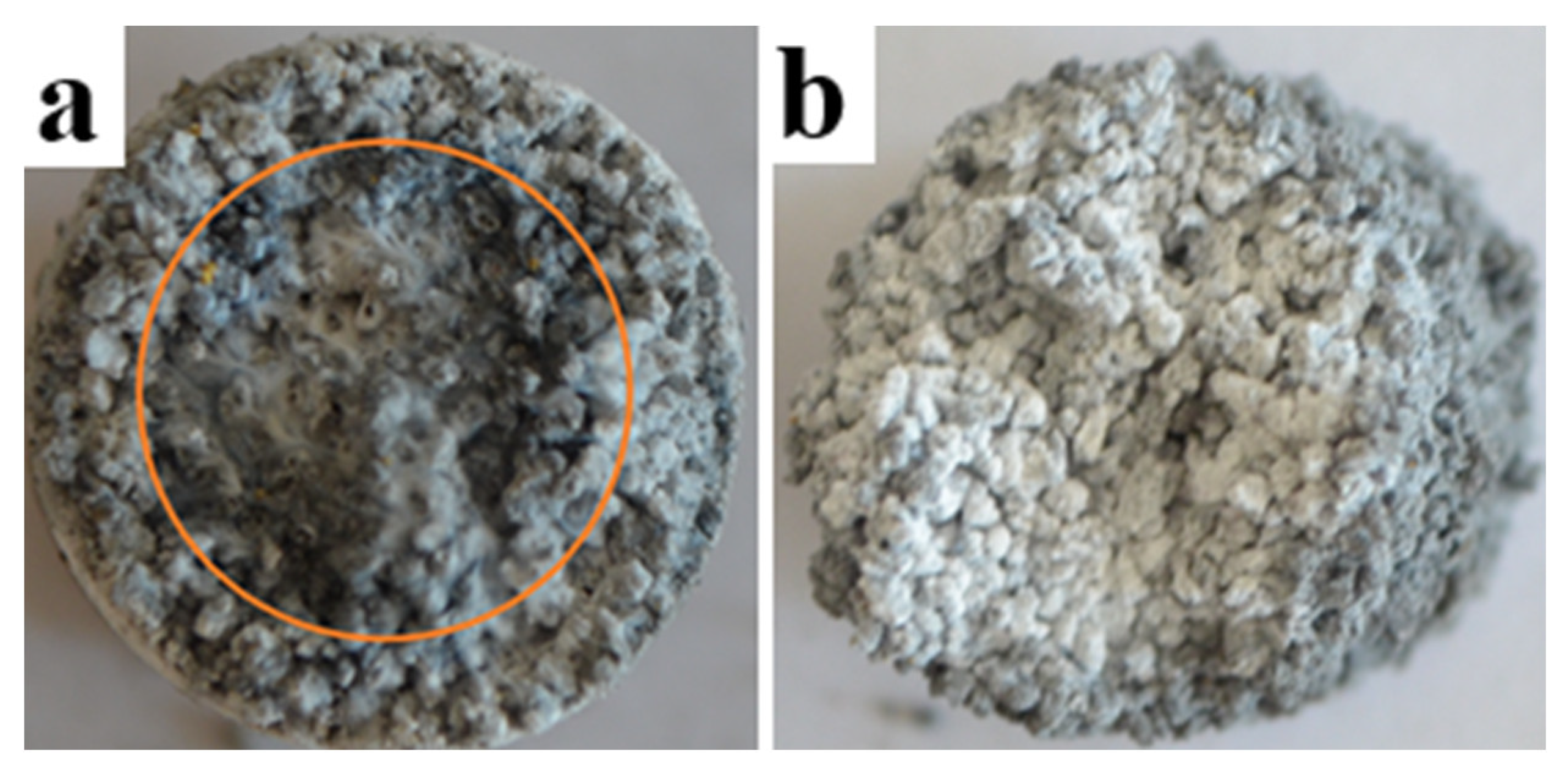
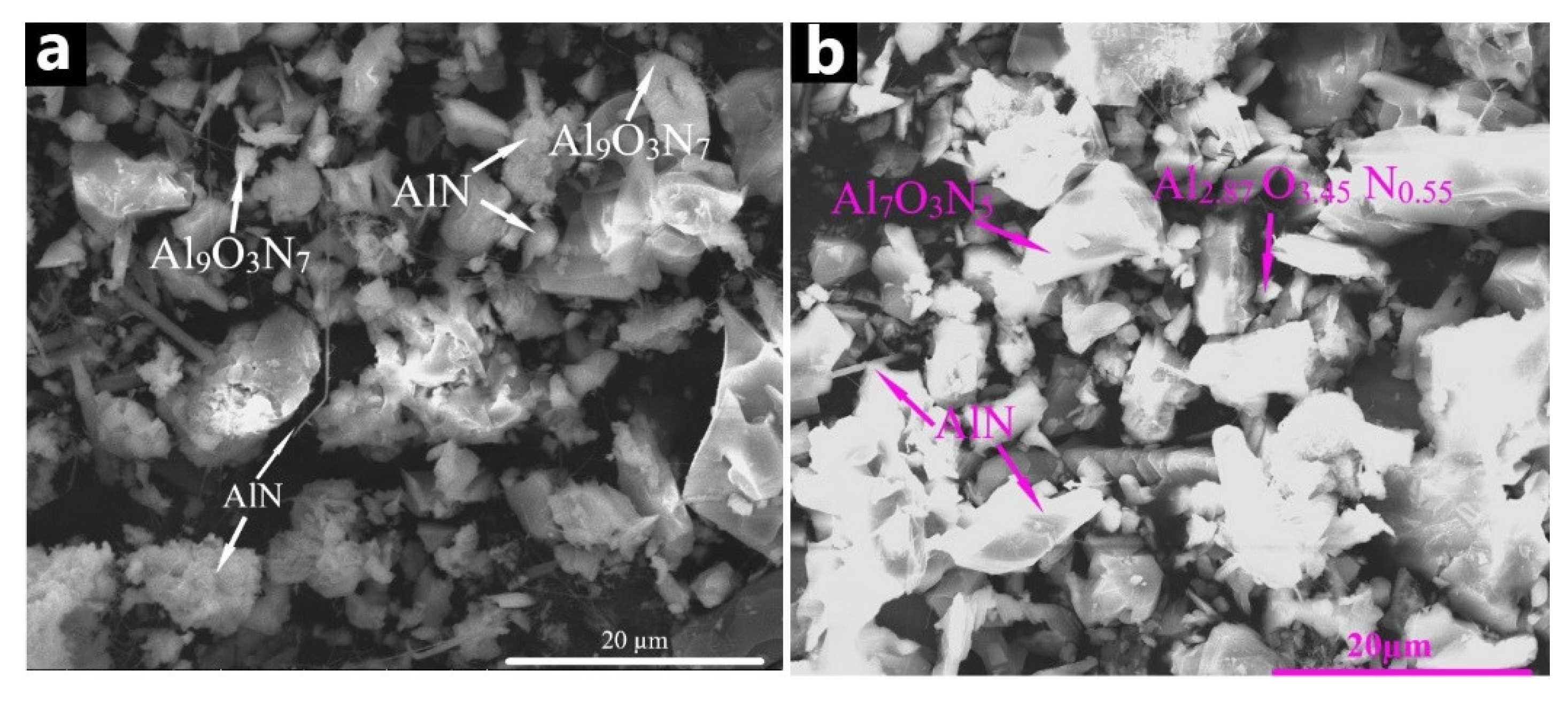
3.6. The Structure of Combustion Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, T.B.; Virkar, A.V.; More, K.L.; Dinwiddie, R.B.; Cutler, R.A. High-Thermal-Conductivity Aluminum Nitride Ceramics: The Effect of Thermodynamic, Kinetic, and Microstructural Factors. J. Am. Ceram. Soc. 1997, 80, 1421–1435. [Google Scholar] [CrossRef]
- Lee, H.M.; Bharathi, K.; Kim, D.K. Processing and Characterization of Aluminum Nitride Ceramics for High Thermal Conductivity. Adv. Eng. Mater. 2014, 16, 655–669. [Google Scholar] [CrossRef]
- Watari, K.; Kawamoto, M.; Ishizaki, K. Sintering Chemical Reactions to Increase Thermal Conductivity of Aluminum Nitride. J. Mater. Sci. 1991, 26, 4727–4732. [Google Scholar] [CrossRef]
- Baranda, P.S.; Cnudsen, A.K.; Rah, E. Effect of Silica on the Thermal Conductivity of Aluminum Nitride. J. Am. Ceram. Soc. 1993, 76, 1761–1771. [Google Scholar] [CrossRef]
- Watari, K.; Brito, M.; Yasuoka, M.; Valecillos, M.C.; KANZAKI, S. Influence of Powder characteristics on Sintering Process and Thermal Conductivity of Aluminum Nitride Ceramics. J. Cer. Soc. Jap. 1995, 103, 891–900. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Hwan, D.; Kim, Y. Aluminum Nitride Whisker Formation during Combustion Synthesis. J. Am. Ceram. Soc. 2000, 83, 1117–1121. [Google Scholar] [CrossRef]
- Xu, K.Z.; Tang, Z.X.; Zhang, Y.; Tan, H.H.; Zhang, H. Synthesis of Aluminum Nitride Powder by Carbothermal Reduction. Adv. Mater. Res. 2012, 562, 461–464. [Google Scholar] [CrossRef]
- Novikov, P.A.; Kim, A.E.; Ozerskoi, N.E.; Wang, Q.S.; Popovich, A.A. Plasma Chemical Synthesis of Aluminum Nitride Nanopowder. Key Eng. Mater. 2019, 822, 628–633. [Google Scholar] [CrossRef]
- Greil, P.; Kulig, M.; Hotza, D.; Lange, H.; Tischtau, R. Aluminium nitride ceramics with high thermal conductivity from gas-phase synthesized powders. J. Eur. Ceram. Soc. 1994, 13, 229–237. [Google Scholar] [CrossRef]
- Amosov, A.P.; Borovinskaya, I.P.; Merzhanov, A.G. Powder Technology of Self-Propagating High-Temperature Synthesis of Materials: A Reference Book; Mashinostroenie: Moscow, Russia, 2007; p. 567. [Google Scholar]
- Nikitin, P.Y.; Matveev, A.E.; Zhukov, I.A. Energy-effective AlMgB14 production by self-propagating high-temperature synthesis (SHS) using the chemical furnace as a source of heat energy. Ceram. Int. 2021, 47, 21698–21704. [Google Scholar] [CrossRef]
- Matveev, A.E.; Nikitin, P.Y.; Zhukov, I.A.; Zhukov, A.S. The use of plastic waste as carbon raw materials to obtain TiC-based powders. Ceram. Int. 2021, 47, 21140–21146. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Matveev, A.E.; Sokolov, S.D.; Boldin, M.S.; Vorozhtsov, A.B. AlMgB14–TiB2 composite materials obtained by self-propagating high-temperature synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 22733–22737. [Google Scholar] [CrossRef]
- Matveev, A.; Zhukov, I.; Ziatdinov, M.; Zhukov, A. Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials 2020, 13, 1050. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Ahn, D.H.; Shin, M.S.; Kim, Y.S. Self-Propagating High-Temperature Synthesis of Aluminum Nitride under Lower Nitrogen Pressures. J. Am. Ceram. Soc. 2000, 83, 1021–1028. [Google Scholar] [CrossRef]
- Chung, S.; Yu, W.; Lin, C. A self-propagating high-temperature synthesis method for synthesis of AlN powder. J. Mater. Res. 1999, 14, 1928–1933. [Google Scholar] [CrossRef]
- Braverman, B.; Ziatdinov, M.; Maksimov, Y. Chromium combustion in nitrogen. Combust. Explos. Shock Waves 1999, 35, 501–505. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Seplyarskii, B.S.; Shkadinskii, K.G. Theory of filtrational combustion. Combust. Explos. Shock Waves 1980, 16, 33–40. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Merzhanov, A.G.; Seplyarskii, B.S. Theory of filtration combustion of metals. Combust. Explos. Shock Waves 1976, 12, 285–294. [Google Scholar] [CrossRef]
- Aldushin, A.P. New results in the theory of filtration combustion. Combust. Flame 1993, 94, 308–320. [Google Scholar] [CrossRef]
- Aldushin, A.P.; Ivleva, T.P. Hydrodynamic instability of the coflow filtration combustion: Numerical simulation. Dokl. Phys. Chem. 2013, 451, 157–160. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Kochetkov, R.A.; Lisina, T.G. Convective Combustion of a Ti + 0.5C Granulated Mixture. Domain of Existence and Fundamental Phenomena. Combust. Explos. Shock Waves 2019, 55, 295–299. [Google Scholar] [CrossRef]
- Seplyarskii, B.S.; Kochetkov, R.A. A Study of the Characteristics of the Combustion of Ti + xC (x > 0.5) Powder and Granular Compositions in a Gas Coflow. Russ. J. Phys. Chem. B 2017, 11, 798–807. [Google Scholar] [CrossRef]
- Evseev, N.; Ziatdinov, M.; Romandin, V.; Zhukov, A.; Tolynbekov, A.; Ryzhikh, Y. Process of Obtaining Chromium Nitride in the Combustion Mode under Conditions of Co-Flow Filtration. Processes 2020, 8, 1056. [Google Scholar] [CrossRef]
- Ziatdinov, M.K. Chromium combustion in a nitrogen coflow. Combust. Explos. Shock Waves 2016, 52, 418–426. [Google Scholar] [CrossRef]



| q, cm3/(s∙cm2) | υ, mm/s |
|---|---|
| 1.5 | 0.7 |
| 3 5 | 1.45 1.5 |
| q, cm3/(s∙cm2) | Tmax, °C |
|---|---|
| 1.5 | 1831 |
| 3 5 | 1860 1915 |
| Ar, wt.15% | |
|---|---|
| q, cm3/(s∙cm2) | υ, mm/s |
| 3 5 | 0.68 1.27 |
| Ar, wt.15% | |
|---|---|
| q, cm3/(s∙cm2) | Tmax, °C |
| 3 5 | 1661 1792 |
| q, cm3/(s∙cm2) | Phase | Weight Fraction, % |
|---|---|---|
1.5 | AlN Al2.87 O3.45 N0.55 Al7O3N5 | 77 15 8 |
| 5.0 | AlN Al9 O3 N7 | 95 5 |
| q, cm3/(s∙cm2) | Phase | Weight Fraction, % |
|---|---|---|
3.0 | AlN Al2.87 O3.45 N0.55 Al7O3N5 Al | 72 17 11 <1 |
| 5.0 | AlN Al2.87 O3.45 N0.55 Al7O3N5 Al | 74 7 17 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evseev, N.; Nikitin, P.; Ziatdinov, M.; Zhukov, I.; Vakutin, A. AlN Production in Co-Flow Filtration Mode at Low Pressures. Materials 2021, 14, 5482. https://doi.org/10.3390/ma14195482
Evseev N, Nikitin P, Ziatdinov M, Zhukov I, Vakutin A. AlN Production in Co-Flow Filtration Mode at Low Pressures. Materials. 2021; 14(19):5482. https://doi.org/10.3390/ma14195482
Chicago/Turabian StyleEvseev, Nikolay, Pavel Nikitin, Mansur Ziatdinov, Ilya Zhukov, and Alexei Vakutin. 2021. "AlN Production in Co-Flow Filtration Mode at Low Pressures" Materials 14, no. 19: 5482. https://doi.org/10.3390/ma14195482






