Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Disk Preparation
2.2. Osteoblast Cell Culturing
2.3. Titanium Surface Hydrophilicity
2.4. Initial Cell Attachment
2.5. Statistical Analyses
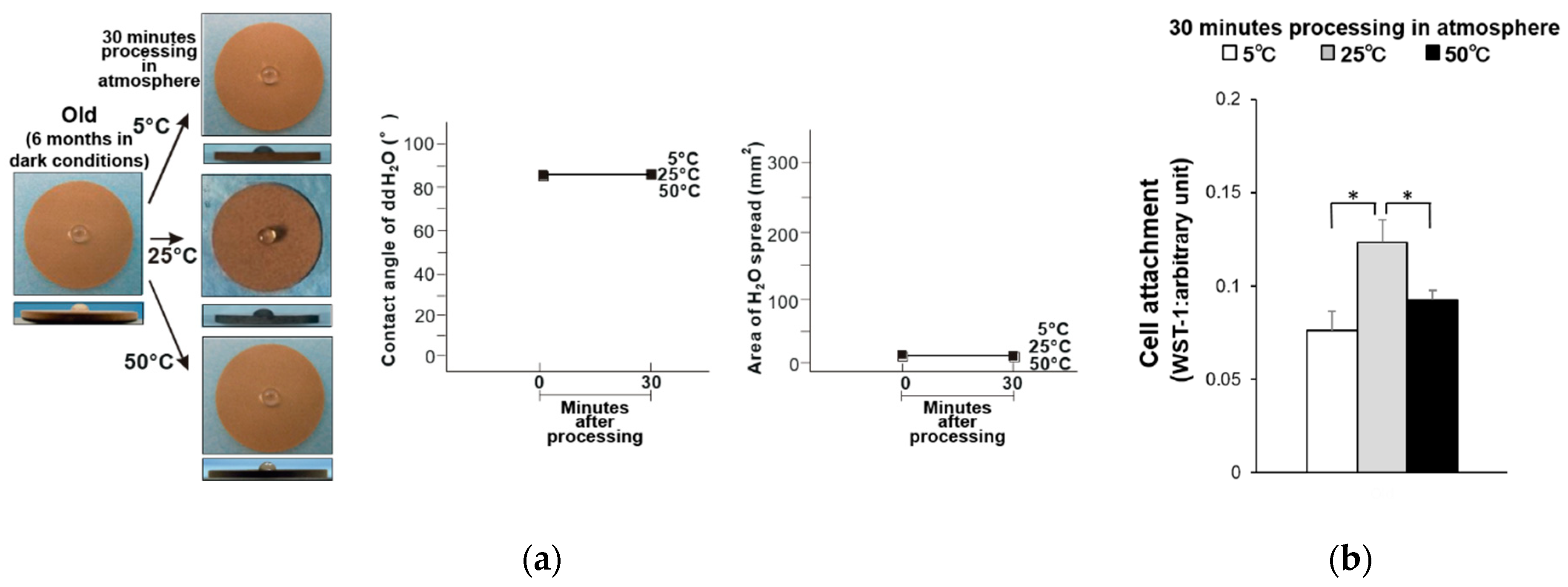
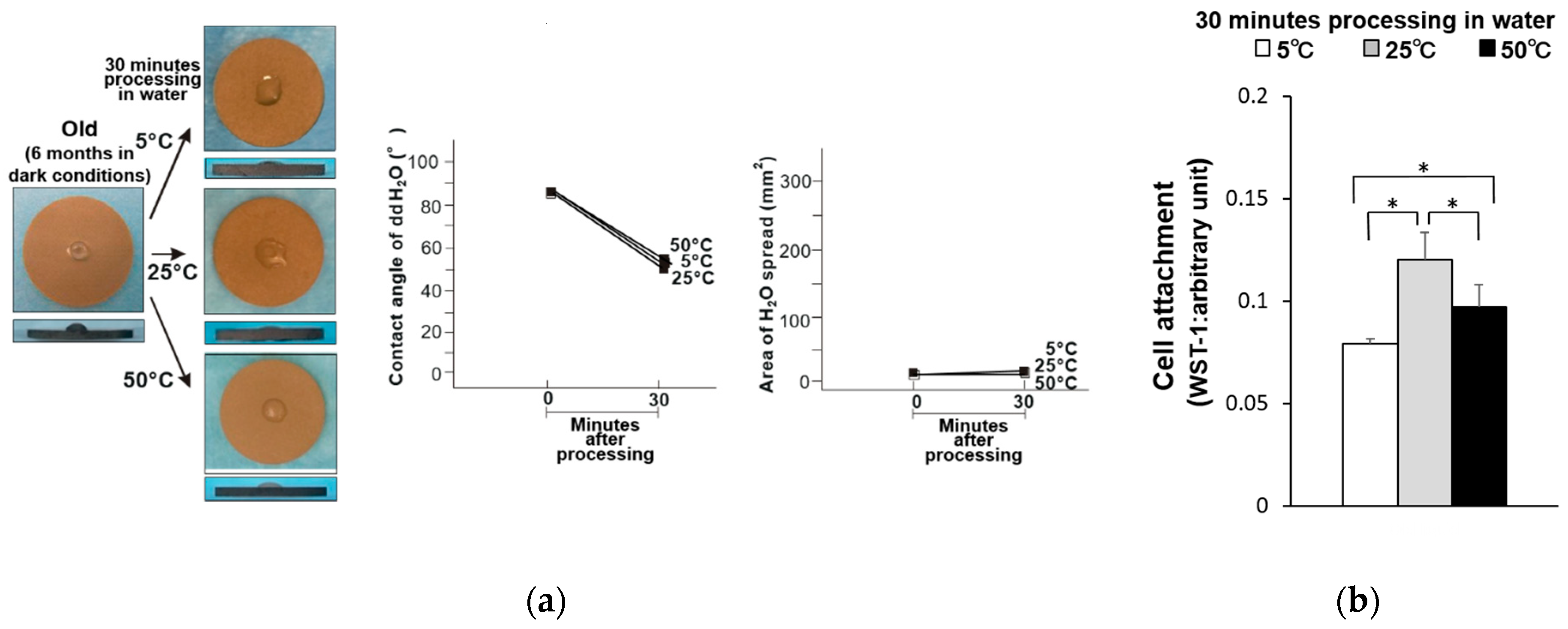
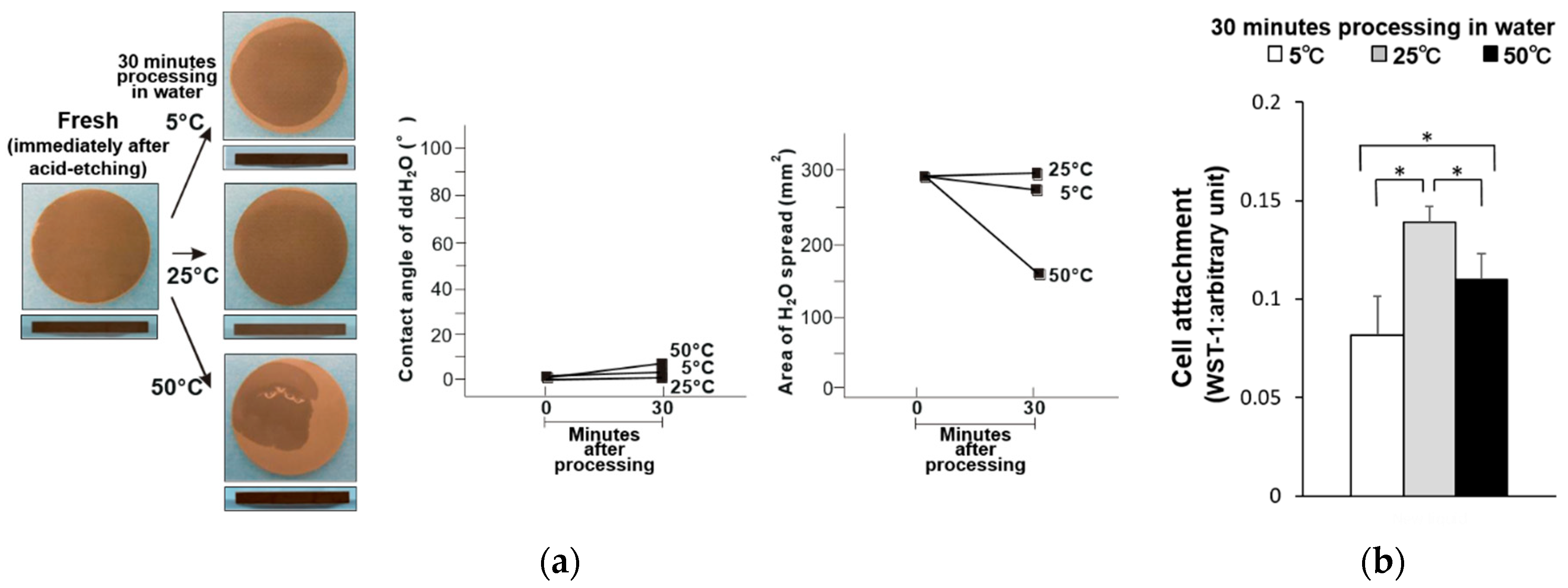
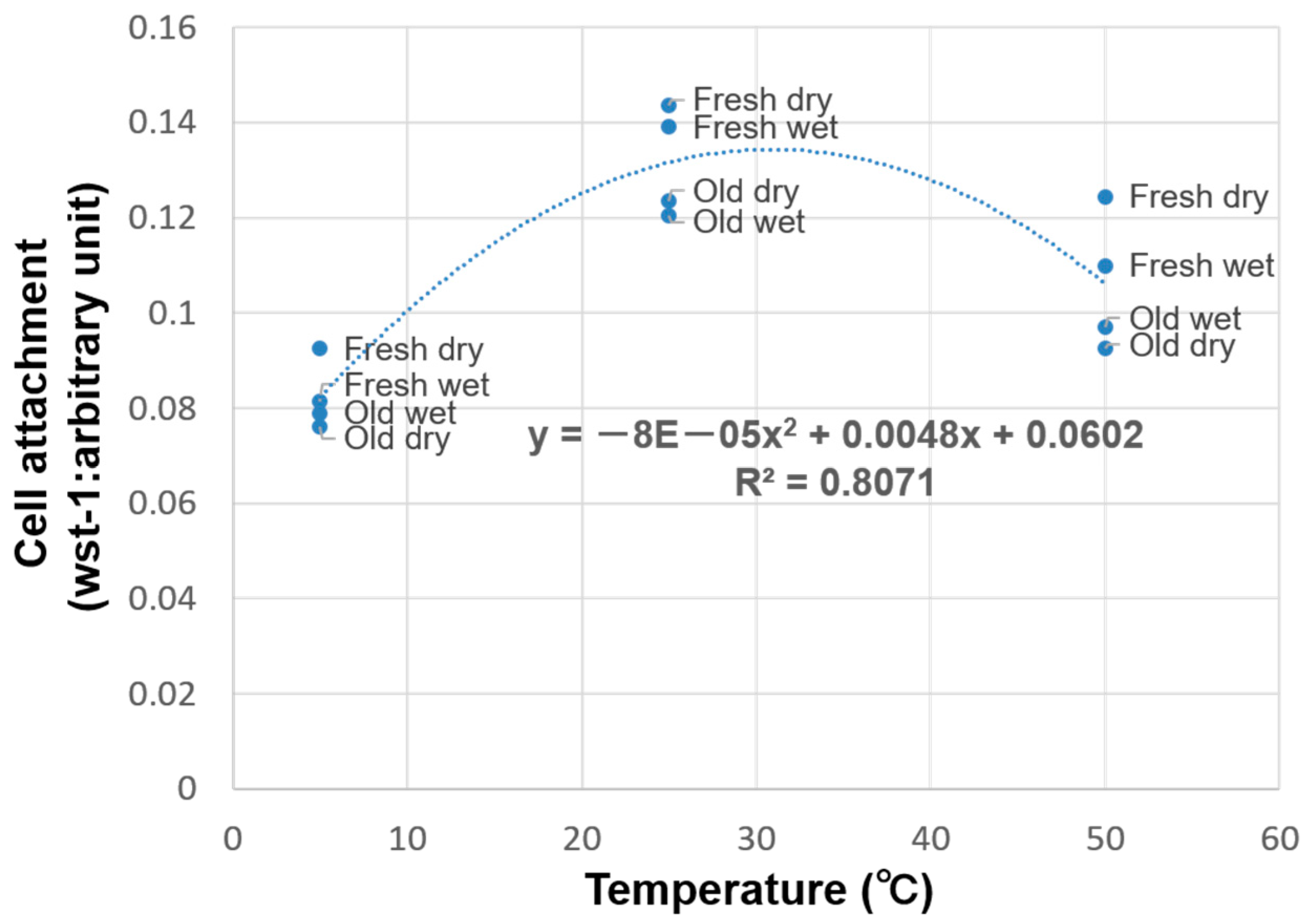
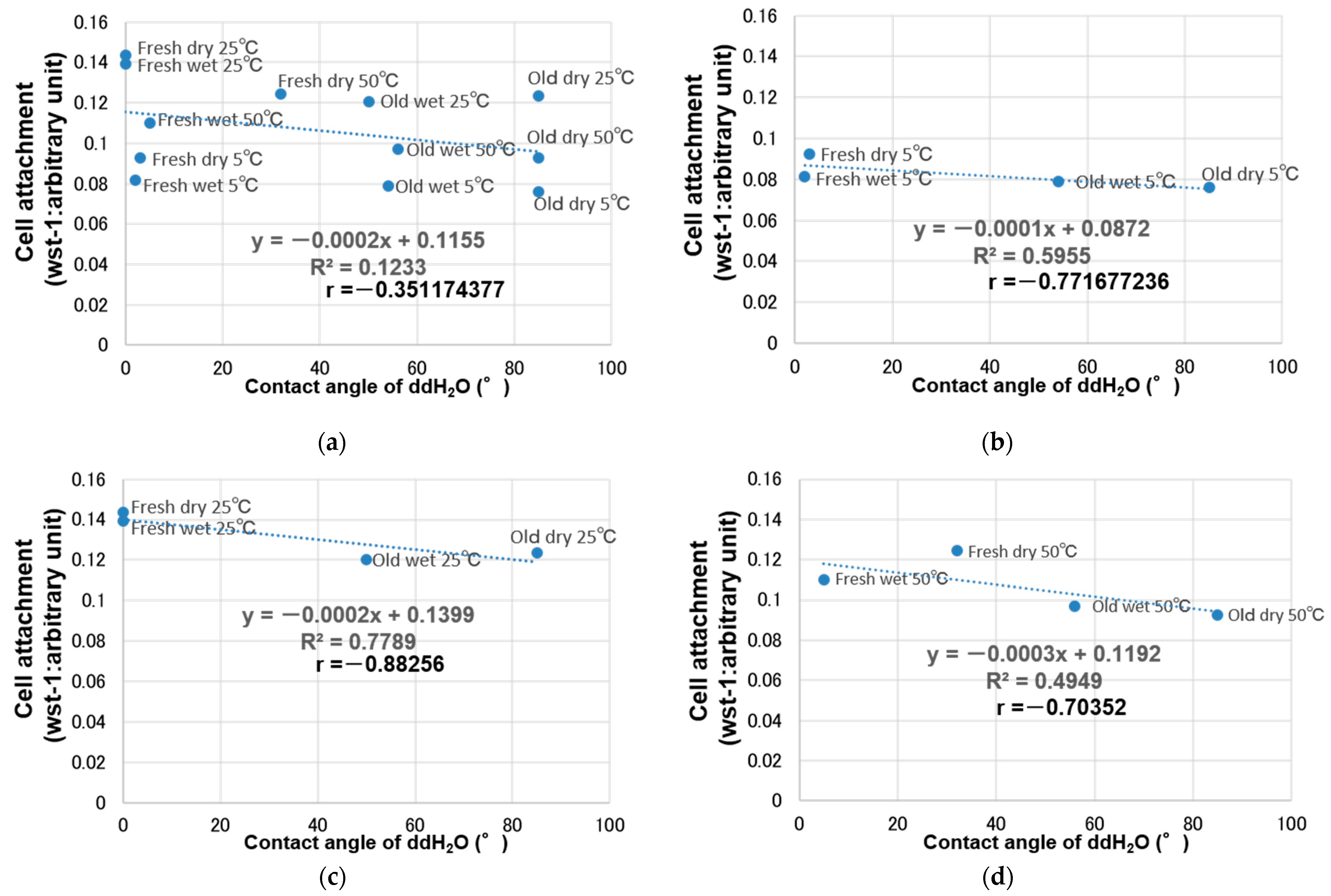
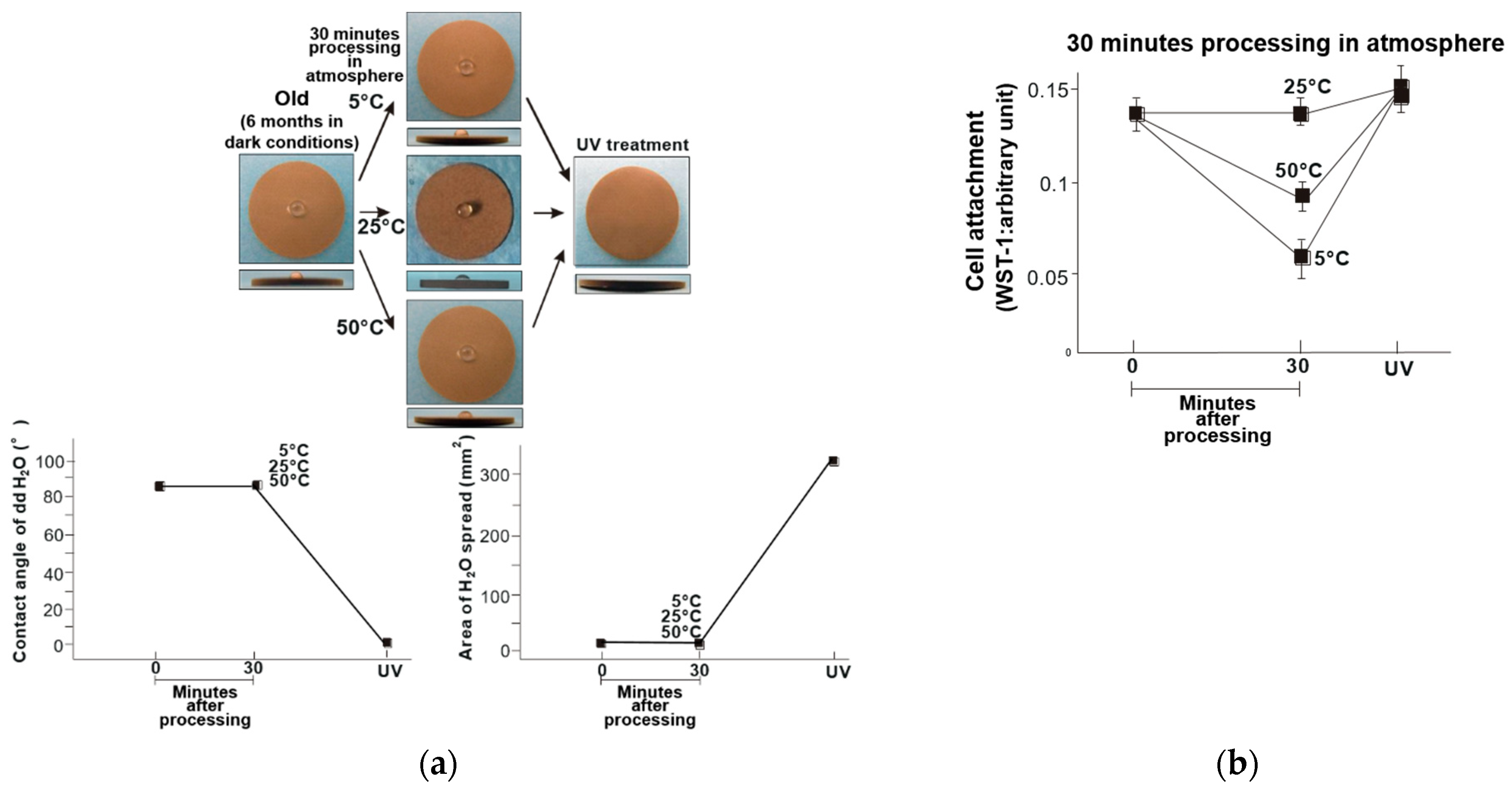
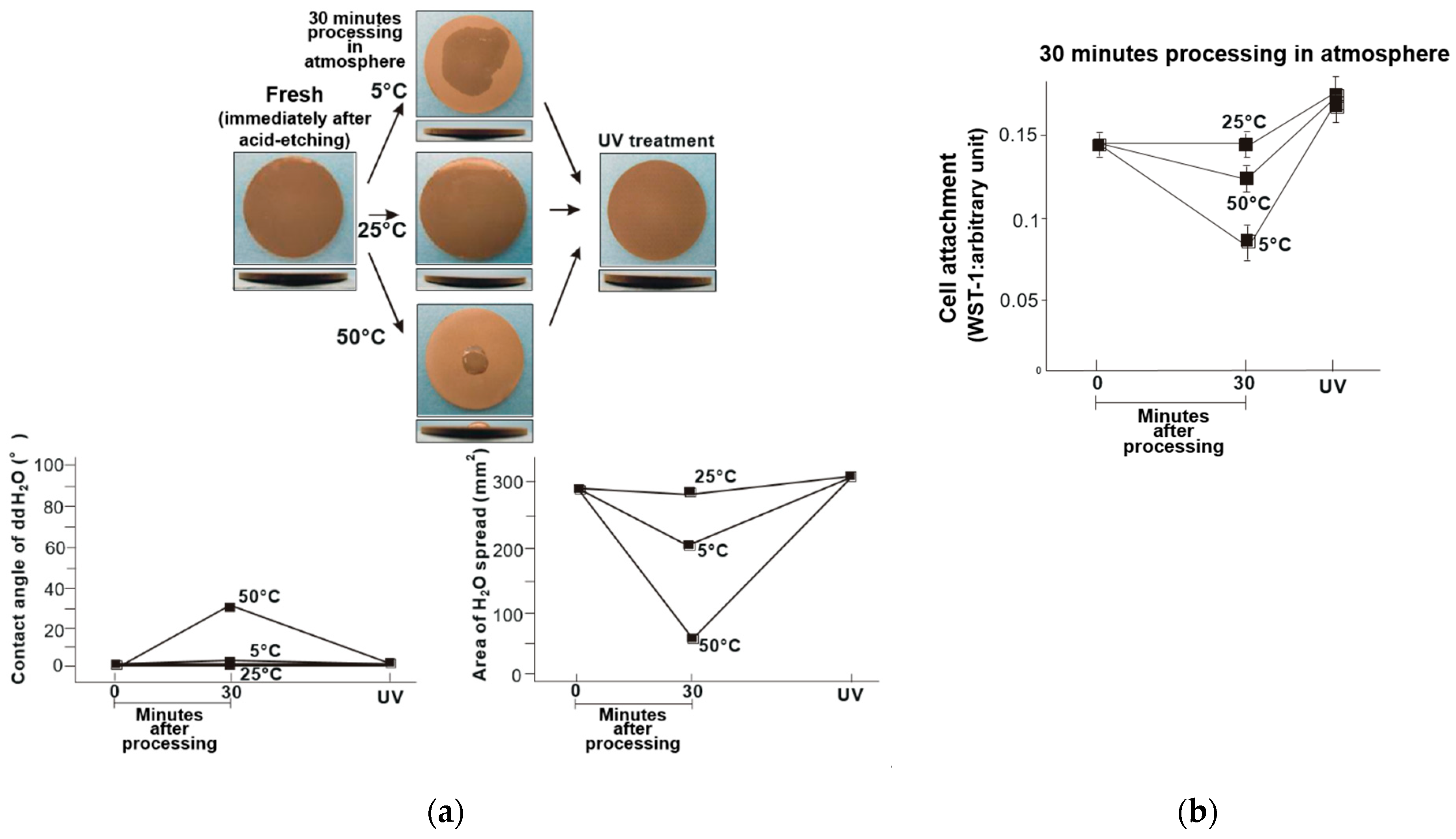
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarb, G.A.; Schmitt, A.; Baker, G. Tissue-Integrated Prostheses: Osseointegration Research in Toronto. Int. J. Periodontics Restor. Dent. 1987, 7, 8–35. [Google Scholar]
- Zarb, G.A. Clinical Application of Osseointegration. An Introduction. Swed. Dent. J. Suppl. 1985, 28, 7–9. [Google Scholar] [PubMed]
- Brånemark, P.I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Butz, F.; Ogawa, T.; Chang, T.L.; Nishimura, I. Three-Dimensional Bone-Implant Integration Profiling Using Micro-Computed Tomography. Int. J. Oral Maxillofac. Implant. 2006, 21, 687–695. [Google Scholar]
- Ogawa, T.; Nishimura, I. Different Bone Integration Profiles of Turned and Acid-Etched Implants Associated with Modulated Expression of Extracellular Matrix Genes. Int. J. Oral Maxillofac. Implant. 2003, 18, 200–210. [Google Scholar]
- Ogawa, T.; Nishimura, I. Genes Differentially Expressed in Titanium Implant Healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef]
- Ogawa, T.; Ozawa, S.; Shih, J.H.; Ryu, K.H.; Sukotjo, C.; Yang, J.M.; Nishimura, I. Biomechanical Evaluation of Osseous Implants Having Different Surface Topographies in Rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef]
- Tsukimura, N.; Kojima, N.; Kubo, K.; Att, W.; Takeuchi, K.; Kameyama, Y.; Maeda, H.; Ogawa, T. The Effect of Superficial Chemistry of Titanium on Osteoblastic Function. J. Biomed. Mater. Res. A 2008, 84, 108–116. [Google Scholar] [CrossRef]
- Ogawa, T.; Sukotjo, C.; Nishimura, I. Modulated Bone Matrix-Related Gene Expression Is Associated with Differences in Interfacial Strength of Different Implant Surface Roughness. J. Prosthodont. 2002, 11, 241–247. [Google Scholar] [CrossRef]
- Nakamura, H.K.; Butz, F.; Saruwatari, L.; Ogawa, T. A Role for Proteoglycans in Mineralized Tissue-Titanium Adhesion. J. Dent. Res. 2007, 86, 147–152. [Google Scholar] [CrossRef]
- Saruwatari, L.; Aita, H.; Butz, F.; Nakamura, H.K.; Ouyang, J.; Yang, Y.; Chiou, W.A.; Ogawa, T. Osteoblasts Generate Harder, Stiffer, and More Delamination-Resistant Mineralized Tissue on Titanium than on Polystyrene, Associated with Distinct Tissue Micro- and Ultrastructure. J. Bone Miner. Res. 2005, 20, 2002–2016. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced Intrinsic Biomechanical Properties of Osteoblastic Mineralized Tissue on Roughened Titanium Surface. J. Biomed. Mater. Res. A 2005, 72, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Tsukimura, N.; Suzuki, T.; Ogawa, T. Effect of Supramicron Roughness Characteristics Produced by 1- and 2-Step Acid Etching on the Osseointegration Capability of Titanium. Int. J. Oral Maxillofac. Implant. 2007, 22, 719–728. [Google Scholar]
- Nakamura, H.; Saruwatari, L.; Aita, H.; Takeuchi, K.; Ogawa, T. Molecular and Biomechanical Characterization of Mineralized Tissue by Dental Pulp Cells on Titanium. J. Dent. Res. 2005, 84, 515–520. [Google Scholar] [CrossRef]
- Miyauchi, T.; Yamada, M.; Yamamoto, A.; Iwasa, F.; Suzawa, T.; Kamijo, R.; Baba, K.; Ogawa, T. The Enhanced Characteristics of Osteoblast Adhesion to Photofunctionalized Nanoscale TiO2 Layers on Biomaterials Surfaces. Biomaterials 2010, 31, 3827–3839. [Google Scholar] [CrossRef]
- Yamada, M.; Miyauchi, T.; Yamamoto, A.; Iwasa, F.; Takeuchi, M.; Anpo, M.; Sakurai, K.; Baba, K.; Ogawa, T. Enhancement of Adhesion Strength and Cellular Stiffness of Osteoblasts on Mirror-Polished Titanium Surface by UV-Photofunctionalization. Acta Biomater. 2010, 6, 4578–4588. [Google Scholar] [CrossRef] [Green Version]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-Dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Hori, N.; Iwasa, F.; Ueno, T.; Takeuchi, K.; Tsukimura, N.; Yamada, M.; Hattori, M.; Yamamoto, A.; Ogawa, T. Selective Cell Affinity of Biomimetic Micro-Nano-Hybrid Structured TiO2 Overcomes the Biological Dilemma of Osteoblasts. Dent. Mater. 2010, 26, 275–287. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Suzuki, T.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; Ogawa, T. Ultraviolet Light Treatment for the Restoration of Age-Related Degradation of Titanium Bioactivity. Int. J. Oral Maxillofac. Implant. 2010, 25, 49–62. [Google Scholar]
- Iwasa, F.; Tsukimura, N.; Sugita, Y.; Kanuru, R.K.; Kubo, K.; Hasnain, H.; Att, W.; Ogawa, T. TiO2 Micro-Nano-Hybrid Surface to Alleviate Biological Aging of UV-Photofunctionalized Titanium. Int. J. Nanomed. 2011, 6, 1327–1341. [Google Scholar] [CrossRef] [Green Version]
- Minamikawa, H.; Att, W.; Ikeda, T.; Hirota, M.; Ogawa, T. Long-Term Progressive Degradation of the Biological Capability of Titanium. Materials 2016, 9, 102. [Google Scholar] [CrossRef]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet Treatment Overcomes Time-Related Degrading Bioactivity of Titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]
- Att, W.; Ogawa, T. Biological Aging of Implant Surfaces and Their Restoration with Ultraviolet Light Treatment: A Novel Understanding of Osseointegration. Int. J. Oral Maxillofac. Implant. 2012, 27, 753–761. [Google Scholar]
- Ogawa, T. UV-Photofunctionalization of Titanium Implants. Oral Craniofac. Tissue Eng. 2012, 2, 151–158. [Google Scholar]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The Effect of UV-Photofunctionalization on the Time-Related Bioactivity of Titanium and Chromium-Cobalt Alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-Dependent Degradation of Titanium Osteoconductivity: An Implication of Biological Aging of Implant Materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Suzuki, T.; Kubo, K.; Hori, N.; Yamada, M.; Kojima, N.; Sugita, Y.; Maeda, H.; Ogawa, T. Nonvolatile Buffer Coating of Titanium to Prevent Its Biological Aging and for Drug Delivery. Biomaterials 2010, 31, 4818–4828. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.; Ogawa, T. Electrostatic Control of Protein Adsorption on UV-Photofunctionalized Titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ogawa, T. The Biological Aging of Titanium Implants. Implant Dent. 2012, 21, 415–421. [Google Scholar] [CrossRef]
- Ghassemi, A.; Ishijima, M.; Hasegawa, M.; Mohammadzadeh Rezaei, N.; Nakhaei, K.; Sekiya, T.; Torii, Y.; Hirota, M.; Park, W.; Miley, D.D.; et al. Biological and Physicochemical Characteristics of 2 Different Hydrophilic Surfaces Created by Saline-Storage and Ultraviolet Treatment. Implant Dent. 2018, 27, 405–414. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet Light-Mediated Photofunctionalization of Titanium to Promote Human Mesenchymal Stem Cell Migration, Attachment, Proliferation and Differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The Effect of Ultraviolet Functionalization of Titanium on Integration with Bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization Increases the Bioactivity and Osteoconductivity of the Titanium Alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef]
- Saita, M.; Ikeda, T.; Yamada, M.; Kimoto, K.; Lee, M.C.; Ogawa, T. UV Photofunctionalization Promotes Nano-Biomimetic Apatite Deposition on Titanium. Int. J. Nanomed. 2016, 11, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Synergistic Effects of UV Photofunctionalization and Micro-Nano Hybrid Topography on the Biological Properties of Titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Hamajima, K.; Tanaka, M.; Sekiya, T.; Hirota, M.; Ogawa, T. UV Light-Generated Superhydrophilicity of a Titanium Surface Enhances the Transfer, Diffusion and Adsorption of Osteogenic Factors from a Collagen Sponge. Int. J. Mol. Sci. 2021, 22, 6811. [Google Scholar] [CrossRef]
- Aita, H.; Oh, W.; Kubo, K.; Tsukimura, N.; Maeda, H.; Ogawa, T. Light-Induced Bone Cement-Philic Titanium Surface. J. Mater. Sci. 2008, 43, 1552–1558. [Google Scholar] [CrossRef]
- Okubo, T.; Ikeda, T.; Saruta, J.; Tsukimura, N.; Hirota, M.; Ogawa, T. Compromised Epithelial Cell Attachment After Polishing Titanium Surface and Its Restoration by UV Treatment. Materials 2020, 13, 3946. [Google Scholar] [CrossRef]
- Okubo, T.; Tsukimura, N.; Taniyama, T.; Ishijima, M.; Nakhaei, K.; Rezaei, N.M.; Hirota, M.; Park, W.; Akita, D.; Tateno, A.; et al. Ultraviolet Treatment Restores Bioactivity of Titanium Mesh Plate Degraded by Contact with Medical Gloves. J. Oral Sci. 2018, 60, 567–573. [Google Scholar] [CrossRef]
- Nakhaei, K.; Ishijima, M.; Ikeda, T.; Ghassemi, A.; Saruta, J.; Ogawa, T. Ultraviolet Light Treatment of Titanium Enhances Attachment, Adhesion, and Retention of Human Oral Epithelial Cells via Decarbonization. Materials 2020, 14, 151. [Google Scholar] [CrossRef]
- Hirota, M.; Ikeda, T.; Tabuchi, M.; Iwai, T.; Tohnai, I.; Ogawa, T. Effect of Ultraviolet-Mediated Photofunctionalization for Bone Formation Around Medical Titanium Mesh. J. Oral Maxillofac. Surg. 2014, 72, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Yamada, M.; Sugita, Y.; Ogawa, T. Effect of Photofunctionalization on Fluoride-Treated Nanofeatured Titanium. J. Biomater. Appl. 2014, 28, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ikeda, T.; Hirota, M.; Nakagawa, K.; Park, W.; Miyazawa, K.; Goto, S.; Ogawa, T. Effect of UV Photofunctionalization on Biologic and Anchoring Capability of Orthodontic Miniscrews. Int. J. Oral Maxillofac. Implant. 2015, 30, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyo, S.W.; Park, Y.B.; Moon, H.S.; Lee, J.H.; Ogawa, T. Photofunctionalization Enhances Bone-Implant Contact, Dynamics of Interfacial Osteogenesis, Marginal Bone Seal, and Removal Torque Value of Implants: A Dog Jawbone Study. Implant Dent. 2013, 22, 666–675. [Google Scholar] [CrossRef]
- Funato, A.; Ogawa, T. Photofunctionalized Dental Implants: A Case Series in Compromised Bone. Int. J. Oral Maxillofac. Implant. 2013, 28, 1589–1601. [Google Scholar] [CrossRef]
- Hirota, M.; Ikeda, T.; Tabuchi, M.; Nakagawa, K.; Park, W.; Ishijima, M.; Tsukimura, N.; Hagiwara, Y.; Ogawa, T. Bone Generation Profiling Around Photofunctionalized Titanium Mesh. Int. J. Oral Maxillofac. Implant. 2016, 31, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Hirota, M.; Ikeda, T.; Tabuchi, M.; Ozawa, T.; Tohnai, I.; Ogawa, T. Effects of Ultraviolet Photofunctionalization on Bone Augmentation and Integration Capabilities of Titanium Mesh and Implants. Int. J. Oral Maxillofac. Implant. 2017, 32, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Hirota, M.; Ozawa, T.; Iwai, T.; Mitsudo, K.; Ogawa, T. UV-Mediated Photofunctionalization of Dental Implant: A Seven-Year Results of a Prospective Study. J. Clin. Med. 2020, 9, 2733. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Implant Stability Development of Photofunctionalized Implants Placed in Regular and Complex Cases: A Case-Control Study. Int. J. Oral Maxillofac. Implant. 2016, 31, 676–686. [Google Scholar] [CrossRef]
- Hori, N.; Iwasa, F.; Tsukimura, N.; Sugita, Y.; Ueno, T.; Kojima, N.; Ogawa, T. Effects of UV Photofunctionalization on the Nanotopography Enhanced Initial Bioactivity of Titanium. Acta Biomater. 2011, 7, 3679–3691. [Google Scholar] [CrossRef]
- Ishijima, M.; de Avila, E.D.; Nakhaei, K.; Shi, W.; Lux, R.; Ogawa, T. Ultraviolet Light Treatment of Titanium Suppresses Human Oral Bacterial Attachment and Biofilm Formation: A Short-Term In Vitro Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 1105–1113. [Google Scholar] [CrossRef]
- Ishijima, M.; Ghassemi, A.; Soltanzadeh, P.; Tanaka, M.; Nakhaei, K.; Park, W.; Hirota, M.; Tsukimura, N.; Ogawa, T. Effect of UV Photofunctionalization on Osseointegration in Aged Rats. Implant Dent. 2016, 25, 744–750. [Google Scholar] [CrossRef]
- Iwasa, F.; Baba, K.; Ogawa, T. Enhanced Intracellular Signaling Pathway in Osteoblasts on Ultraviolet Lighttreated Hydrophilic Titanium. Biomed. Res. 2016, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of Osteoblast Adhesion to UV-Photofunctionalized Titanium via an Electrostatic Mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Iwasaki, C.; Hirota, M.; Tanaka, M.; Kitajima, H.; Tabuchi, M.; Ishijima, M.; Park, W.; Sugita, Y.; Miyazawa, K.; Goto, S.; et al. Tuning of Titanium Microfiber Scaffold with UV-Photofunctionalization for Enhanced Osteoblast Affinity and Function. Int. J. Mol. Sci. 2020, 21, 738. [Google Scholar] [CrossRef] [Green Version]
- Soltanzadeh, P.; Ghassemi, A.; Ishijima, M.; Tanaka, M.; Park, W.; Iwasaki, C.; Hirota, M.; Ogawa, T. Success Rate and Strength of Osseointegration of Immediately Loaded UV-Photofunctionalized Implants in a Rat Model. J. Prosthet. Dent. 2017, 118, 357–362. [Google Scholar] [CrossRef]
- Sugita, Y.; Honda, Y.; Kato, I.; Kubo, K.; Maeda, H.; Ogawa, T. Role of Photofunctionalization in Mitigating Impaired Osseointegration Associated with type 2 Diabetes in Rats. Int. J. Oral Maxillofac. Implant. 2014, 29, 1293–1300. [Google Scholar] [CrossRef]
- Taniyama, T.; Saruta, J.; Mohammadzadeh Rezaei, N.; Nakhaei, K.; Ghassemi, A.; Hirota, M.; Okubo, T.; Ikeda, T.; Sugita, Y.; Hasegawa, M.; et al. UV-Photofunctionalization of Titanium Promotes Mechanical Anchorage in A Rat Osteoporosis Model. Int. J. Mol. Sci. 2020, 21, 1235. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Yamada, M.; Hori, N.; Suzuki, T.; Ogawa, T. Effect of Ultraviolet Photoactivation of Titanium on Osseointegration in a Rat Model. Int. J. Oral Maxillofac. Implant. 2010, 25, 287–294. [Google Scholar]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of Bone-Titanium Integration Profile with UV-Photofunctionalized Titanium in a Gap Healing Model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef]
- Ishikawa, T.; Vela, X.; Kida, K.; Moroi, H.; Kitajima, H.; Ogawa, T. Restoration of Optimum Esthetics in Complex Clinical Situations Using an Interdisciplinary Strategy in Combination with Advanced Techniques and Technologies in Regenerative Medicine. J. Cosmet. Dent. 2014, 29, 60–72. [Google Scholar]
- Kitajima, H.; Ogawa, T. The Use of Photofunctionalized Implants for Low or Extremely Low Primary Stability Cases. Int. J. Oral Maxillofac. Implant. 2016, 31, 439–447. [Google Scholar] [CrossRef]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M.; Minamikawa, H.; Sugita, Y.; Yamada, M.; Wakabayashi, N.; Ogawa, T. Novel Antioxidant Capability of Titanium Induced by UV Light Treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef]
- Tsukimura, N.; Yamada, M.; Aita, H.; Hori, N.; Yoshino, F.; Chang-Il Lee, M.; Kimoto, K.; Jewett, A.; Ogawa, T. N-Acetyl Cysteine (NAC)-Mediated Detoxification and Functionalization of Poly(Methyl Methacrylate) Bone Cement. Biomaterials 2009, 30, 3378–3389. [Google Scholar] [CrossRef]
- Nakamura, H.; Shim, J.; Butz, F.; Aita, H.; Gupta, V.; Ogawa, T. Glycosaminoglycan Degradation Reduces Mineralized Tissue-Titanium Interfacial Strength. J. Biomed. Mater. Res. A 2006, 77, 478–486. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-Acetyl Cysteine Protects Osteoblastic Function from Oxidative Stress. J. Biomed. Mater. Res. A 2011, 99, 523–531. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Sato, N.; Iwasa, F.; Hori, N.; Ogawa, T. N-Acetyl Cysteine Alleviates Cytotoxicity of Bone Substitute. J. Dent. Res. 2010, 89, 411–416. [Google Scholar] [CrossRef]
- Aita, H.; Tsukimura, N.; Yamada, M.; Hori, N.; Kubo, K.; Sato, N.; Maeda, H.; Kimoto, K.; Ogawa, T. N-Acetyl Cysteine Prevents Polymethyl Methacrylate Bone Cement Extract-Induced Cell Death and Functional Suppression of Rat Primary Osteoblasts. J. Biomed. Mater. Res. A 2010, 92, 285–296. [Google Scholar] [CrossRef]
- Tsukimura, N.; Ueno, T.; Iwasa, F.; Minamikawa, H.; Sugita, Y.; Ishizaki, K.; Ikeda, T.; Nakagawa, K.; Yamada, M.; Ogawa, T. Bone Integration Capability of Alkali- and Heat-Treated Nanobimorphic Ti-15Mo-5Zr-3Al. Acta Biomater. 2011, 7, 4267–4277. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Minamikawa, H.; Ikeda, T.; Nakagawa, K.; Ogawa, T. Early-Stage Osseointegration Capability of a Submicrofeatured Titanium Surface Created by Microroughening and Anodic Oxidation. Clin. Oral Implant. Res. 2013, 24, 991–1001. [Google Scholar] [CrossRef]
- Kojima, N.; Ozawa, S.; Miyata, Y.; Hasegawa, H.; Tanaka, Y.; Ogawa, T. High-Throughput Gene Expression Analysis in Bone Healing Around Titanium Implants by DNA Microarray. Clin. Oral Implant. Res. 2008, 19, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, N.M.; Hasegawa, M.; Ishijima, M.; Nakhaei, K.; Okubo, T.; Taniyama, T.; Ghassemi, A.; Tahsili, T.; Park, W.; Hirota, M.; et al. Biological and Osseointegration Capabilities of Hierarchically (Meso-/micro-/nanoscale) Roughened Zirconia. Int. J. Nanomed. 2018, 13, 3381–3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Cellular Behavior on TiO2 Nanonodular Structures in a Micro-to-Nanoscale Hierarchy Model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Saruwatari, L.; Takeuchi, K.; Aita, H.; Ohno, N. Ti Nano-Nodular Structuring for Bone Integration and Regeneration. J. Dent. Res. 2008, 87, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Tsukimura, N.; Yamada, M.; Ogawa, T. Enhanced Bone-Integration Capability of Alkali- and Heat-Treated Nanopolymorphic Titanium in Micro-to-Nanoscale Hierarchy. Biomaterials 2011, 32, 7297–7308. [Google Scholar] [CrossRef]
- Yamada, M.; Ueno, T.; Tsukimura, N.; Ikeda, T.; Nakagawa, K.; Hori, N.; Suzuki, T.; Ogawa, T. Bone Integration Capability of Nanopolymorphic Crystalline Hydroxyapatite Coated on Titanium Implants. Int. J. Nanomed. 2012, 7, 859–873. [Google Scholar] [CrossRef] [Green Version]
- Uno, M.; Hayashi, M.; Ozawa, R.; Saruta, J.; Ishigami, H.; Ogawa, T. Mechanical Interlocking Capacity of Titanium with Respect to Surface Morphology and Topographical Parameters. J. Dent. Oral Biol. 2020, 5, 1163. [Google Scholar]
- Uno, M.; Ozawa, R.; Hamajima, K.; Saruta, J.; Ishigami, H.; Ogawa, T. Variation in Osteoblast Retention Ability of Titanium Surfaces with Different Topographies. J. Dent. Oral Biol. 2020, 5, 1169. [Google Scholar]
- Butz, F.; Ogawa, T.; Nishimura, I. Interfacial Shear Strength of Endosseous Implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 746–751. [Google Scholar]
- Ozawa, S.; Ogawa, T.; Iida, K.; Sukotjo, C.; Hasegawa, H.; Nishimura, R.D.; Nishimura, I. Ovariectomy Hinders the Early Stage of Bone-Implant Integration: Histomorphometric, Biomechanical, and Molecular Analyses. Bone 2002, 30, 137–143. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, N.; Ishijima, M.; Okubo, T.; Hirota, M.; Ogawa, T. Disproportionate Effect of Sub-Micron Topography on Osteoconductive Capability of Titanium. Int. J. Mol. Sci. 2019, 20, 4027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Kubo, K.; Yamada, M.; Hori, N.; Suzuki, T.; Maeda, H.; Ogawa, T. Osteoblast Mechanoresponses on Ti with Different Surface Topographies. J. Dent. Res. 2009, 88, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Rodan, G.A. Type beta transforming growth factor (TGF beta) regulation of alkaline phosphatase expression and other phenotype-related mRNAs in osteoblastic rat osteosarcoma cells. J. Cell. Physiol. 1987, 133, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.A.; Tanq, W.; Horowitz, M.C. Cytokine and hormonal stimulation of human osteosarcoma interleukin-11 production. Endcrinology 1995, 136, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, R.; Nagasawa, T.; Kiji, M.; Hormdee, D.; Kobayashi, H.; Koshy, G.; Nitta, H.; Ishikawa, I. Transforming growth factor-beta stimulates interleukin-11 production by human periodontal ligament and gingival fibroblasts. J. Clin. Periodontol. 2006, 33, 165–171. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokeen, B.; Zamani, L.; Zadmehr, S.; Pouraghaie, S.; Ozawa, R.; Yilmaz, B. Surface characterization and assessment of biofilm formation on two titanium-based implant coating materials. Front. Dent. Med. 2021, 2, 51. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Prechtl, C.; Möst, T.; Seidl, C.; Lutz, R.; von Wilmowsky, C. Osseointegration of SLActive Implants in Diabetic Pigs. Clin. Oral Implant. Res. 2013, 24, 128–134. [Google Scholar] [CrossRef]
- Zinelis, S.; Silikas, N.; Thomas, A.; Syres, K.; Eliades, G. Surface Characterization of SLActive Dental Implants. Eur. J. Esthet. Dent. 2012, 7, 72–92. [Google Scholar]
- Anselme, K. Osteoblast Adhesion on Biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and Non-Thermal Plasma Activation of Titanium Surfaces. Clin. Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 2533:1975 Standard Atmosphere, 1st ed.; International Organization for Standardization: Geneva, Switzerland, 1975. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Okubo, T.; Saruta, J.; Hirota, M.; Kitajima, H.; Yanagisawa, N.; Ogawa, T. Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization. Materials 2021, 14, 5493. https://doi.org/10.3390/ma14195493
Ikeda T, Okubo T, Saruta J, Hirota M, Kitajima H, Yanagisawa N, Ogawa T. Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization. Materials. 2021; 14(19):5493. https://doi.org/10.3390/ma14195493
Chicago/Turabian StyleIkeda, Takayuki, Takahisa Okubo, Juri Saruta, Makoto Hirota, Hiroaki Kitajima, Naoki Yanagisawa, and Takahiro Ogawa. 2021. "Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization" Materials 14, no. 19: 5493. https://doi.org/10.3390/ma14195493
APA StyleIkeda, T., Okubo, T., Saruta, J., Hirota, M., Kitajima, H., Yanagisawa, N., & Ogawa, T. (2021). Osteoblast Attachment Compromised by High and Low Temperature of Titanium and Its Restoration by UV Photofunctionalization. Materials, 14(19), 5493. https://doi.org/10.3390/ma14195493






