Mechanism of Microwave Activation on Molybdenite
Abstract
:1. Introduction
2. Experiment Procedure
2.1. Materials
2.2. Preparation of Microwave Activated Molybdenite
2.3. Oxidization Roasting Experiment and Determination of Sulfur Content
2.4. Analysis and Characterization
3. Results and Discussion
3.1. Sulfur Content of Molybdenum Calcine Samples
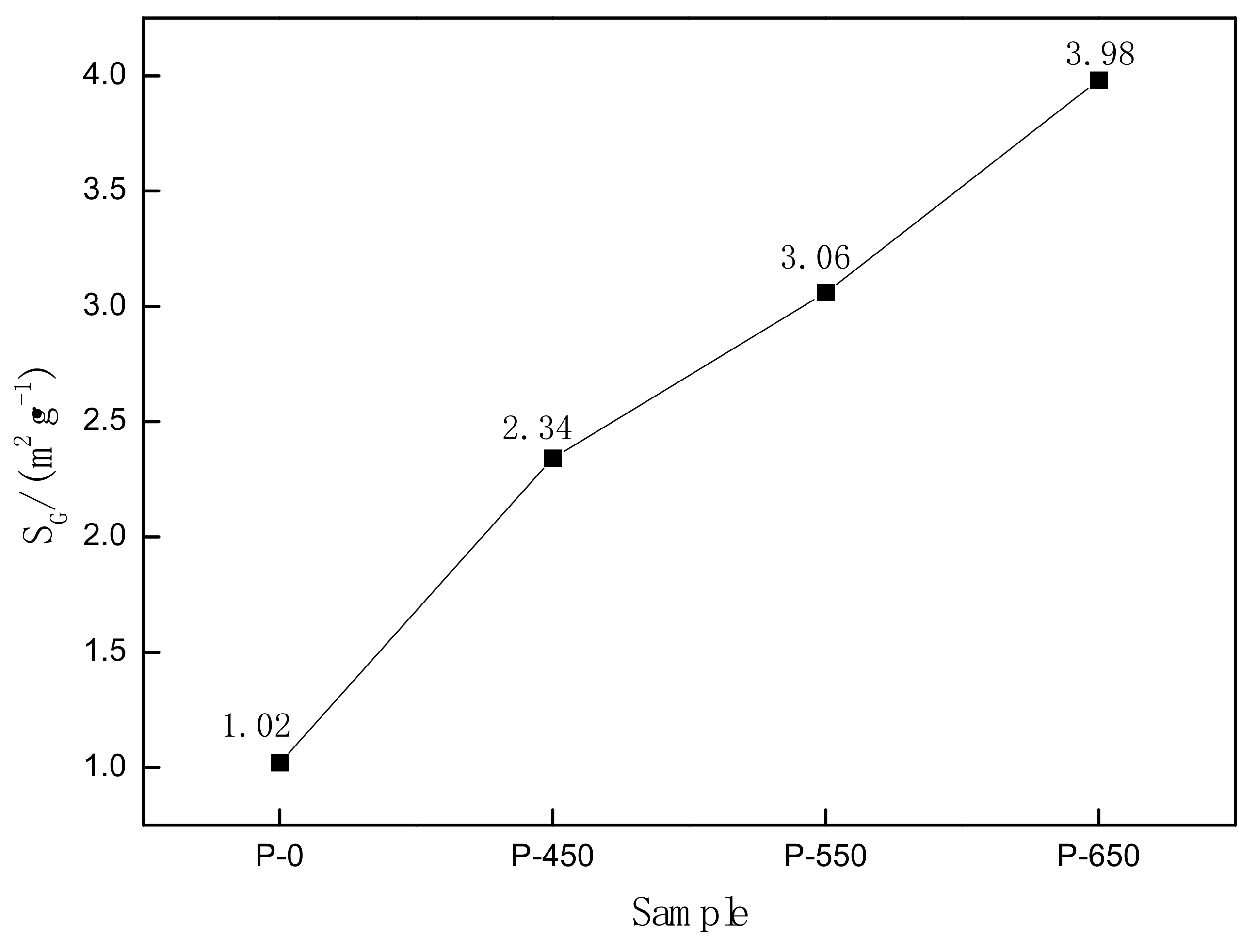
3.2. Specific Surface Area (SV)
- (1)
- In the microwave field, MoS2 and gangue have different heating rates and they can be heated up to different temperatures because of their different abilities to absorb microwaves. The obvious temperature difference between them can produce thermal stress, which can generate cracks when thermal stress reaches a certain level. The generation of cracks can effectively promote the monomer dissociation of MoS2, thereby increasing the specific surface area of the molybdenite [22].
- (2)
- Due to the uneven distribution of the molybdenite components, the dispersed distribution of MoS2 can also lead to an obvious selective heating phenomenon, making internal cracks and increasing the specific surface area of the molybdenite [23].
- (3)
- According to the isothermal gas equation, P·V = n·R·T, the higher is the temperature, the greater is the pressure of the gas. Microwave heating occurs from the inside out, so the internal gas pressure is greater than that outside. The internal gas tends to spread outward and promote the generation of cracks. In addition, the higher the microwave power, the more obvious the change is. This can be attributed to the microwave field, the material absorbing energy can be expressed by Equation (1) [24], ε0 is the permittivity of vacuum, ε0 = 8.85 × 10−12 F/m; where ε is the loss factor, F/m; tgδ is the loss of dielectric tangent; E is the electric field intensity of internal material, V/m; and V is the volume of material, m3. It can be seen that if f and V remain unchanged, increasing the microwave power can strengthen the capacity of the material to absorb the microwave directly. The selective heating effect will be more obvious, and the specific surface area of molybdenite will gradually increase.
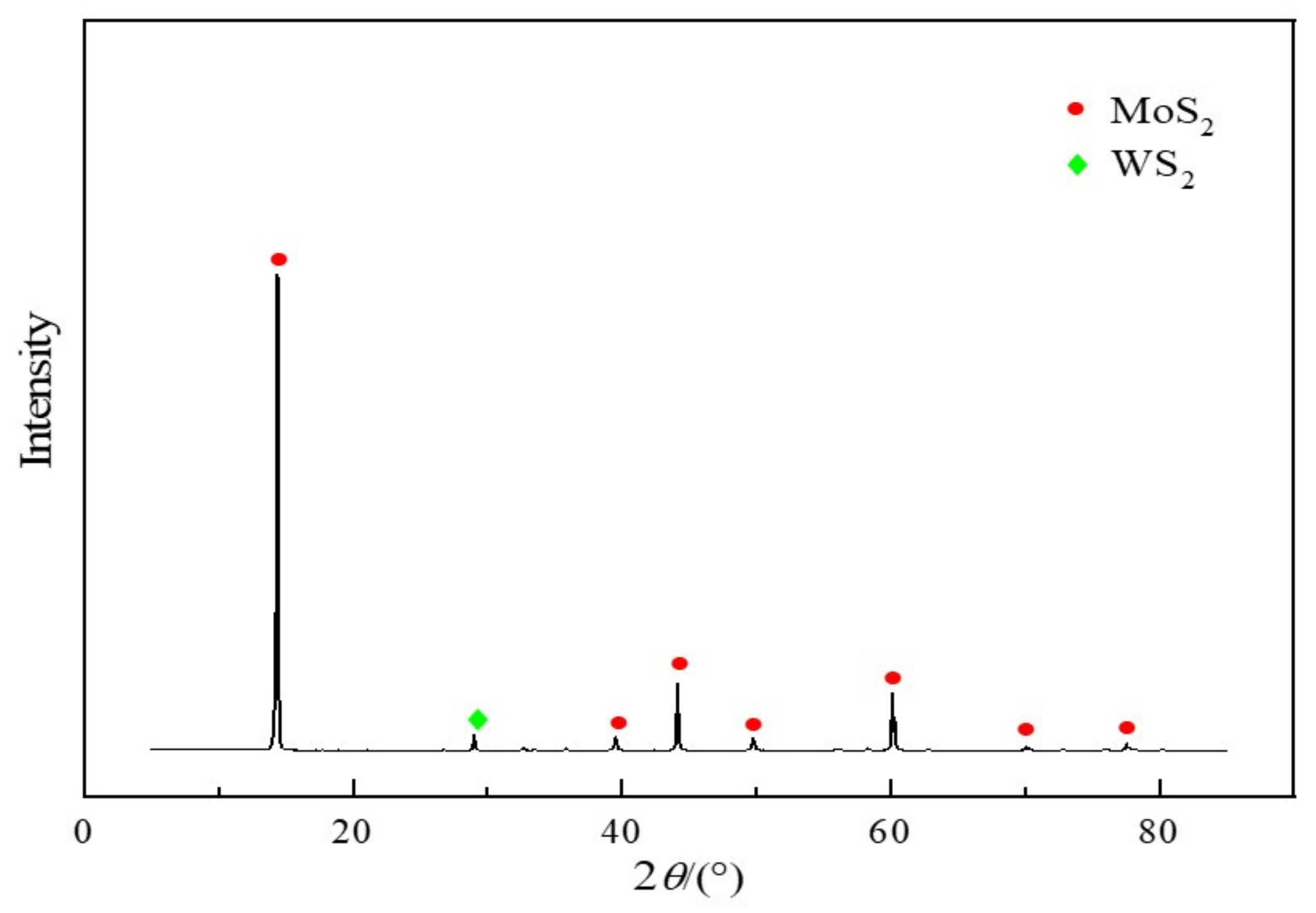
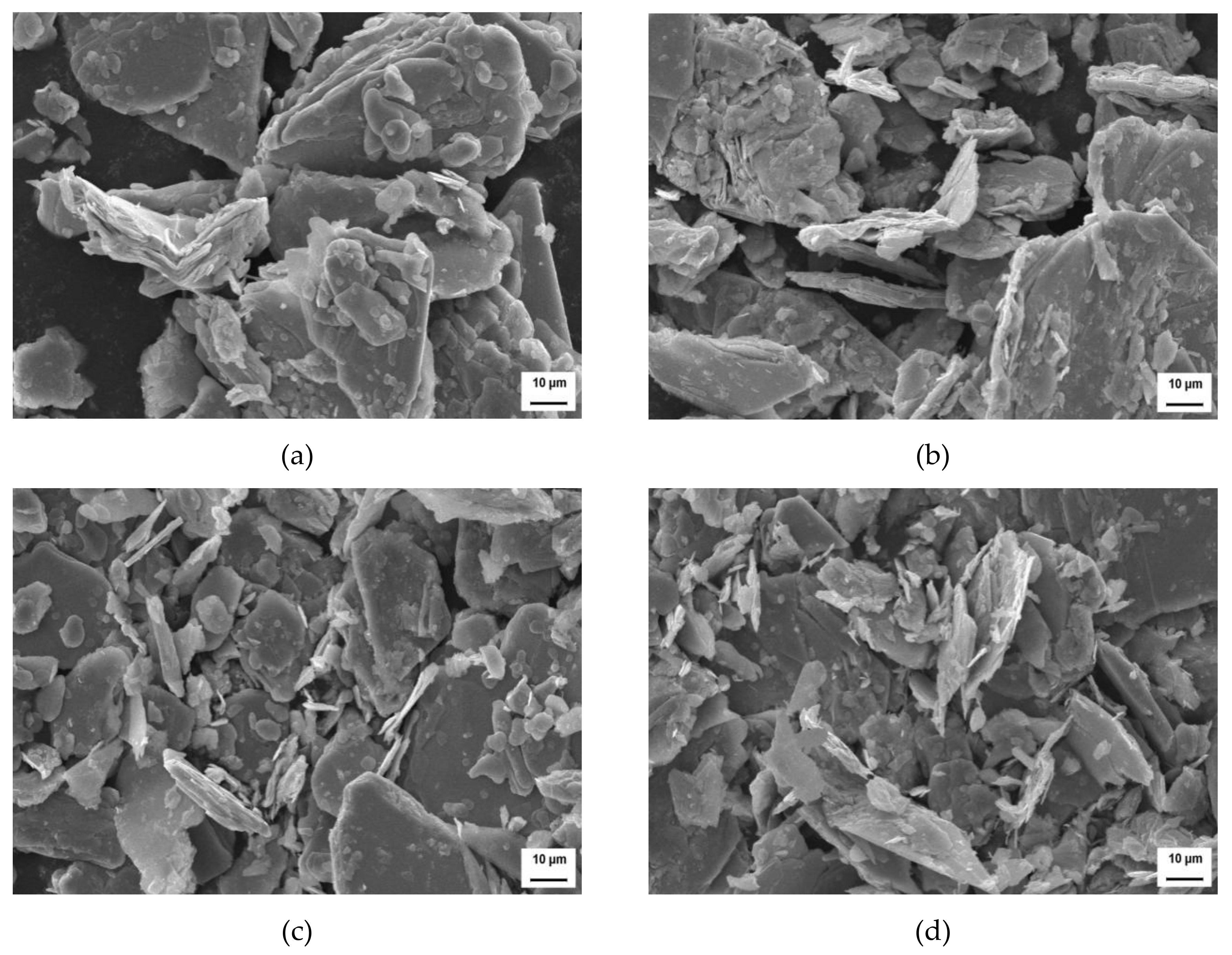
3.3. Analysis of XRD and SEM
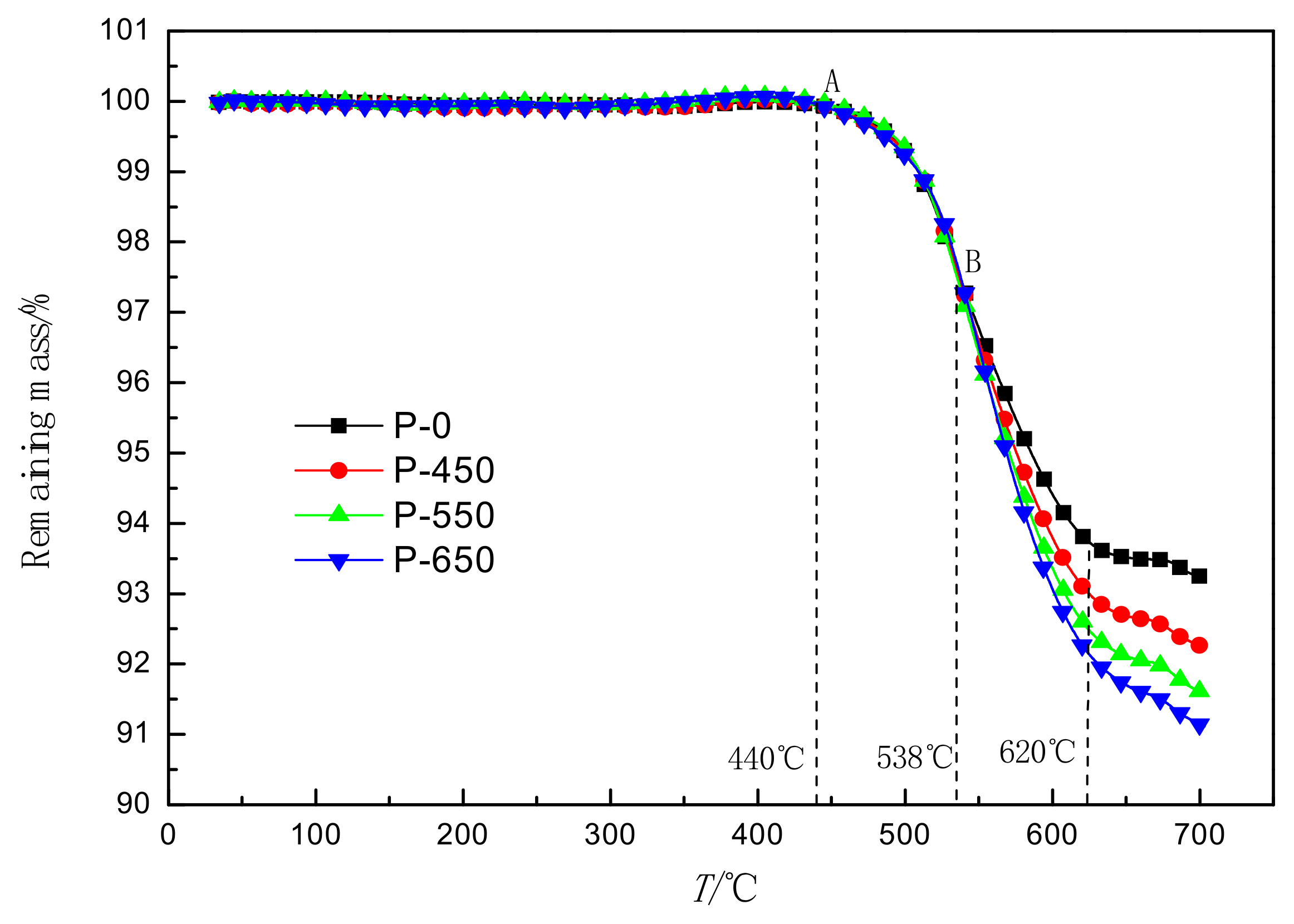
3.4. Analysis of the TG Curve
- (1)
- The increase in the specific surface area and the value of ε with the increased power of microwave add an effective reaction area between the molybdenite and oxygen.
- (2)
- In the presence of the microwave, both polar and nonpolar molecules are polarized, which results in the exchange between microwave energy stored in the molecule and the average kinetic free energy of the molecule, reducing the activation energy and promoting the reaction process.
- (3)
4. Conclusions
- (1)
- Microwave activation can effectively reduce the sulfur content of molybdenum calcine samples obtained from molybdenite. The reason is that microwave activation can improve the thermodynamic and kinetic conditions of the molybdenite oxidation roasting process and oxidize molybdenite more thoroughly than before treatment.
- (2)
- After microwave activation, the specific surface area of molybdenite increased. The structural parameters and material properties of MoS2, the main component of molybdenite, were changed by microwave activation.
- (3)
- With the extension of microwave activated power, the crystal cell volume and grain size of MoS2 reduced, the microstrain increased slightly, and morphologic features of the surface of molybdenite became looser.
- (4)
- Microwave activation significantly changed the oxidation characteristics of molybdenite and promoted its oxidation reaction above 538 °C. The weight loss rate increased from 6.177% to 7.718% at 620 °C. The extension of activation time improved the conversion of molybdenite and accelerated the reaction rate of molybdenite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.-X.; Yin, Z.-L.; Chen, Y.G.; Xiong, L.-Z. Leaching kinetics of molybdenum from Ni-Mo ore in sulfuric acid solution with sodium peroxodisulfate as oxidant. J. Cent. South Univ. 2015, 22, 874. (In Chinese) [Google Scholar] [CrossRef]
- Lindstrom, R.E.; Scheiner, B.J. Extraetion of molybdenum from ores by electro-oxidation. Bur. Mines Met. Reeovery Progr. USBM Tech. Prog. Rep. 1972, 10, 4–7. [Google Scholar]
- Hou, X.-C.; Yang, Y.-D.; Li, H.; Zeng, L.; Xiao, L.-S. Kinetics study of leaching arsenic from Ni-Mo ore roasting in dust mixture of hydrochloric and sulfuric acids. J. Cent. South Univ. 2014, 21, 2176. (In Chinese) [Google Scholar] [CrossRef]
- Sharma, T. Physico-chemical processing of low grade manganese ore. Int. J. Miner. Process. 1992, 35, 191–203. [Google Scholar] [CrossRef]
- Ren, B.-J. Production practice of molybdenum concentrate roasting by rotary kiln. Nonferrous Met. Extr. Met. 1999, 2, 18–20. (In Chinese) [Google Scholar]
- Mehugh, L.F.; Barchers, D.E. Roasting of Molybdenite on Centrates Containing Flotation Oils. U.S. Patent 4,523,948, 18 June 1985. [Google Scholar]
- Mehugh, L.F.; Godssehalk, J.; Kuzior, M. Climax conversionⅢ. In Proceedings of the Vancouver: The XVI Canadian Institute of Mining and Metallurgy Annu Meeting, Vancouver, DC, Canada, 8–10 August 1977. [Google Scholar]
- Maheshc, J.; May, W.A. Fluidized-Bed Roasting of Molybdenites Concentrates. U.S. Patent 6,190,625, 20 February 2001. [Google Scholar]
- Cao, Z.-F.; Zhong, H.; Liu, G.-Y. Electric oxidation kinetics of molybdenite concent rate in acidic NaCl solution. Can. J. Chem. Eng. 2009, 87, 93–94. (In Chinese) [Google Scholar]
- Kim, B.-S.; Lee, H.-I.; Choi, Y.-Y.; Kim, S. Kinetics of the Oxidative Roasting of Low Grade Mongolian Molybdenite Concentrate. Mater. Trans. 2009, 50, 2669–2674. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rehim, A.M. Thermal Analysis and X-ray Diffraction of Roasting of Egyptian Molybdenite. J. Therm. Anal. Calorim. 1999, 57, 415–431. [Google Scholar] [CrossRef]
- Ebrahimi, K.; Reza, A.; Mohammad, H.; Saidi, A. Model-Fitting Approach to Kinetic Analysis of Non-Isothermal Oxidation of Molybdenite. Iran. J. Chem. Chem. Eng. 2007, 26, 119–123. [Google Scholar]
- Gan, M.; Fan, X.-H.; Zhang, L.; Jiang, T.; Qiu, G.-Z.; Wang, Y.; Deng, Q.; Chen, X.-L. Reaction behavior of low grade molybdenum concentrates in oxidation roasting process. Chin. J. Nonferrous Met. 2014, 24, 3115–3122. (In Chinese) [Google Scholar]
- Chen, X.-L.; Wang, H.-B.; Gan, M.; Fan, X.-H.; Zhang, L.; Deng, Q.; Wang, Y.; Zeng, J.-L. Extraction of molybdenum from low grade molybdenum concentrates by calciun-based roasting and acid leaching process. Chin. J. Nonferrous Met. 2015, 25, 2913–2920. (In Chinese) [Google Scholar]
- Hu, H.-P.; Chen, Q.-Y.; Yin, Z.-L.; Gunter, G.; Zhang, P.-M.; Guo, G.-H. Structural Change of Mechanically Activated Molybdenite and the Effect of Mechanical Activation on Molybdenite. Met. Mater. Trans. B 2004, 12, 1203–1207. [Google Scholar] [CrossRef]
- Yi, J.-H.; Tang, X.-W.; Luo, S.-D. Development and trend of microwave sintering technology. Powder Met. Technol. 2003, 21, 351–353. (In Chinese) [Google Scholar]
- Yu, Q.; Ding, D.-X.; Zhang, J.; Hu, N.; Yang, Y.-S.; Wang, H. Application status and development trend of microwave irradiation technology in mining industry. Gold Sci. Technol. 2017, 25, 112–120. (In Chinese) [Google Scholar]
- Kelly, R.M.; Rowson, N.A. Microwave reduction of oxidized ilmenite concentrates. Miner. Eng. 1995, 8, 1427–1438. [Google Scholar] [CrossRef]
- Mourao, M.B.; Carvalho, I.P.; Takano, C. Carbothermic Reduction by Microwave Heating. ISIJ Int. 2001, 41, S27–S30. [Google Scholar] [CrossRef] [Green Version]
- Farahat, M.; Elmahdy, A.M.; Hirajima, T. Influence of microwave radiation on the magnetic properties of molybdenite and arsenopyrite. Powder Technol. 2017, 315, 276–281. [Google Scholar] [CrossRef]
- Guo, G.-Z.; Deng, G.-Y.; Tang, H.-Y. The characteristics of microwave drying process for molybdenum. J. Henan Univ. Sci. Technol. Nat. Sci. 2014, 35, 5–8. (In Chinese) [Google Scholar]
- Jing, Q.-H. Chemistry of Microwave; Science Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Kenkre, V.M.; Skal, A.L.; Weiser, M.W. Theory of microwave interactions in ceramic materials:the phenomenon of thermal runaway. Int. J. Refract. Met. Hard Mater. 2010, 28, 180–186. [Google Scholar]
- Zhou, J.; Cheng, J.-P.; Fu, W.-B.; Dong, X.-B.; Mei, B.-C. Research on Improving Single Mode Cavity Type Microwave Sintering System with 2450 MHz/5 kW. J. Wuhan Univ. Technol. 1999, 21, 2. (In Chinese) [Google Scholar]
- Wang, M.; Yang, S.-P.; Guo, S.-Q.; Cao, S.-W.; He, K. Production of low-sulfur molybdenum oxide by microwave-activated pre-calcination of molybdenite. Rare Met. Mater. Eng. 2020, 49, 48–58. (In Chinese) [Google Scholar]
- Alexiew, V.; Prins, R.; Webzer, T. Ab initio study of MoS2 and Li adsorbed on the face of MoS2. Phys. Chem. Chem. Phys. 2000, 2, 1815–1827. [Google Scholar] [CrossRef]
- Luo, J.; Cai, C.; Lv, C.-X. Recent development of microwave-induced organic reaction chemistry. Chin. J. Synth. Chem. 2002, 10, 17–24. (In Chinese) [Google Scholar]
- Zhou, X.-P.; Yan, L.-M. Investigation on microwave reaction mechanism of thiophene and its derivatives. J. Fuel Chem. Technol. 1998, 26, 506–509. (In Chinese) [Google Scholar]
- Zhang, H.-L.; Hu, X.-M.; Lai, S.-L. The research into kinetic principle of microwave effect on chemical reaction. J. South China Univ. Technol. (Nat. Sci.) 1997, 25, 46–50. (In Chinese) [Google Scholar]


| Mo | S | Cu | Pb | WO3 | Bi | C | K | Fe | SiO2 | CaO | MgO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52.21 | 34.83 | 0.11 | 0.09 | 0.10 | 0.05 | 0.40 | 0.09 | 0.33 | 5.20 | 1.70 | 4.89 |
| Mass Fraction/% | ||
|---|---|---|
| >0.044 mm | 0.036~0.044 mm | <0.036 mm |
| 0.14 | 8.66 | 91.2 |
| System | a = b/ nm | c/ nm | α = β/ (°) | γ/ (°) | Lattice Volume/ nm3 | D/ nm | ε/ % |
|---|---|---|---|---|---|---|---|
| P-0 | 0.31659 | 1.23142 | 90 | 120 | 0.10689 | 86.9 | 0 |
| P-450 | 0.31646 | 1.23156 | 90 | 120 | 0.10681 | 79.1 | 0.104 |
| P-550 | 0.31644 | 1.23055 | 90 | 120 | 0.10672 | 74.0 | 0.119 |
| P-650 | 0.31640 | 1.22947 | 90 | 120 | 0.10662 | 72.4 | 0.148 |
| Samples | P-0 | P-450 | P-550 | P-650 |
|---|---|---|---|---|
| Mass loss ratio/% | 6.177 | 6.881 | 7.386 | 7.718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, T.; Liu, S.; Sun, H. Mechanism of Microwave Activation on Molybdenite. Materials 2021, 14, 5486. https://doi.org/10.3390/ma14195486
Yang S, Zhang T, Liu S, Sun H. Mechanism of Microwave Activation on Molybdenite. Materials. 2021; 14(19):5486. https://doi.org/10.3390/ma14195486
Chicago/Turabian StyleYang, Shuangping, Tiantian Zhang, Shouman Liu, and Haixing Sun. 2021. "Mechanism of Microwave Activation on Molybdenite" Materials 14, no. 19: 5486. https://doi.org/10.3390/ma14195486





